Abstract
AIM: To explore the expression and clinicopathological significance of cyclooxygenase-2 (COX-2) and microvessel density (MVD) in gastric carcinogenesis, and to investigate their roles in the invasion and the relationship between biological behaviors and prognosis of gastric cancer.
METHODS: Using Envision immunohistochemistry, COX-2 and CD34 expressions in gastric cancer tissue array were examined. MVD was counted and the relationship between the biological behaviors and prognosis was analyzed.
RESULTS: The expression of COX-2 in gastric cancer tissue was significantly higher than that in normal mucosa (χ2 = 12.191, P < 0.05). The over-expression of COX-2 in gastric cancer was obviously related to metastasis and depth of invasion (χ2 = 6.315, P < 0.05), but not related to the histological type and Borrmann type (χ2 = 5.391 and χ2 = 2.228, respectively). Moreover, MVD in gastric cancer tissues was significantly higher than that in the normal mucosa (65.49 ± 20.64 vs 36.21 ± 18.47, t/F = 7.53, P < 0. 05). MVD was related to the histologic type and metastasis (t/F = 3.68 and t/F = 4.214, respectively, P < 0. 05), but not related to the depth of invasion and Borrmann type (t/F = 0.583 and t/F = 0.459, respectively). MVD in COX-2-positive tissues was markedly higher compared to COX-2-negative tissues, indicating a positive correlation between COX-2 expression and MVD (t = 13.12, P < 0. 05).
CONCLUSION: Tissue microarray (TMA) is a powerful tool for rapid identification of the molecular alterations in gastric cancer. COX-2 expression, via inducing angiogenesis, may play an important role in gastric carcinogenesis. It could be served as a determinant factor for clinical prognosis and curative effect.
Keywords: Gastric cancer, Tissue microarray, COX-2, Immunohistochemistry, CD34, Microvessel density
INTRODUCTION
Gastric cancer is one of the most common malignant gastrointestinal tumors, the incidence and mortality of gastric cancer show an upward tendency. The unlimited growth of the tumor cell and the metastasis are the important trait of the tumor, are the main cause of cancer-related death induced by the failure of treatment of gastric cancer. Growth and metastasis of tumor cell are regulated by many factors[1]. The tissue array/tissue microarrays (TMA) is a new technique, with numerous advantages including, decreased assay volume, amplification of resource information, high laboratory efficiency, flexible design, experimental uniformity[2,3]. Many researches have suggested the TMA is very useful in the oncology-pathologic research and clinical-pathologic research. Cyclooxygenase-2 (COX-2) is the central enzyme in the biosynthetic pathway to prostaglandins (PGs) from arachidonic acid (AA). Studies from different laboratories suggested that over-expression of COX-2 was detected in colon tumors and other tumors[4-8]. Non-steroid anti-inflammatory drugs (NSAIDs) are the COX-2 inhibitors which have been used as a chemical preventive agent in the animal models of colonic tumors[9,10]. It is well known that tumor growth and angiogenesis are inter-dependent. In this study, using Envision immunohistochemistry and TMA, we aimed to explore the expression and clinicopathological significance of COX-2 and angiogenesis in the gastric carcinogenesis, and further to investigate their roles in the cancer invasion and the relationship between biological behaviors and prognosis of gastric cancer.
MATERIALS AND METHODS
Materials
One hundred and four gastric cancer specimens and 79 normal gastric tissues (resected concurrently 5 cm from the tumor tissues) surgically resected in the First Affiliated Hospital of China Medical University from December 2003 to May 2004 were used. 100 mL/L formalin-fixed and paraffin-embedded tissues were subjected to routine sectioning of 4 μm thickness and HE staining for specific locations. Undiluted rabbit anti-human COX-2 monoclonal antibody and undiluted rat anti-human CD34 monoclonal antibody were purchased from Maixin Bio, China. UltraSensitiveTM PV9000 kit was purchased from Zhongshan Biological Inc., Beijing, China. Tissue arrayer and punches in size 1 mm diameter were from Beecher Instruments, USA.
Tissue microarray preparation
The needed spots were chosen under microscopy from each case and marked on the corresponding spot on the tissue block. The needed spots included the typical tumor, intestinal metaplasia, dysplasia and normal tissue. Then cyclinderic tissue columns (1 mm diameter, 4 mm height) were punctured with tissue arrayer in the marked area and transferred to corresponding receiver pore of the prepared block. The two tissue array blocks, which had 225 specimens, were then completed according to the predetermined scheme. The blocks were heated at 42°C for 3 min, and then were routinely sectioned at 4-μm thickness.
Immunohistochemical staining
Gastric cancer tissue arrays were subjected to Envision immunohistochemical staining. Rabbit anti-human COX-2 monoclonal antibody was used to detect COX-2 protein expression. About 200 cells were counted in two representative high-power fields to calculate COX-2 positive rate as follows: positive rate < 5% = negative (-); 5%-25% = weakly positive (+); 25%-50% = moderately positive (++); and > 50% = strongly positive (+++). The microvessel density (MVD) was determined by immunostaining for CD34 which was expressed in the cytoplasm and membrane of endothelial cells. Any more than three brown-stained endothelial cells or endothelial cell cluster that was clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements was considered a single, countable microvessel. The vessels with area more than the diameter of 8 red cells, and the vessels with thick tunica media were not considered microvessel[11,12]. The areas of highest neovascularization were identified as regions of invasive carcinoma with the highest numbers of discrete microvessels stained for CD34. Each count was expressed as the highest number of microvessels identified at a magnification of × 400. At least two fields were analyzed for each tumor. All counts were performed by two investigators simultaneously and independently. The primary antibody was replaced by PBS for negative control.
Statistical analysis
Data were analyzed by Chi-square test. P < 0.05 was considered statistically significant. All analyses were performed with the SPSS statistical software (Ver. 12.0).
RESULTS
Quality of tissue array
The gastric precancerous lesions and gastric cancer tissue array had good morphology. The HE-stained tissue arrays were highly representative of their donor tissues. Some gastric cancer tissue samples fell off or became invalid during the immunohistochemical process, and the other samples displayed intense signals with clear background (Figures 1 and 2).
Figure 1.

Scanning image of tissue array. A: HE Staining; B: COX-2 immuno-histochemical staining.
Figure 2.

Scanning image of tissue array. A: HE, × 100; B: COX-2 immuno-histochemical staining, × 100.
Expression of COX-2 in the gastric cancer and normal mucosa
Eighty-seven gastric cancer samples and 37 distal normal mucosa samples were valid for the immuno-histochemical staining of COX-2 antibody-stained array. Immunohistochemical tissue array demonstrated that COX-2 protein was located in the cytoplasm and nuclear membrane, mostly in the cancerous tissue (Figure 3). COX-2 expression was obviously higher in gastric cancer tissues compared to the normal mucosa (P < 0.05). The over-expression of COX-2 in gastric cancer was significantly related to metastasis and the depth of invasion (P < 0.05), but not related to the histological type and Borrmann type (Table 1).
Figure 3.
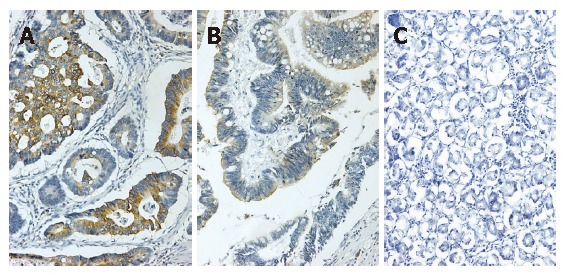
COX-2 expression in gastric cancer. A: Positive COX-2 expression in the well-differentiated adenocarcinoma (Envision, × 200); B: Positive COX-2 expression in the moderately-differentiated adenocarcinoma (Envision, × 200); C: Negative COX-2 expression in the normal gastric mucosa (Envision, × 200).
Table 1.
Expression of COX-2 protein in the gastric cancer and normal mucosa
| Variable | n |
COX-2 expression |
COX-2-positive rate (%) | χ2 | P | |
| - | + - +++ | |||||
| Tissue character | 12.191 | 0.000 | ||||
| Normal mucosa | 37 | 15 | 22 | 59.46 | ||
| Gastric cancer | 87 | 11 | 76 | 87.36 | ||
| Histologic type | 5.391 | 0.495 | ||||
| Papillary adenocarcinoma | 2 | 0 | 2 | 100.00 | ||
| Well-differentiated adenocarcinoma | 8 | 2 | 6 | 75.00 | ||
| Moderately-differentiated adenocarcinoma | 28 | 4 | 24 | 85.71 | ||
| Poorly-differentiated adenocarcinoma | 29 | 2 | 27 | 91.10 | ||
| Mucin adenocarcinoma | 12 | 0 | 12 | 100.00 | ||
| Signet-ring cell carcinoma | 5 | 1 | 4 | 80.00 | ||
| Undifferentiated carcinoma | 2 | 1 | 1 | 50.00 | ||
| Metastasis | 5.502 | 0.019 | ||||
| Negative | 21 | 6 | 15 | 71.43 | ||
| Positive | 66 | 5 | 61 | 92.42 | ||
| Depth of invasion | 6.315 | 0.043 | ||||
| Muscle invasion | 3 | 0 | 3 | 10.00 | ||
| Subserosa invasion | 68 | 20 | 48 | 70.59 | ||
| Serosa exposure invasion | 23 | 2 | 21 | 91.30 | ||
| Borrman type | 2.228 | 0.328 | ||||
| Borrman II | 7 | 0 | 7 | 100.00 | ||
| Borrman III | 85 | 9 | 76 | 89.41 | ||
| Borrman IV | 4 | 1 | 3 | 75.00 | ||
MVD in the gastric cancer and normal mucosa
Ninety-two gastric cancer samples and 38 distal normal mucosa samples were valid for the immunohistochemical staining of anti-CD34 antibody-stained array. Immuno-histochemical tissue array demonstrated that brown-stained CD34 protein expression was located in the microvessels (Figure 4). MVD in gastric cancer tissues (65.49 ± 20.64, ranged from 16 to 100, median 65.5) was significantly higher than that in the normal mucosa (36.21 ± 18.47, P < 0.05). MVD was obviously related to the histological type and metastasis (P < 0. 05), but not to the depth of invasion and Borrmann type. Moreover, MVD was obviously higher in the poorly-differentiated gastric cancer tissues compared to the well-differentiated gastric cancer tissues (P < 0.05) (Table 2).
Figure 4.
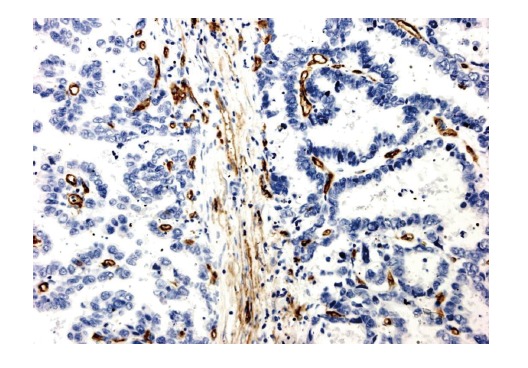
CD34 expression in the gastric tissue. Immunostaining of CD34 in the cytoplasm and membrane of endothelial cells (Envision, × 200).
Table 2.
MVD in the gastric cancer and normal mucosa (mean ± SD)
| Variable | n | MVD | t/F | P |
| Tissue character | 7.53 | 0.000 | ||
| Normal mucosa | 38 | 36.21 ± 18.47 | ||
| Gastric cancer | 92 | 65.49 ± 20.64 | ||
| Histologic type | 3.68 | 0.003 | ||
| Papillary adenocarcinoma | 2 | 39.50 ± 7.78 | ||
| Well-differentiated adenocarcinoma | 9 | 42.56 ± 20.35 | ||
| Moderately-differentiated adenocarcinoma | 28 | 56.79 ± 20.97 | ||
| Poor-differentiated adenocarcinoma | 31 | 72.65 ± 20.49 | ||
| Mucin adenocarcinom | 14 | 63.64 ± 23.02 | ||
| Signet-ring cell carcinoma | 5 | 74.40 ± 33.56 | ||
| Undifferentiated carcinoma | 3 | 44.67 ± 20.23 | ||
| Histologic type | 4.83 | 0.000 | ||
| Moderately or well-differentiated | 39 | 54.68 ± 19.78 | ||
| Poorly or undifferentiated | 53 | 73.88 ± 17.25 | ||
| Metastasis | 4.214 | 0.043 | ||
| Negative | 26 | 54.08 ± 24.42 | ||
| Positive | 66 | 65.08 ± 22.63 | ||
| Organ metastasis | 1.14 | 0.355 | ||
| Lymph nodes metastasis | 66 | 65.08 ± 22.63 | ||
| Liver metastasis | 4 | 82.00 ± 10.61 | ||
| Ovary metastasis | 1 | 100 | ||
| Peritoneal metastasis | 9 | 68.44 ± 26.16 | ||
| Depth of invasion | 0.583 | 0.627 | ||
| Muscle invasion | 4 | 68.00 ± 19.13 | ||
| Subserosa invasion | 63 | 60.33 ± 24.28 | ||
| Serosa exposure invasion | 24 | 65.96 ± 22.68 | ||
| Borrman type | 0.459 | 0.634 | ||
| Borrman II | 7 | 55.71 ± 17.17 | ||
| Borrman III | 79 | 62.92 ± 23.89 | ||
| Borrman IV | 6 | 61.97 ± 23.55 |
Relation between COX-2 expression and MVD in the gastric cancer
MVD in the COX-2-positive tissues was markedly higher than that in the COX-2-negative tissue, indicating an obvious positive correlation between COX-2 expression and MVD (P < 0. 05) (Table 3).
Table 3.
Correlation between COX-2 protein expression and MVD in gastric cancer
| Degree of staining | MVD | t | P | |
| COX-2-negative | - | 25.82 ± 7.76 | 13.12 | 0.000 |
| COX-2-positive | + - +++ | 68.59 ± 19.8 |
DISCUSSION
The tissue array/tissue microarray (TMA) is a technique which is to re-locate a large numbers of tissues in the predicted order on a slide. It is a high-throughput analysis tool of multi-sample. It gets over the lag manner of the conventional pathological technique, decreases the time and money consumption, and promotes the laboratory rate to enable 'genome-scale' molecule pathology studies. It also facilitates the analysis of molecular alteration in thousands of tissue specimens in a massively parallel fashion, avoids the result errors of different experiment conditions. It can analyze a specific gene at DNA, RNA, protein and antibody levels with conventional pathologic technique, histochemistry and immuno-histochemistry[13-15]. Moch et al[16] screened 89 genes with differential expression between the renal cancer cell line and normal kidney tissue by cDNA array analysis, one of them coded for vimentin, a cytoplasmic intermediate filament. Then a renal cancer tissue array containing 532 renal cell carcinoma specimens was used to determine vimentin expression by immunohistochemistry. The over-expression of vimentin was seen in the most renal cancer, and it was significantly associated with poor prognosis of patients. Gene chip can screen the genes with differential expression between the cancer and normal tissue, and tissue array can investigate prevalence or prognostic significance of the genes' molecular changes. Taken together, they can facilitate the translation of findings from basic research into clinical applications. Schraml et al[17] made a tissue array containing 397 tumor specimens from 17 different tumor types, amplification of three extensively studied oncogenes (CCND1, CMYC, and ERBB2) was analyzed in three fluorescence in situ hybridization experiments from consecutive sections cut from the tissue array. Amplification of CCND1 was found in the breast, lung, head and neck, and bladder cancer, as well as in melanoma. CMYC was amplified in the breast, colon, kidney, lung, ovary, bladder, head and neck, and endometrial cancer. ERBB2 was amplified in the bladder, breast, colon, stomach, testis, and lung cancer. These results confirm and even extend existing data in the literature on such amplifications. And this study revealed, for the first time, an ERBB2 amplification in an embryonal carcinoma of the testis. In the tumor research, tissue array is ideally suitable for genomics-based diagnostic and drug target discovery by revealing the cellular localization, prevalence and clinical significance of candidate cancer genes. We constitute a tissue array consisting of 225 samples from 104 gastric cancer and normal gastric mucosa, to detect the expression of COX-2 and CD34.
Cyclooxygenase (COX) is one of the rate-limiting enzymes in metabolism of arachidonic acid that catalyzes the arachidonic acid into a series of products, such as prostaglandins and other eicosanoids. It has two isoforms in human, constitutive COX-1 and inducible COX-2. COX-2 maps to 1q25.2-q25.3, contains 11 exons and 10 introns, is 8.3 kb in size[9,10]. COX-1 is now known to be present in most tissues as the housekeeper enzyme, to maintain the normal physiological function. It maintains normal gastric mucosa and influences kidney function. COX-2 is considered "the immediate early gene", it is composed when the cell is stimulated and it takes part in many pathophysiologic processes, such as carcinogenesis and inflammation[18]. The regulation of COX-2 expression is mainly on the level of transcription, in the other words, the signal transduction pathway leading to COX-2 protein expression is initiated when the cell is stimulated. It has been documented that COX-2 plays an important role in the development of human tumors[19,20]. The high expression of COX-2 is the early process of carcinogensis in general[21]. Many researches verified the relation between COX-2 and the gross type, pathologic type, TNM stage and lymph-node metastasis. Murata et al[22] reported that COX-2 over-expression in gastric cancer was significantly correlated with tumor invasion into the lymphatic vessels in the gastric wall and metastasis to the lymph nodes, but not correlated with histopathological grading, surface size, and venous vessel invasion of the tumors. Fosslien et al[23] reviewed that the COX-2 produced by a malignant tumor and COX-2 produced by the surrounding host tissue both contribute to new vessel formation, which explains how selective COX-2 inhibition reduces tumor growth where the tumor COX-2 gene has been silenced by methylation. COX-2 could induce tumor angiogenesis by the over-expression of prostaglandin (PG) in the tumor tissue, it is perhaps the basement of the growth, invasion and metastasis of tumor. Ohno et al[24] detected the expression of COX-1 and COX-2 concomitantly in 33 surgical specimens (including carcinomas and corresponding non-cancerous mucosa) by RT-PCR and immunohistochemistry and HE. The COX-2 index in gastric carcinoma was significantly higher than in normal mucosa (3.4 ± 0.7 vs 2.2 ± 0.7; P < 0.05). In addition, COX-2 indices were significantly higher in gastric carcinoma tissues with deep invasion (P < 0.05). Immunohistochemistry demonstrated COX-2 protein located diffusely in the cytoplasm of tumor cells, but not in surrounding stroma or in non-cancerous mucosa. Lim et al[25] measured COX-2 expression in 104 human gastric carcinoma tissues by immunohistochemical analysis and Western blot analysis, demonstrating that COX-2 protein was over-expressed in the gastric cancer tissues as compared to the normal gastric mucosa. Similarly, we found that the expression of COX-2 in the gastric cancer tissue was obviously higher than that in normal mucosa. The over-expression of COX-2 in gastric cancer was related to the metastasis and depth of invasion, but not to the histological type and Borrmann type.
MVD is a reliable index of tumor angiogenesis. The tumor growth and metastasis is dependent upon the new blood vessel formation. Tumor angiogenesis is associated with the production of highly permeable and poorly-formed vasculature, thereby facilitates the metastasis of tumor cells via the bloodstream[26-28]. So, the detection of gastric cancer MVD can reflect the degree of blood vessel, the growth and metastasis capability of gastric cancer in some extent. Our study showed that MVD in gastric cancer tissues (65.49 ± 20.64) was significantly higher than that in the normal mucosa (36.21 ± 18.47). Moreover, MVD was related to the histological type and metastasis, but not to the depth of invasion and Borrmann type and the organ of metastasis. MVD in the COX-2-positive tissues was markedly higher than that in the COX-2-negative tissues, thereby suggesting a positive correlation between COX-2 expression and MVD.
In conclusion, our study demonstrates that COX-2 protein expression may play an important role in the angiogenesis of gastric cancer, it has an obvious relation with the proliferation of gastric cancer cells and the lymph node metastasis. It could be served as a determinant factor for clinical prognosis and curative effect.
COMMENTS
Background
The tissue array/tissue microarray (TMA) is a technique which is to re-locate a large numbers of tissues in the predicted order on a slide. It is a high-throughput analysis tool of multi-sample. We constituted a tissue array consisting of 225 samples from 104 gastric cancers and normal gastric mucosa, to detect the expression of COX-2 and CD34. Tissue array can help us complete the time- and people-consuming work.
Research frontiers
Tissue array is ideally suitable for genomics-based diagnostic and drug target discovery by revealing the cellular localization, prevalence and clinical significance of candidate cancer genes. In this study, we detected two hot molecules expressions by using the tissue microarray which allows rapid visualization of molecular targets at a time in the same level.
Innovations and breakthroughs
The expressions of these molecules were detected by using tissue micoarray. In this study, we explored the expression and clinicopathological significance of COX-2 and MVD in gastric carcinogenesis, investigated their roles in the invasion and the relationship between biological behaviors and prognosis of gastric cancer.
Applications
Tissue array can facilitate rapid translation of molecular discoveries to clinical applications used. So we can speculate that the tissue microarray technology, which is fast, convenient and economic, may have potential dominant position in macro-scale detection of tissue specimens in cancer studies. Moreover, COX-2 protein expression may play an important role in the angiogenesis of gastric cancer. It may be served as an important molecule on the clinical prognosis and curative effect.
Terminology
In 1998, Kononen et al developed tissue array whereby an ordered array of tissue samples are placed on a single slide. Once constructed, it can be probed with a molecular target (DNA, RNA or protein) for analysis by immunohistochemistry, fluorescence in situ hybridization (FISH) or other molecular detection methods, enabling a high-throughput in situ analysis of specific molecular targets in hundreds or even thousands of tissue specimens.
Peer review
Mao and colleagues looked at the expression of COX-2 gene in gastric cancer, and its relationship with angiogenesis by using tissue microarray. They found a significant higher expression of COX-2 in gastric cancer tissue than that in normal mucosa, which was positively associated with tumor metastasis, invasion and angiogenesis.
Footnotes
S- Editor Liu Y L- Editor Kumar M E- Editor Lu W
References
- 1.Han CB, Mao XY, Xin Y, Wang SC, Ma JM, Zhao YJ. Quantitative analysis of tumor mitochondrial RNA using microarray. World J Gastroenterol. 2005;11:36–40. doi: 10.3748/wjg.v11.i1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng M, Simon R, Kononen J, Sauter G, Mihatsch MJ, Moch H. [Analysis of gene expression profiles among 3 epithelial ovarian tumor subtypes using cDNA and tissue microarrays] Ai Zheng. 2004;23:771–776. [PubMed] [Google Scholar]
- 3.Andersen CL, Monni O, Wagner U, Kononen J, Bärlund M, Bucher C, Haas P, Nocito A, Bissig H, Sauter G, et al. High-throughput copy number analysis of 17q23 in 3520 tissue specimens by fluorescence in situ hybridization to tissue microarrays. Am J Pathol. 2002;161:73–79. doi: 10.1016/S0002-9440(10)64158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamradt SC, Feeley BT, Liu NQ, Roostaeian J, Lin YQ, Zhu LX, Sharma S, Dubinett SM, Lieberman JR. The effect of cyclooxygenase-2 (COX-2) inhibition on human prostate cancer induced osteoblastic and osteolytic lesions in bone. Anticancer Res. 2005;25:107–115. [PubMed] [Google Scholar]
- 5.Hasegawa K, Ohashi Y, Ishikawa K, Yasue A, Kato R, Achiwa Y, Nishio E, Udagawa Y. Expression of cyclooxygenase-2 in uterine endometrial cancer and anti-tumor effects of a selective COX-2 inhibitor. Int J Oncol. 2005;26:1419–1428. [PubMed] [Google Scholar]
- 6.Block KI. Inflammation, COX-2 inhibitors, and cancer. Integr Cancer Ther. 2005;4:3–4. doi: 10.1177/1534735405274664. [DOI] [PubMed] [Google Scholar]
- 7.Wendum D, Masliah J, Trugnan G, Fléjou JF. Cyclooxygenase-2 and its role in colorectal cancer development. Virchows Arch. 2004;445:327–333. doi: 10.1007/s00428-004-1105-2. [DOI] [PubMed] [Google Scholar]
- 8.Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 9.Hutchison R. COX-2--selective NSAIDs. Am J Nurs. 2004;104:52–55; quiz 55-6. doi: 10.1097/00000446-200403000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Evans JF, Kargman SL. Cancer and cyclooxygenase-2 (COX-2) inhibition. Curr Pharm Des. 2004;10:627–634. doi: 10.2174/1381612043453126. [DOI] [PubMed] [Google Scholar]
- 11.Boxer GM, Tsiompanou E, Levine T, Watson R, Begent RH. Immunohistochemical expression of vascular endothelial growth factor and microvessel counting as prognostic indicators in node-negative colorectal cancer. Tumour Biol. 2005;26:1–8. doi: 10.1159/000084180. [DOI] [PubMed] [Google Scholar]
- 12.Preziosi R, Sarli G, Paltrinieri M. Prognostic value of intratumoral vessel density in cutaneous mast cell tumors of the dog. J Comp Pathol. 2004;130:143–151. doi: 10.1016/j.jcpa.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Sun Y, Kong QY, Zhang KL, Wang XW, Chen XY, Wang Q, Liu J. Combination of nucleic acid and protein isolation with tissue array construction: using defined histologic regions in single frozen tissue blocks for multiple research purposes. Int J Mol Med. 2003;12:299–304. [PubMed] [Google Scholar]
- 14.Lusis EA, Chicoine MR, Perry A. High throughput screening of meningioma biomarkers using a tissue microarray. J Neurooncol. 2005;73:219–223. doi: 10.1007/s11060-004-5233-y. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Li JY, He JS, Zhou LX, Chen K. Tissue microarray analysis of multiple gene expression in intestinal metaplasia, dysplasia and carcinoma of the stomach. Histopathology. 2005;46:505–514. doi: 10.1111/j.1365-2559.2005.02111.x. [DOI] [PubMed] [Google Scholar]
- 16.Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T, Mihatsch MJ, Kallioniemi OP, Sauter G. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol. 1999;154:981–986. doi: 10.1016/S0002-9440(10)65349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schraml P, Kononen J, Bubendorf L, Moch H, Bissig H, Nocito A, Mihatsch MJ, Kallioniemi OP, Sauter G. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res. 1999;5:1966–1975. [PubMed] [Google Scholar]
- 18.Mohammed NA, Abd El-Aleem SA, El-Hafiz HA, McMahon RF. Distribution of constitutive (COX-1) and inducible (COX-2) cyclooxygenase in postviral human liver cirrhosis: a possible role for COX-2 in the pathogenesis of liver cirrhosis. J Clin Pathol. 2004;57:350–354. doi: 10.1136/jcp.2003.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393–1399. [PubMed] [Google Scholar]
- 20.Eibl G, Takata Y, Boros LG, Liu J, Okada Y, Reber HA, Hines OJ. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Res. 2005;65:982–990. [PubMed] [Google Scholar]
- 21.Yazawa K, Tsuno NH, Kitayama J, Kawai K, Okaji Y, Asakage M, Sunami E, Kaisaki S, Hori N, Watanabe T, et al. Selective inhibition of cyclooxygenase-2 inhibits colon cancer cell adhesion to extracellular matrix by decreased expression of beta1 integrin. Cancer Sci. 2005;96:93–99. doi: 10.1111/j.1349-7006.2005.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. doi: 10.1111/j.1572-0241.1999.876_e.x. [DOI] [PubMed] [Google Scholar]
- 23.Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci. 2001;31:325–348. [PubMed] [Google Scholar]
- 24.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 25.Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519–525. [PubMed] [Google Scholar]
- 26.Arbiser JL. Implications of Epstein-Barr Virus (EBV)-induced carcinogenesis on cutaneous inflammation and carcinogenesis: evidence of recurring patterns of angiogenesis and signal transduction. J Invest Dermatol. 2005;124:xi–xii. doi: 10.1111/j.0022-202X.2005.23695.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoshiji H, Noguchi R, Kuriyama S, Yoshii J, Ikenaka Y. Combination of interferon and angiotensin-converting enzyme inhibitor, perindopril, suppresses liver carcinogenesis and angiogenesis in mice. Oncol Rep. 2005;13:491–495. [PubMed] [Google Scholar]
- 28.Onizuka S, Kawakami S, Taniguchi K, Fujioka H, Miyashita K. Pancreatic carcinogenesis: apoptosis and angiogenesis. Pancreas. 2004;28:317–319. doi: 10.1097/00006676-200404000-00020. [DOI] [PubMed] [Google Scholar]


