Abstract
AIM: To investigate a specific association between hepatic steatosis and hepatitis C virus (HCV) core.
METHODS: HeLa cells and primary mouse hepatocytes were transfected with HCV core plasmid, and conditional transgenics in which hepatic over-expression of HCV core is regulated by the tetracycline-off system, were developed. The expression of the HCV core was assessed over one to six months after withdrawal of doxycycline (dox) by immunohistochemistry (IHC) and Western blotting and by sequential liver biopsy. Hepatic steatosis was evaluated using oil red stain. 8-hydroxydeoxyguanosine (8-OHdG) stains and caspase levels were conducted to clarify hepatic oxidative stress and apoptosis rate. Serum aminotransferase was checked.
RESULTS: The transfected hepatocytes had globular cores under the lipid vesicles. In transgenic mice on control diet, core expression was robust, localized to the cytoplasmic vesicle membrane and strongly associated with microvesicular steatosis, which was gradually replaced by macrovesicular steatosis. However, both steatosis and core positive hepatocytes diminished with time. Increases in aminotransferase, caspase and 8-OHdG were associated with peak core expression.
CONCLUSION: The core protein was readily detected and morphologically associated with steatosis in individual hepatocytes both in vitro and in vivo. In vivo, oxidative stress caused by the core potentially reduced the number of core positive hepatocytes and in parallel the level of steatosis. To our knowledge, this is the first animal model that directly shows topological relationship between HCV core and hepatic lipid vesicles.
Keywords: Hepatic steatosis, Conditional transgenic mice, HCV core, Oxidative stress
INTRODUCTION
Infection with the hepatitis C virus (HCV) causes chronic hepatic fibrosis, cirrhosis and hepatocellular carcinoma[1]. While the cellular pathogenesis of these events are incompletely understood, chronic inflammation is believed to play a central role that involves the presence of T cell subsets with specific reactivity toward HCV gene products[2]. An HCV-infected liver, however, exhibits not only inflammation but also steatosis. Also, diabetes is considerably more commonly associated with this disease than other forms of chronic hepatitis[3]. The "metabolic" changes associated with HCV, notably fatty liver and diabetes, are less well understood.
HCV core protein, a structural protein that modulates cellular processes, was shown to be the predictor of human steatosis[4]. Many in vitro studies also documented its association with cellular lipid droplet[5,6], its unique sequence motifs required for lipid droplet association and protein stability[7] and the intramembrane proteolysis promotes trafficking of it to lipid droplets[8].
Transgenic mice that express HCV-cores exhibit certain features of the human infection, such as steatosis[9,10], insulin resistance[11] and ultimately cancer[12]. However, a problem associated with the published transgenics is the level of HCV-core expression, which is detectable by either RT-PCR or Western blotting[9-12]. Staining for core protein in liver sections is rarely strong enough to observe its association with cellular lipid directly. Furthermore, these transgenics are conventional, with expression of core protein during the latter part of gestation as well as following birth. As models, they differ from human HCV infection in that in humans, virtually all HCV is acquired postnatally, and most is acquired in adulthood. The fetus appears to be protected. The virus does not cross the placenta, and perinatal infection is rare[13]. These facts raise the question of whether the presentation of HCV-core in the developing liver has unique metabolic effects or underlies the low level of viral protein expression in the transgenic mice reported to date.
Although core expression has been documented both in vitro and in vivo, a definite topological relationship between the HCV core protein and the intrahepatic cellular lipid vesicles has never been established in vivo. Mice that over-express the HCV core protein were developed to address this question. The transgene can be suppressed until the animal is weaned and fully developed. When the mouse is placed on standard chow, the core protein appears over the subsequent one to five months. This model more closely replicates the timing of typical hepatitis C infection in humans than constitutive transgenics. Core expression is robust, allowing its subcellular localization to be studied. The results provide morphological evidence of the direct effect of the HCV core on hepatic lipid vesicle formation.
MATERIALS AND METHODS
Transgenic mice
Mice, in which the expression of the hepatitis C core gene was suppressible by tetracycline, were raised. The details will be described elsewhere. Briefly, the HCV core gene sequence was isolated by RT-PCR from the plasma of a patient with chronic hepatitis C, genotype 1b. It was cloned into the PUGH16-3 vector, which contains the tetracycline response element (TRE)[14]. Fertilized ova from FVB/N mice were injected with the construct, and several founder mice were obtained. These were crossed with a second transgenic line that is homozygous for the tetracycline transactivator (tTA) under control of the liver activator protein (LAP) promoter which is hepatocyte-specific[15]. The LAP-tTA mice were generously provided by JM Bishop and Rong Wang (University of California, San Francisco). Unless otherwise indicated, mating pairs were maintained on doxycycline (dox)-containing chow, to suppress the HCV-core during development and through weaning. At approximately one month of age, dox was withdrawn.
Cell culture and transfection
HeLa cells that express tTA (Clontech) and primary hepatocytes isolated from LAP-tTA mice were used for transfection of both PUGH16-3-core and PUGH16-3 plasmids. Primary hepatocytes were prepared by perfusion of the liver with collagenase, as described elsewhere[16] with a perfusion rate of 5 mL/min. Transfection was carried out with Lipofectin 2000 (Invitrogen) following the vendor's protocol. HeLa cells or hepatocytes were plated at a density of 5000000 and 2500000 respectively, on glass coverslips in 6-well plates. The cells were transfected with plasmids, with or without the addition of doxycycline to the culture medium. At 48 h after transfection, the cells were harvested to extract protein, or fixed for IHC studies.
HCV core protein expression
A wedge biopsy of the liver, kidney, spleen, intestine and thymus (ca. 100 mg) were taken from the mouse under enflurane anesthesia. The remainder underwent histological examination. Serial liver biopsy was performed every two months in the transgencis. For Western blotting, cultured cells or mouse liver was extracted. After electrophoresis, the protein was transferred to a PVDF membrane (Biorad), and incubated with a 1:250 dilution of monoclonal mouse anti-HCV core antibody (Anogen). After washing, the membrane was incubated with secondary antibody (Biorad) and developed with an ECL kit (Amersham). A Fluor-S multiImager and Quality One Software were used to quantify core staining according to the manufacturer's protocol (Bio-Rad).
Liver biopsies or cells in culture were fixed in freshly prepared 4% paraformaldehyde (PFA) for IHC examination. The cells were permeabilized with 0.1% Triton-100; incubated with HCV core antibody; washed, and then incubated with fluoresceinated secondary antibody (Caltag).
Transfected cultured cells were examined by immunoelectron microscopy. The cells were fixed in 4% PFA and the pellets were dehydrated in methanol and embedded using Lowicryl K 4 M (Chemische). The grids were incubated with monoclonal mouse HCV core antibody (Affinity Bioreagents); and incubated with goat anti-mouse IgG labeled with 10 nm gold particles (Biocell).
Hepatic inflammation evaluation
The histology of hepatic inflammation was evaluated by standard hematoxylin and eosin staining according to hepatic activity index score. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured using a Vitros DT60 II Chemistry System (Johnson-Johnson).
Hepatic apoptosis evaluation
Caspase-9 was measured with a colorimetric assay (Promega) for the frozen liver homogenates according to the vendor's protocol.
Oxidative stress evaluation
Sections fixed in PFA were doubly stained for 8-hydroxydeoxyguanosine (8-OHdG) and HCV core to evaluate the oxidative stress. After staining for HCV core protein, the samples were incubated with monoclonal mouse 8-OHdG Ab (Oxis Corp.). The positive rates were defined as the number of 8-OHdG (+) hepatocytes divided by total number of hepatocytes within the same field in 3 randomly selected fields.
Fat stain
Fat vesicles were identified by oil-red-O stain in the frozen liver section using the commercial kit (Biogenex Lab) according to the manufacturer's protocol.
RESULTS
Conditional expression of HCV core protein in cell culture
The extracts were prepared from transfected HeLa cells for Western blot of HCV core protein. As displayed in Figure 1A (lane 1), a prominent band of 21 kDa was observed (arrows); it was undetectable in cells exposed to doxycycline (lane 2). IHC (Figure 2A) revealed that HCV core protein was located exclusively in the cytoplasm, but its apparent level varied from cell to cell within the same culture. Also, two patterns were noted: condensed perinuclear (Figure 2A) and disseminated granular/particulate (Figure 2B). Different results were obtained when primary hepatocytes were used in place of HeLa cells. When hepatocytes were transfected in primary culture with the HCV core construct in the absence of doxycycline, both granular and globular (arrows) fluorescence was seen (Figure 2C). Immunoelectronic microscopy verified that globular staining is caused by the association of the HCV core with the membranes of the lipid vesicles (Figure 2E, arrows).
Figure 1.
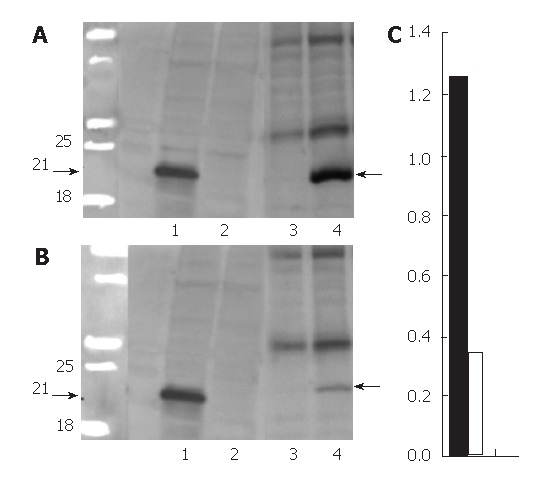
Western blotting for protein extracted from transfected HeLa cells and representative transgenic mice liver. A: two months old mice; B: the faint 21-KD-core band (line 4) in six months old mice. Lanes 1 and 2: proteins from transfected HeLa cells without and with the administration of doxycycline which served as positive and negative controls. Lanes 3 and 4: proteins from the transgenic mice fed with and without doxycycline chow, respectively; C: The quantitative comparison of the blotting. Black bar, female 2 mo old DTM liver protein (1.26 ± 0.08); white bar: female 6 mo old DTM liver protein (0.34 ± 0.05), P = 0.002 between black and white bars (mean ± SD, n = 3).
Figure 2.
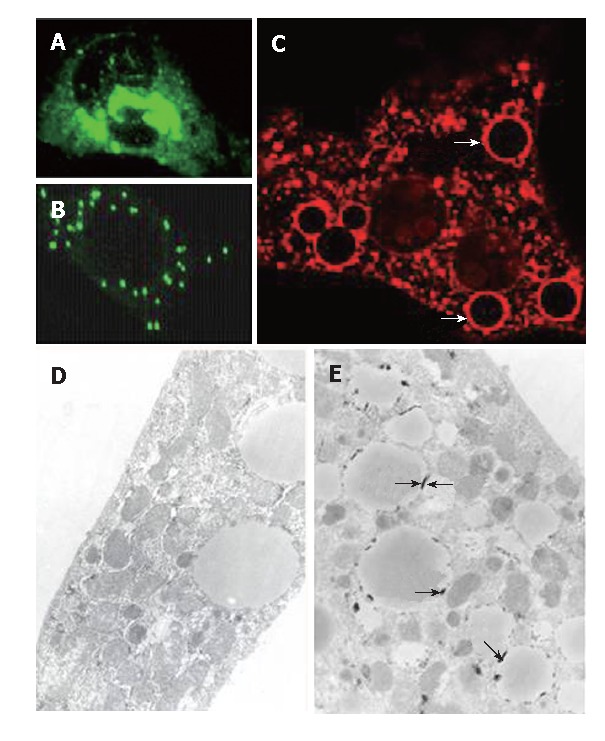
A and B: Immunofluorescent stain for transfected HeLa cells and primary hepatocytes (C) (× 60). Two cytoplasmic distribution patterns of the core protein in HeLa cells were obtained-condensed perinuclear (A) and disseminated granular (B) patterns. The core protein was present exclusively in the cytoplasm of hepatocytes and some of it exhibited the vesicular pattern (C, × 60, arrows). Under the electron microscope (D and E, × 100), the vesicular structures were found to be lipid vesicles and the core protein was located just underneath the surface of the vesicles (E, arrows), whereas the cells transfected with empty plasmid (PUGH16-3) showed no core associated with the lipid vesicles (D).
Conditional expression of HCV core protein in vivo
Doubly transgenic mice (DTM) (LAP-tTA × HCV core) were raised as described; some were removed from dox-containing chow at weaning (one month old). One month later (two months old), a liver biopsy was taken. A portion was processed for Western blot (Figure 1, lane 3-4) and another was microscopically studied. IHC caused the HCV core to be readily detected and showed that it was entirely cytoplasmic (Figure 3A and B). In the liver from mice on chow without doxycyline, the proportions of core-positive hepatocytes were around 50%-60%; the distribution of positive cells within the lobules appeared to be random. The staining pattern was both granular and globular
Figure 3.
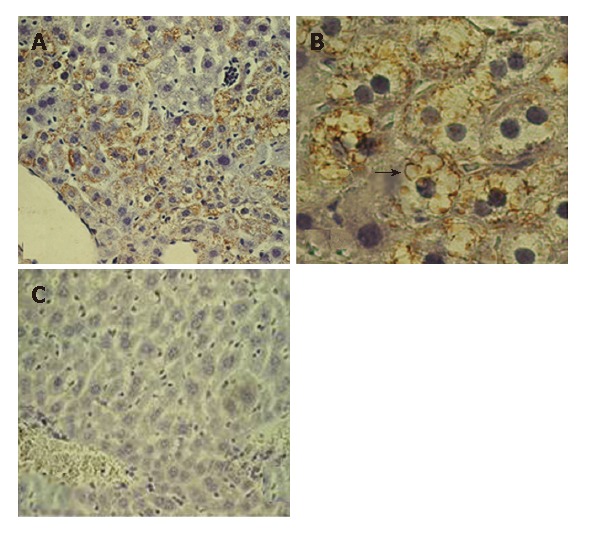
Immunostaining of the female 2 mo old DTM liver (without Dox). A: The core positive hepatocytes are grouped near a central vein (× 20); B: The characteristic globular core expression is shown clearly (arrow, × 60); C: The sex-age matched DTM on Dox chow mouse liver served as negative control.
(Figure 3B, arrows), as in the primary transfected hepatocytes. No definite core expression could be detected in other tissues by either IHC or western blottings (data not shown).
Decrease in HCV-core expression over time in vivo
Figure 1 (lanes 3 and 4) presents a Western blot of liver extracts from a set of DTM with (lane 3) and without dox chow (lane 4) that were biopsied once at 1 mo (2 mo old) (Figure 1A) after withdrawal of doxycycline and again at 5 mo (6 mo old) (Figure 1B). The Western blot provides a semiquantitative estimate of the core expression and indicates that it fell over this interval. The quantitative comparison for the blotting of three female two months old DTM without dox chow (Figure 1C, black bar) and three six months old DTM without dox chow (Figure 1C, white bar) mice liver proteins also showed compatible data (Figure 1C). The core was histologically detectable in all liver samples, but the number of positive cells declined gradually (Figure 4C, E and G). In mice with a negative Western blot, in the second biopsy, an acinar unit contained, on average only one or two positive cells (data not shown). These findings suggest that the reason for decreased HCV-core expression is not inactivation of the transgene but rather loss of the core-expressing hepatocytes.
Figure 4.
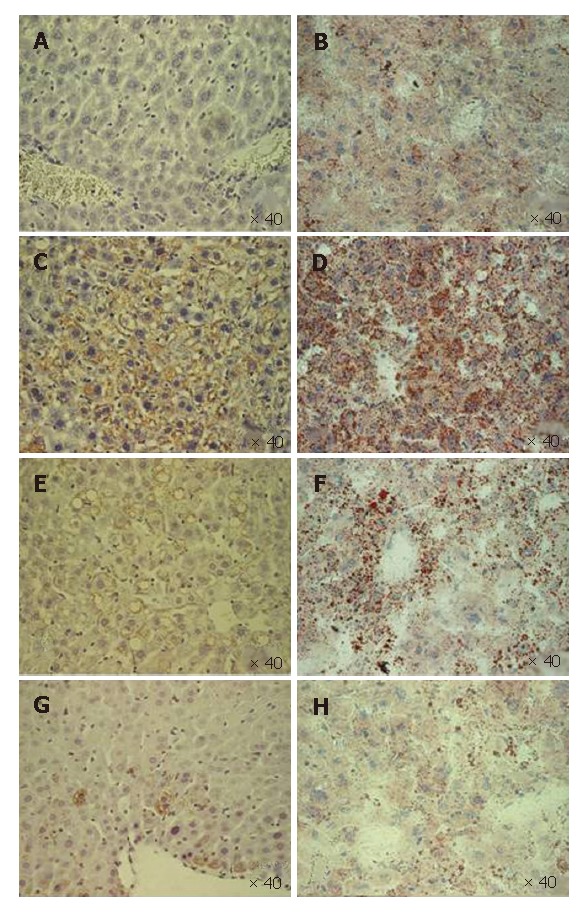
Immunostaining for core protein in core-expressing mice (DTM) livers fixed in 4% PFA (A, C, E and G) and the corresponding oil-red-O stain for fat in the livers of the frozen section (B, D, F and H). The livers were from the mice on doxycycline (A and B) and those not on doxycycline (C and D) at an age of 2 mo, 4 mo (E and F) and 6 mo (G and H). The core expression parallels the degree of hepatic steatosis. Both peaked at the age of 2 mo (C and D) when the hepatic steatosis was microvesicular (D), and diminished gradually (E and H). Macrovesicular hepatic steatosis (E and F) replaced microvesicluar steatosis during evolution.
Hepatic inflammation evaluation
The HE stain did not indicate any significant hepatic inflammation. The aminotransferase levels of the DTM exceeded those of LAP-tTA transgenics at an age of two months; however, the difference diminished gradually with time (Table 1).
Table 1.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of three female mice for the LAP-tTA transgenics and DTM, respectively
|
Age |
||||
| 2 mo | 3 mo | 4 mo | ||
| ALT | LAP-tTA (n = 3) | 51.0 ± 12.2 | 54.0 ± 12.1 | 51.3 ± 2.8 |
| LAP-tTA-HCV core (n = 3) | 211.6 ± 25.1 | 62.3 ± 2.0 | 53.3 ± 2.0 | |
| P value | 0.01 | 0.3 | 0.38 | |
| AST | LAP-tTA (n = 3) | 63.6 ± 7.0 | 67.3 ± 8.3 | 64.6 ± 15.0 |
| LAP-tTA-HCV core (n = 3) | 116.3 ± 12.2 | 80.0 ± 1.0 | 81.3 ± 6.6 | |
| P value | 0.03 | 0.06 | 0.15 | |
Apoptosis evaluation
In serial liver biopsies taken over a period of several months and analyzed for caspase-9, the activity was elevated significantly in the DTM in the first two months but the difference between the DTM and LAP-tTA transgenics declined with time (Figure 5).
Figure 5.
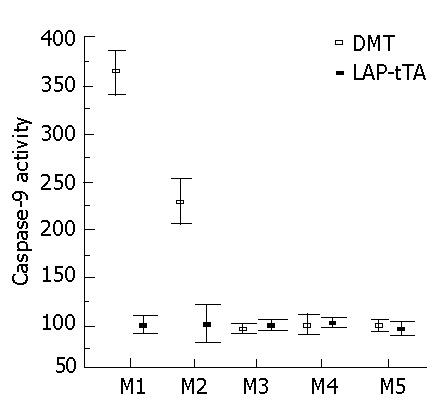
Caspase-9 activity after release of DTM from dox suppression. Caspase-9 activity is significantly increased in DTM mice at 4 and 8 wk after release from dox suppression (mean ± SE).
Decreasing hepatic steatosis with time in transgenics
In two months old DTM, the hepatic steatosis pattern evolved from prominent, microvesicular (Figure 4C and D) to low-level macrovesicular in two months (Figure 4E and F). Steatosis also declined gradually and finally disappeared (Figure 4H), in parallel with the drop in the number of core positive hepatocytes (Figure 4C, E and G)
Oxidative stress evaluation
The DTM liver contained significantly more 8-OHdG positive hepatocytes (65% hepatocytes showed positive 8-OHdG nuclear stain under 20 × 20 power field) than the LAP-tTA control transgenics (No hepatocytes showed positive 8-OHdG nuclear stain under 20 × 20 power field). Most of the 8-OHdG positive hepatocytes also expressed core protein (Figure 6B, arrows).
Figure 6.
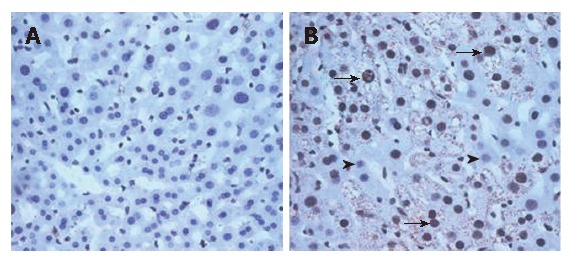
Double stains for core and 8-OHdG at an age of 2 mo for LAP-tTA transgenics (A) and DTM (B). The density of 8-OHdG was significantly higher in the DTM (B, representatively dark nuclei, arrows, 65% ± 2.4%) than in the LAP-tTA transgenics (2% ± 0.1%). Most 8-OHdG positive cells exhibited robust expression of HCV core. The 8-OHdG negative nuclei were indicated by arrow heads (B).
DISCUSSION
The in vitro data obtained indicate that HCV-core is entirely cytoplasmic. The disseminated granular and condensed perinuclear core suggests that the ER, the Golgi apparatus and the mitochondria are possible subcellular locations of core protein in transiently transfected HeLa cells. In fact, all of the above organelles have been reported to be the locations of core storage, with ER being the most abundant one[17,18]. Although some early studies reported nuclear staining, depending on the size of the core protein[19], the current consensus from studies of cell culture and human tissue is that the core is cytoplasmic[20]. In addition to granular core expression, the primary transiently transfected hepatocytes exhibited the characteristic globular pattern of core protein, which was found to be due to core localization to the membrane of fat-containing vesicles. However, this does not prove that core is responsible for generating lipid vesicles. Primary hepatocytes acquire lipid vesicles during their isolation, regardless of HCV core expression (unpublished data). Thus, the association of HCV core with lipid vesicles in primary culture could be fortuitous.
An association of chronic hepatitis C with hepatocellular steatosis and insulin resistance in human infection has been firmly established[21], and is modeled by various HCV core transgenic mouse lines[11]. However, few data exist on the subcellular localization of the HCV-core in vivo. In our model of conditional expression of HCV-core, expression was suppressed from breeding to weaning. Core protein would appear only in the context of the adult liver, as typically occurs in human infection. Western blot of liver extracts and IHC revealed that suppression is complete when mice are on the dox-containing diet, as reported by others[15]. When mice are fed standard chow at around two months of age, the HCV core becomes readily detectable. This system circumvents the possible artifacts associated with hepatocyte isolation and transfection in vitro. Sequential examination of the transgenic liver revealed not only a positive topological relationship between the core protein and the lipid vesicle, but also the simultaneous diminution of the steatosis and core positive hepatocytes with time. Trivial, macrovesicular steatosis replaced prominent, microvesicular steatosis during this process. The LAP-tTA transgenics, which received the same course of dox administration showed no evidence of hepatic steatosis (data not shown). Hence, as well as colocalizing with the hepatic lipid vesicles, the core protein appears to cause hepatic steatosis, followed by apoptosis.
The mechanism of apoptosis in core-expressing hepatocytes appears to involve oxidative stress, and indicated by 8-OHdG staining, as suggested by others[22-24]. Previous investigations have demonstrated that HCV-core enhances apoptosis, mediated by either TNF-alpha[25] or FAS[26]. On the other hand, in mice expressing the entire HCV polypeptide conferred resistance to apoptosis associated with the administration of adenovirus; the effect was selective for FAS-mediated apoptosis[27]. As the authors of the latter study suggest, other viral proteins may in some way override the pro-apoptotic effect of the core, but the mechanism remains to be determined. Also, the level of core expression and its localization in this model are as yet unclarified, as is the effect of transgene expression during development.
No significant hepatic inflammation and only modest aminotransferase elevation occurred in those mice with robust core expression. The difference in aminotransferase between the LAP-tTA-HCV core transgenics and LAP-tTA transgenics fell with time, as did hepatic steatosis.
In contrast to our doubly transgenic mice, the transgenics with constitutive HCV core expression developed by Moriya et al[9] showed progressive hepatic steatosis rather than gradual diminution of steatosis. We proposed that differences in the level and timing of core expression of the constitutive and conditional transgenics might account for the contrasting evolution of hepatic steatosis.
In summary, the newly developed conditional transgenic mice permit morphological analysis of the subcellular distribution of the hepatitis C core gene. The close association of lipid vesicles and core protein and their parallel evolution with time supports the view that the HCV core protein affects hepatocellular lipid metabolism, probably through stimulating oxidative stress. To the best of our knowledge, this is the first animal model that directly elucidates topological and evolutional relationships between core protein and hepatic steatosis.
Footnotes
Supported by grants from National Science Council, Taiwan, China. No. NSC 93-2314-B-182A-148, 94-2314-B-182A-185 and 95-3112-B-182A-002 and Chang Gung Memorial Hospital, Taoyuan, Taiwan, China. No. CMRPG 33014, CMRPG 340341 and SMRPG350081
S- Editor Zhu LH L- Editor Alpini GD E- Editor Ma WH
References
- 1.Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology. 2004;127:S62–S71. doi: 10.1053/j.gastro.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 3.Zein CO, Levy C, Basu A, Zein NN. Chronic hepatitis C and type II diabetes mellitus: a prospective cross-sectional study. Am J Gastroenterol. 2005;100:48–55. doi: 10.1111/j.1572-0241.2005.40429.x. [DOI] [PubMed] [Google Scholar]
- 4.Fujie H, Yotsuyanagi H, Moriya K, Shintani Y, Tsutsumi T, Takayama T, Makuuchi M, Matsuura Y, Miyamura T, Kimura S, et al. Steatosis and intrahepatic hepatitis C virus in chronic hepatitis. J Med Virol. 1999;59:141–145. doi: 10.1002/(sici)1096-9071(199910)59:2<141::aid-jmv3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, et al. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulant S, Montserret R, Hope RG, Ratinier M, Targett-Adams P, Lavergne JP, Penin F, McLauchlan J. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J Biol Chem. 2006;281:22236–22247. doi: 10.1074/jbc.M601031200. [DOI] [PubMed] [Google Scholar]
- 7.Hope RG, McLauchlan J. Sequence motifs required for lipid droplet association and protein stability are unique to the hepatitis C virus core protein. J Gen Virol. 2000;81:1913–1925. doi: 10.1099/0022-1317-81-8-1913. [DOI] [PubMed] [Google Scholar]
- 8.McLauchlan J, Lemberg MK, Hope G, Martoglio B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. EMBO J. 2002;21:3980–3988. doi: 10.1093/emboj/cdf414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78(Pt 7):1527–1531. doi: 10.1099/0022-1317-78-7-1527. [DOI] [PubMed] [Google Scholar]
- 10.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 11.Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 12.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 13.Dal Molin G, D'Agaro P, Ansaldi F, Ciana G, Fertz C, Alberico S, Campello C. Mother-to-infant transmission of hepatitis C virus: rate of infection and assessment of viral load and IgM anti-HCV as risk factors. J Med Virol. 2002;67:137–142. doi: 10.1002/jmv.2202. [DOI] [PubMed] [Google Scholar]
- 14.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci USA. 1996;93:10933–10938. doi: 10.1073/pnas.93.20.10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissell DM, Wang SS, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moradpour D, Englert C, Wakita T, Wands JR. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology. 1996;222:51–63. doi: 10.1006/viro.1996.0397. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki R, Sakamoto S, Tsutsumi T, Rikimaru A, Tanaka K, Shimoike T, Moriishi K, Iwasaki T, Mizumoto K, Matsuura Y, et al. Molecular determinants for subcellular localization of hepatitis C virus core protein. J Virol. 2005;79:1271–1281. doi: 10.1128/JVI.79.2.1271-1281.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q, Tackney C, Bhat RA, Prince AM, Zhang P. Regulated processing of hepatitis C virus core protein is linked to subcellular localization. J Virol. 1997;71:657–662. doi: 10.1128/jvi.71.1.657-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouillé Y, Helle F, Delgrange D, Roingeard P, Voisset C, Blanchard E, Belouzard S, McKeating J, Patel AH, Maertens G, et al. Subcellular localization of hepatitis C virus structural proteins in a cell culture system that efficiently replicates the virus. J Virol. 2006;80:2832–2841. doi: 10.1128/JVI.80.6.2832-2841.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zekry A, McHutchison JG, Diehl AM. Insulin resistance and steatosis in hepatitis C virus infection. Gut. 2005;54:903–906. doi: 10.1136/gut.2004.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, Buscail L, Bartenschlager R, Ichas F, Rizzuto R, et al. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24:4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- 23.Chou AH, Tsai HF, Wu YY, Hu CY, Hwang LH, Hsu PI, Hsu PN. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- 24.Schwer B, Ren S, Pietschmann T, Kartenbeck J, Kaehlcke K, Bartenschlager R, Yen TS, Ott M. Targeting of hepatitis C virus core protein to mitochondria through a novel C-terminal localization motif. J Virol. 2004;78:7958–7968. doi: 10.1128/JVI.78.15.7958-7968.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai MM. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorman JP, Prayther D, McVay D, Hahn YS, Hahn CS. The C-terminal region of hepatitis C core protein is required for Fas-ligand independent apoptosis in Jurkat cells by facilitating Fas oligomerization. Virology. 2003;312:320–329. doi: 10.1016/s0042-6822(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 27.Disson O, Haouzi D, Desagher S, Loesch K, Hahne M, Kremer EJ, Jacquet C, Lemon SM, Hibner U, Lerat H. Impaired clearance of virus-infected hepatocytes in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2004;126:859–872. doi: 10.1053/j.gastro.2003.12.005. [DOI] [PubMed] [Google Scholar]


