Abstract
A 41-year old female with metastatic gastrointestinal stromal tumor was referred to 18F-FDG-positron emission tomography and computed tomography (PET/CT) scan before and after one-month treatment with imatinib (Glivec®, Gleevec®, Novartis, Basel, Switzerland), a tyrosine kinase inhibitor (400 mg/d). Metabolic response was evaluated before and after one month of therapy. The decrease of the maximum standardised uptake value (SUV) was 79% (from 9.8 to 2.1). Positron emission tomography demonstrated complete metabolic response after one-month of imatinib treatment. Additionally, the previous lesion was compared with the coronal computerized tomographic image. There was no difference in the size of the tumor before and after therapy according to CT images. However, metabolic activity was inhibited. 18F-FDG-PET is a valuable method for the detection of response to one-month imatinib treatment in patients with gastrointestinal stromal tumors.
Keywords: 18F-fluorodeoxyglucose, Positron emission tomography, Computerized tomography, Gastrointestinal stromal tumor, Imatinib
INTRODUCTION
18F-FDG (fluoro-deoxyglucose)-positron emission tomography and computed tomography (PET/CT) may prove useful in staging and treating malignant gastro-intestinal stromal tumor (GIST)[1,2]. GIST is a rare aggres-sive mesenchymal tumor in the gastrointestinal system and can metastasize[3-6]. In this case, FDG-PET/CT scans were performed before and after one- month treatment with imatinib, a tyrosine-kinase inhibitor, demonstrating a significant, nearly complete metabolic response to imatinib treatment.
CASE REPORT
A 41-year old female with biopsy-proven malignant GIST was referred to 18F-FDG-PET scan before and after one-month chemotheraphy. 18F-FDG-PET/CT scans were performed by using a Siemens Biograph 2 LSO entegrated PET/CT camera (Siemens, Knoxville, Tennessee, USA). FDG-PET images were obtained approximately 60 min after intravenous injection of 481 MBq (13 mCi) F-18 FDG with the patient fasted for 8 h. Oral contrast was given for CT part of the PET/CT. The first PET/CT was performed before imatinib treatment and the coronal image (Figure 1) demonstrated abnormal and markedly increased FDG uptake, consistent with metastatic disease involving abdominal, pelvic and inferior parts of the left thoracic areas. Maximum standardised uptake value (SUV) for the lesion area was 9.8. The second FDG-PET/CT scan was performed one month after imatinib treatment and the coronal image (Figure 2) demonstrated a significant, complete metabolic response to imatinib treatment. Maximum SUV for the lesion area was 2.1.
Figure 1.
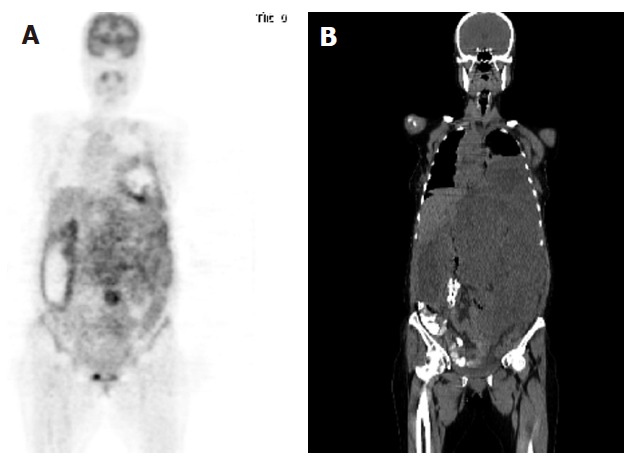
Coronal PET image demonstrating abnormal FDG uptake (A) and coronal CT image showing a large lesion occupying the same areas (B).
Figure 2.
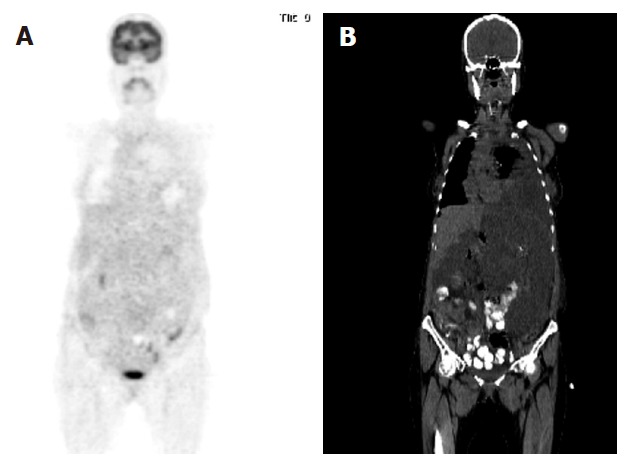
Coronal PET image demonstrating a significant metabolic response to imatinib treatment (A) and coronal CT image showing a large lesion (B). There was no difference in dimensions before and after imatinib treatment although metabolic activity was inhibited.
DISCUSSION
Invasive surgical resection is used as a choice of primary treatment for gastrointestinal stromal tumor, because standard chemotherapy and radiotherapy cannot improve the overall survival of patients with GIST[3,4]. Recently trials using imatinib, a tyrosine-kinase inhibitor have shown a significant response in patients with metastatic GIST[7,8]. The extent of GIST is usually assessed by CT scans, showing typical intramural or extramural nodules with areas of necrosis[9].
There are a number few studies in the literature reporting the role of FDG PET in GIST[1,2,10]. Van Oosterom et al[10] investigated imatinib, a tyrosine-kinase inhibitor in the treatment of metastatic GIST and performed FDG PET for response assessment, showing that FDG PET may prove useful in staging and treating GIST. Prenen et al[2] have established a mouse GIST model and evaluated the response to imatinib treatment by small animal positron emission tomography. Small animal PET revealed that FDG up-take in xenografts was significantly decreased after 24 h treatment with imatinib. This model can be used to study imatinib resistance and evaluate novel targeted therapies. Heinicke et al[1] detected the early response to imatinib treatment in gastrointestinal stromal tumors using 18F-PET. The mean decrease in SUV before and after one-week imatinib treatment was 60% (range 43%-77%). In our case, FDG-PET/CT scans were performed before and one month after imatinib treatment, demonstrating a significant, nearly complete metabolic response to imatinib treatment. The decrease in the SUV was 79% (9.8 vs 2.1).
Additionally, the previous lesion was compared with the coronal computerized tomographic image. There was no difference in size of GIST before and after imatinib treatment according to CT images. However, metabolic activity was inhibited. If the tumor was evaluated using the sum of the longest dimensions (RECIST), the patient was categorized as having stable disease, despite the fact that 79% reduction in the mean SUVmax on FDG PET. Thus, tumor size determined using the RECIST is not reliable and underestimates the tumor response to imatinib treatment during the early post-treatment stage in patients with metastatic GIST[11].
FDG PET is a sensitive and specific method to eva-luate tumor response on the basis of changes in tumor metabolism. In our study, a dramatic decrease in FDG uptake was observed early after imatinib treatment. FDG PET may be useful in detecting the response of patients to imatinib treatment one month later.
Footnotes
S- Editor Liu Y L- Editor Wang XL E- Editor Liu Y
References
- 1.Heinicke T, Wardelmann E, Sauerbruch T, Tschampa HJ, Glasmacher A, Palmedo H. Very early detection of response to imatinib mesylate therapy of gastrointestinal stromal tumours using 18fluoro-deoxyglucose-positron emission tomography. Anticancer Res. 2005;25:4591–4594. [PubMed] [Google Scholar]
- 2.Prenen H, Deroose C, Vermaelen P, Sciot R, Debiec-Rychter M, Stroobants S, Mortelmans L, Schöffski P, Van Oosterom A. Establishment of a mouse gastrointestinal stromal tumour model and evaluation of response to imatinib by small animal positron emission tomography. Anticancer Res. 2006;26:1247–1252. [PubMed] [Google Scholar]
- 3.Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000;7:705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571) Ann Surg Oncol. 2002;9:831–839. doi: 10.1007/BF02557518. [DOI] [PubMed] [Google Scholar]
- 6.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118–8121. [PubMed] [Google Scholar]
- 8.Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898–3905. doi: 10.1200/JCO.2002.03.095. [DOI] [PubMed] [Google Scholar]
- 9.Nishida T, Kumano S, Sugiura T, Ikushima H, Nishikawa K, Ito T, Matsuda H. Multidetector CT of high-risk patients with occult gastrointestinal stromal tumors. AJR Am J Roentgenol. 2003;180:185–189. doi: 10.2214/ajr.180.1.1800185. [DOI] [PubMed] [Google Scholar]
- 10.van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, Martens M, Webb A, Sciot R, Van Glabbeke M, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358:1421–1423. doi: 10.1016/s0140-6736(01)06535-7. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, Macapinlac HA, Podoloff DA. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]


