Abstract
AIM: To examine the association of beta-catenin with the clinicopathologic features and prognosis of esophageal squamous cell carcinoma (ESCC).
METHODS: Beta-catenin mRNA expression level in 40 ESCC patients (28 males and 12 females, age range 38-82 years, median 60 years) was analyzed by real-time PCR. Beta-catenin mRNA expression levels in tumor cells were categorized as weaker (level 1) or equal to or stronger (level 2) than those in endothelial cells. We examined the correlation between the beta-catenin expression and the clinicopathological factors and prognosis of ESCC patients.
RESULTS: Level 2 beta-catenin expression was found in 29 patients. ESCC with level 2 expression had a higher rate of lymphnode metastasis (0.0776 ± 0.0369 vs 0.3413 ± 0.1803, P < 0.001) and deeper tumor invasion (0.0751 ± 0.0356 vs 0.3667 ± 0.1928, P < 0.001), and a poorer survival rate (P = 0.0024) than ESCC with level 1 expression.
CONCLUSION: Beta-catenin expression in ESCC is of great importance.
Keywords: Beta-catenin, mRNA expression level, Esophageal carcinoma, Prognosis
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) shows a poor prognosis because of the occurrence of systemic metastasis, mainly via lymphatic vessels[1-6]. Detection of ESCC at its early stage is possible by X-ray and endoscopic examinations[7,8], but there might be occult micrometastases at the time of surgery even in such cases[9,10]. In this respect, an assessment of metastatic potential is important to establish appropriate therapeutic modalities for ESCC. Previous studies have shown the prognostic significance of several clinicopathologic factors for ESCC, such as tumor size, age at diagnosis, and primary site[11,12]. Among them, depth of cancer invasion in the esophageal wall and lymph node metastasis are the main factors for ESCC recurrence[5,6,11], and these two factors as well as distant metastasis have been included in the pathological tumor-node-metastasis (pTNM) staging for ESCC[12]. However, the prognosis of ESCC, even in cases of early-stage disease (pT1), is heterogeneous, and a strategy to establish appropriate therapeutic modalities for each patient has yet to be formulated.
Several biological indices for predicting the prognosis of ESCC, a number of indices, such as aberrant expression or mutation of the tumor suppressor p53, adhesion molecule Ecadherin, and cell-cycle-related molecules such as p27, have been proposed[3,13,11]. However, the utility of these factors for predicting the prognosis of ESCC is controversial[15].
Recently, beta catenin and elements of the Wnt signaling pathway have been found to play an important role in the pathogenesis of human cancers including ESCC. In the present study, we analyzed beta-catenin mRNA expression level in 40 patients with ESCC and evaluated its correlation with the clinicopathologic features and survival of these patients.
MATERIALS AND METHODS
Patients
In the present study, 40 patients underwent surgery for ESCC at the Nanjing First Hospital Affiliated to Nanjing Medical University from July 1999 to February 2000 were selected. There were 28 males and 12 females (age range 38-82 years, median 60 years). An endoscopic examination of esophageal lesions was performed, and a histologic diagnosis of ESCC was made based on biopsy specimens obtained before surgery. Preoperative diagnostic examinations, including esophagography, computed tomography, and ultrasound, were performed for clinical staging. All patients underwent curative surgery for ESCC. The types of surgery used were subtotal esophagectomy with lymphnode resection in 32 patients, partial esophagectomy in 8 patients. The resected esophagus was examined macroscopically to determine the location and size of the tumor. Tumors were located in the cervical esophagus in 2, the upper third of the thorax in 8, the middle third of the thoraxin in 22, the lower third of the thorax in 8 patients, respectively. The size of the main tumor ranged from 10 to 90 mm (median, 44 mm). Samples obtained from the esophageal lesions were fixed in 10% formalin and processed routinely for paraffin embedding. The number of specimens prepared for histologic examination per case ranged from 6 to 46. Four-μm thick histologic sections were stained with hematoxylin and eosin using immunoperoxidase procedures (the avidin-biotin complex method). Tumor stages were defined based on the pTNM classification[12]. After surgery, all patients underwent laboratory test such as routine peripheral blood cell counts and measurement of serum squamous cell carcinoma antigen levels every 1-6 mo, and chest roentgenography, ultrasonography of the liver, computerized tomographic scan of the thorax and abdomen, and endoscopic examination of the remaining esophagus at 6-12 mo intervals. The follow-up time for survivors ranged from 8 to 62 mo (median 40.0 mo).
RNA extraction and real time-PCR analysis
Real time-PCR was performed on paraffin-embedded sections from 40 ESCC patients. Briefly, total RNA was extracted by Recover AllTM. Total nucleic acid was isolated (Ambion), 10 μg of DNase I-treated total RNA was used for reverse transcription with Superscript III(Invitrogen, Carlsbad, CA). An aliquot representing 100 ng of input RNA was amplified by quantitative real-time PCR using the TaqMan PCR reagent kit and assay-on-demand gene expression products (FAM/Sybr, Foster City, CA). RNA extracted from a noncancerous lesion in one patient was used as a standard. After reverse transcription, standard cDNA was serially diluted to obtain five standard solutions for use in PCR to generate the reference curve. The primary design was shown in Table 1.The relative amount of cDNA in each sample is measured by interpolation using the standard curve (Figure 1), and then the relative ratio of beta-catenin to beta-actin expression was calculated for each ESCC sample.
Table 1.
Primer sequences for real time PCR
| Gene name | Prime | Temp (°C) | bp |
| Beta-actin | F 5' CCTGTACGCCAACACAGTGC 3' | 60 | 211 |
| R 5' ATACTCCTGCTTGCTGATCC 3' | |||
| Beta-catenin | F 5' AAAATGGCAGTGCGTTTAG 3' | 60 | 144 |
| R 5' TTTGAAGGCAGTCTGTCGTA 3' |
Figure 1.
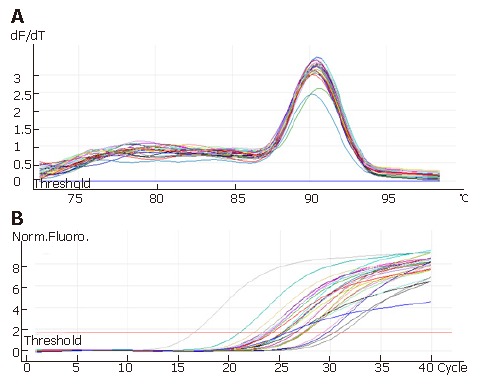
Solution curve of beta-catenin gone PCR products (A and standard curve of beta-catenin gone PCR products (B).
Statistical analysis
Statistical analyses were performed with SPSS 11.5 software for windows. Correlation between the expression levels of beta-catenin determined by reverse transcription-PCR was evaluated by one-way ANOVA test. Chi-square test and Fisher's exact probability test were used to analyze the correlation between beta-catenin expression and the clinicopathologic features of ESCC. The Kaplan-Meier method was used to calculate survival rates, and differences in survival curves were evaluated with the log-rank test. P < 0.05 was considered statistically significant.
RESULTS
Histologic findings
Histologically, 10 were well-differentiated, 16 moderately-differentiated, and 14 poorly-differentiated squamous cell carcinomas. Tumor cells invaded the mucosa or submucosa (pT1) in 6, muscularis propria or subadventitia (pT2) in 22, adventitia (pT3) in 10, and adjacent organs (pT4) in 2 patients, respectively.
Patient outcome
The 5-year survival rate was 37.3%. Seventeen patients showed tumor recurrence, which was found in local sites of 8, in lymph nodes of 5, in liver of 2, and in other organs of 2 patients, respectively. Fifteen patients died.
Beta-catenin expression in ESCC
We used the beta-catenin mRNA expression level in endothelial cells as the standard to divide all samples into two groups. Beta-catenin mRNA expression level in tumor cells was categorized as weaker (level 1) or equal to or stronger (level 2) than that in endothelial cells. The relative ratios of beta-catenin to beta-actin expression in cases with level 1 and 2 expression were 0.6 ± 0.2 and 1.9 ± 1.4 (P < 0.05). The correlation between beta-catenin expression and clinical factors is shown in Figure 2. Compared with patients with ESCC level 1, higher rates of lymph node metastasis were found in 10 of 19 patients (54.4%) and 13 of 17 patients (75.0%), and deep invasion (pT3 and pT4) in 8 of 19 patients (38.5%) and 10 of 17 patients (61.5%) with ESCC level 2. Patients with level 2 ESCC showed higher frequencies of local recurrence and distant metastasis than those with level 1 ESCC (P < 0.01). Patients with level 1 ESCC had better 5-year survival rates than those with level 2 ESCC (P < 0.001).
Figure 2.
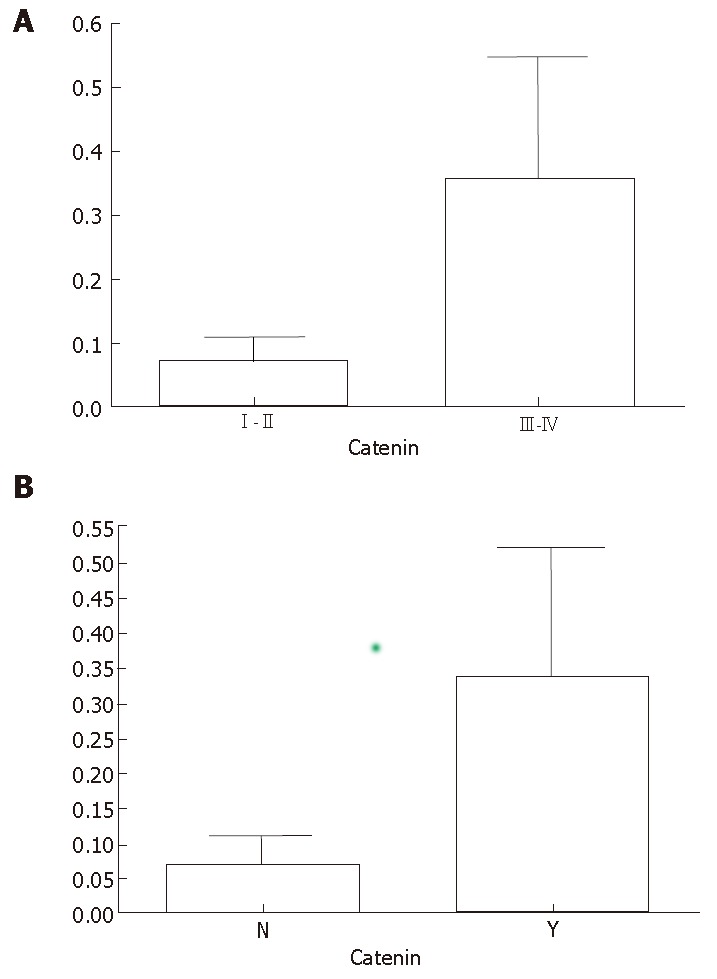
A: ESCC patients at stages III- IV(A) showing higher mRNA expression level of beta-catenin than ESCC patients at stages I-II(B). Y: observed lymph node metastasis, N: no lymph node metastasis. ESCC patients with lymph node metastasis had higher mRNA expression level of beta-catenin than ESCC patients with no lymph node metastasis.
Prognostic significance of beta-catenin expression in pTNM classification
The prognostic significance of beta-catenin expression was further analyzed according to the pTNM classification[12]. A significant difference was found in survival between patients with level 1 and 2 expressions at the early stage of the disease (pT1, P < 0.01). Lymph node metastasis was found in 4 (20.0%) of 20 level 1 patients and in 8 (72.2%) of 11 level 2 patients (P < 0.01). At the advanced stage (pT2-pT4), we observed a significant difference in survival between patients with level 1 and 2 expressions (P < 0.05). The survival curve is shown in Figure 3.
Figure 3.
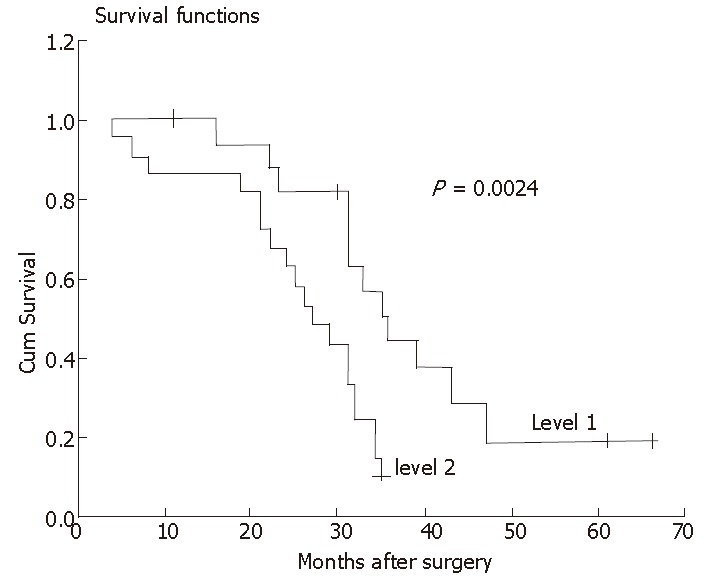
At the advanced stage (pT2-pT4), a significant difference in survival between patients at advanced stage with level 1 and 2 expression.
DISCUSSION
The conventional TNM staging system for ESCC provides useful information for the prediction of tumor recurrence and patient survival[5,6,11]. Recent advances in therapeutic modalities for ESCC, however, have led to new problems to be solved. For example, recently introduced reduction surgeries, such as endoscopic mucosal resection and transhiatal esophagectomy, enable tumor excision without a severe influence on the patient's general condition[14]. However, lymph node metastasis, observed at relatively high frequencies even in early ESCC, becomes a risk factor when these techniques are used. In contrast, the introduction of adjuvant chemoradiotherapy has improved the prognosis of patients with ESCC, particularly those with high potential for lymph node metastasis[16]. Under these circumstances, preoperative assessment of lymph node metastasis and metastatic potential of ESCC is essential for therapeutic decision-making. The characteristics of patients, such as gender, age, primary site, and 5-year survival rate, in the present series are similar to those in previous reports from China and other Asian countries where ESCC is common[1,10,15,20]. ESCC in Western countries, where it is relatively uncommon, shares the above-mentioned characteristics except that tumors are rather frequent in the lower esophagus[5,6]. Beta-catenin is involved in the ubiquitin/proteasome-dependent protein degradation pathway, which plays an essential role in controlling the levels of various cellular proteins and therefore regulates basic cellular processes such as cell cycle progression, signal transduction, and cell transformation[17].
We examined beta-catenin expression at the mRNA level by reverse transcription-PCR. Among the clinicopathologic factors examined, we observed a significant correlation between beta-catenin expression and depth of invasion and lymph node metastasis, indicating that beta-catenin expression is closely related to the growth and invasiveness of ESCC. The uni- and multivariate analyses indicate that the beta-catenin expression level is an independent prognostic factor for ESCC recurrence and patient survival. The 5-year survival rates of patients with beta-catenin level 1 and 2 expressions were 86.3% and 31.2% at stage pT1 and 43.2% and 22.4% at stages pT2 to pT4, respectively. The combination of pTNM classification and beta-catenin expression level in tumor cells is useful for predicting the prognosis of patients with ESCC. Grouping the cases with ESCC based on the present system could be a good guide for choosing the modalities of adjuvant therapy. In early ESCC patients with beta-catenin level 1 expression, a favorable outcome could be expected. Since lymph node metastasis was observed in only 20% of these patients, reduction surgeries might be considered, particularly for those in a poor general condition or with small tumors. In contrast, patients with level 2 ESCC and advanced stage disease might have a dismal prognosis, and the introduction of intensive adjuvant therapies for these patients might be justified.
In conclusion, beta-catenin mRNA expression level is a new prognostic marker for ESCC. The stratification of ESCC patients based on the disease stage and beta-catenin expression level is valuable for predicting tumor recurrence and prognosis. This system might provide a novel way to explore effective treatment modalities for ESCC.
Footnotes
S- Editor Liu Y L-Editor Wang XL E- Editor Wang HF
References
- 1.Dawsey SM, Wang GQ, Weinstein WM, Lewin KJ, Liu FS, Wiggett S, Nieberg RK, Li JY, Taylor PR. Squamous dysplasia and early esophageal cancer in the Linxian region of China: distinctive endoscopic lesions. Gastroenterology. 1993;105:1333–1340. doi: 10.1016/0016-5085(93)90137-2. [DOI] [PubMed] [Google Scholar]
- 2.Faivre J, Forman D, Estève J, Gatta G. Survival of patients with oesophageal and gastric cancers in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:2167–2175. doi: 10.1016/s0959-8049(98)00329-3. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 4.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Ueyama T, Kawamoto K, Yamada Y, Masuda K. Early esophageal carcinoma. Evaluation of the depth of invasion based on double-contrast esophagography. Acta Radiol. 1998;39:133–137. doi: 10.1080/02841859809172166. [DOI] [PubMed] [Google Scholar]
- 6.Lambert R. Diagnosis of esophagogastric tumors. Endoscopy. 2002;34:129–138. doi: 10.1055/s-2002-19852. [DOI] [PubMed] [Google Scholar]
- 7.Nozoe T, Saeki H, Ohga T, Sugimachi K. Clinicopathological features of early esophageal squamous cell carcinoma with subsequent recurrence. Dis Esophagus. 2002;15:145–148. doi: 10.1046/j.1442-2050.2002.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Lam KY, Tsao SW, Zhang D, Law S, He D, Ma L, Wong J. Prevalence and predictive value of p53 mutation in patients with oesophageal squamous cell carcinomas: a prospective clinico-pathological study and survival analysis of 70 patients. Int J Cancer. 1997;74:212–219. doi: 10.1002/(sici)1097-0215(19970422)74:2<212::aid-ijc13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Sanders DS, Bruton R, Darnton SJ, Casson AG, Hanson I, Williams HK, Jankowski J. Sequential changes in cadherin-catenin expression associated with the progression and heterogeneity of primary oesophageal squamous carcinoma. Int J Cancer. 1998;79:573–579. doi: 10.1002/(sici)1097-0215(19981218)79:6<573::aid-ijc4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 10.Bian YS, Osterheld MC, Bosman FT, Fontolliet C, Benhattar J. Nuclear accumulation of beta-catenin is a common and early event during neoplastic progression of Barrett esophagus. Am J Clin Pathol. 2000;114:583–590. doi: 10.1309/3QLC-5MF1-JYXU-A5XX. [DOI] [PubMed] [Google Scholar]
- 11.Wijnhoven BP, Nollet F, De Both NJ, Tilanus HW, Dinjens WN. Genetic alterations involving exon 3 of the beta-catenin gene do not play a role in adenocarcinomas of the esophagus. Int J Cancer. 2000;86:533–537. doi: 10.1002/(sici)1097-0215(20000515)86:4<533::aid-ijc15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.González MV, Artímez ML, Rodrigo L, López-Larrea C, Menéndez MJ, Alvarez V, Pérez R, Fresno MF, Pérez MJ, Sampedro A, et al. Mutation analysis of the p53, APC, and p16 genes in the Barrett's oesophagus, dysplasia, and adenocarcinoma. J Clin Pathol. 1997;50:212–217. doi: 10.1136/jcp.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng H, Shao J, Williams CS, Pereira MA, Taketo MM, Oshima M, Reynolds AB, Washington MK, DuBois RN, Beauchamp RD. Nuclear translocation of beta-catenin in hereditary and carcinogen-induced intestinal adenomas. Carcinogenesis. 1998;19:543–549. doi: 10.1093/carcin/19.4.543. [DOI] [PubMed] [Google Scholar]
- 14.Hourihan RN, O'Sullivan GC, Morgan JG. Transcriptional gene expression profiles of oesophageal adenocarcinoma and normal oesophageal tissues. Anticancer Res. 2003;23:161–165. [PubMed] [Google Scholar]
- 15.Kimura Y, Shiozaki H, Doki Y, Yamamoto M, Utsunomiya T, Kawanishi K, Fukuchi N, Inoue M, Tsujinaka T, Monden M. Cytoplasmic beta-catenin in esophageal cancers. Int J Cancer. 1999;84:174–178. doi: 10.1002/(sici)1097-0215(19990420)84:2<174::aid-ijc14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Roh H, Green DW, Boswell CB, Pippin JA, Drebin JA. Suppression of beta-catenin inhibits the neoplastic growth of APC-mutant colon cancer cells. Cancer Res. 2001;61:6563–6568. [PubMed] [Google Scholar]
- 17.Green DW, Roh H, Pippin JA, Drebin JA. Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res. 2001;101:16–20. doi: 10.1006/jsre.2001.6241. [DOI] [PubMed] [Google Scholar]
- 18.Yan YX, Nakagawa H, Lee MH, Rustgi AK. Transforming growth factor-alpha enhances cyclin D1 transcription through the binding of early growth response protein to a cis-regulatory element in the cyclin D1 promoter. J Biol Chem. 1997;272:33181–33190. doi: 10.1074/jbc.272.52.33181. [DOI] [PubMed] [Google Scholar]
- 19.Jäättelä M. Programmed cell death: many ways for cells to die decently. Ann Med. 2002;34:480–488. doi: 10.1080/078538902321012423. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]


