Abstract
Preservation of the spleen at distal pancreatectomy has recently attracted considerable attention. Since our first successful trial, spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein for tumors of the pancreas and chronic pancreatitis has been performed more frequently. The technique for spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein are outlined. The splenic vein is identified behind the pancreas and within the thin connective tissue membrane. The connective tissue membrane is cut longitudinally above the splenic vein. An important issue is to remove the splenic vein from the body of the pancreas toward the spleen, since a different approach may be very difficult. The pancreas is preferably removed from the splenic artery toward the head of the pancreas itself. This procedure is much easier than removing the pancreas from the vein side. One patient had undergone distal gastrectomy for duodenal ulcer, with reconstruction by Billroth II tehcnique. If distal pancreatectomy with splenectomy had been performed for the lesion of the distal pancreas at the time, the residual stomach would also have to be resected. The potential damage done to the patient by reconstruction of the gastrointestinal tract in combination with distal pancreatectomy and splenectomy would have been much greater than with distal pancreatectomy only with preservation of the spleen and residual stomach. Benign lesions as well as low-grade malignancy of the body and tail of the pancreas may be a possible indication for this procedure.
Keywords: Spleen preservation, Intraductal Papillary-Mucinous Neoplasm, Splenic artery, Splenic vein, The fusion fascia of Treitz and Toldt
INTRODUCTION
For tumors or low-grade malignancies of the body and tail of the pancreas, or for chronic pancreatitis, spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein is performed[1]. This method is already being used worldwide for the treatment of chronic pancreatitis[2,3]. We were the first in the world to perform this procedure for chronic pancreatitis (Figure 1)[1,4]. The patient was a 22-year-old man with familial chronic pancreatitis. Because the patient was very young and had many protein plugs in the dilated ductule of the tail of the pancreas, we decided to perform a Puestow’s procedure with removal of the tail of the pancreas. The postoperative course was uneventful. The splenic function of this young man has been preserved in the all of his life.
Figure 1.
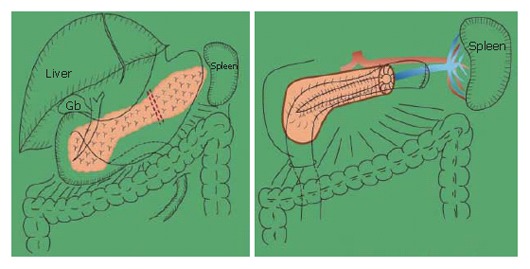
Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein for chronic pancreatitis. A Puestow’s procedure with removal of the tail of the pancreas.
The relevance of preserving the spleen has been shown in Europe, the United States and Japan. Splenic loss causes either changes in the peripheral blood count, infection or sepsis[5-7]. Some authors have also stressed the potential immuno suppression related to splenectomy[8].
SURGICAL ANATOMY
In order to perform the procedure, adequate anatomical knowledge of the pancreas and supplying vessels, especially the fusion fascia of Toldt and fusion fascia of Treitz, is necessary[9].
Figure 2 displays the fusion fascia of the head of the pancreas in an autopsy case[9]. The fascia is composed of loose connective tissue. By Kocher's maneuver, the fascia adheres to the pancreatic parenchymal side, but not to the vena cava side. All the relevant pancreatico-duodenal arcades of arteries, veins, and nerves are situated on this membrane.
Figure 2.
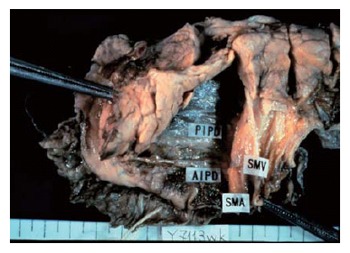
The fusion fascia of the head of the pancreas in an autopsy case.
The composition of this membrane has been previously described. During early development, the pancreas is divided into two buds representing the ventral and dorsal anlagen. The ventral anlage of the pancreas then moves around the duodenum until it comes in contact with the dorsal bud (Figure 3).
Figure 3.
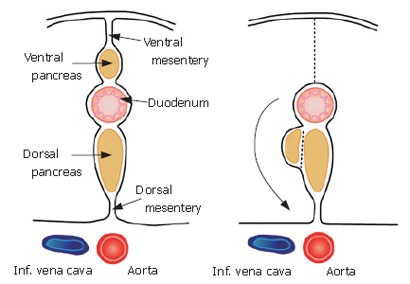
During development, the pancreas is divided into two buds representing the ventral and dorsal anlagen. The ventral anlage of the pancreas then moves around the duodenum until it comes in contact with the dorsal bud.
The membrane of the ventral bud and that of the inferior vena cava and abdominal aorta become fused, and this is reflected by the term "fusion fascia" (Figure 4). The fusion fascia of the head of the pancreas is known as the "fusion fascia of Treitz", while the one of the body and tail of the pancreas is called the "fusion fascia of Toldt".
Figure 4.
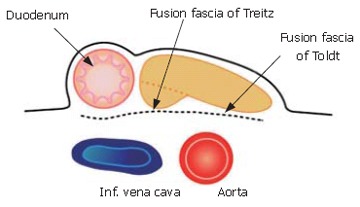
The membrane of the ventral bud and that of the inferior vena cava and abdominal aorta become fused. The fusion fascia of the head of the pancreas is known as the "fusion fascia of Treitz" and that of the body and tail of the pancreas is called the "fusion fascia of Toldt".
Histological findings of the "fusion fascia" of the pancreas are shown in the Figure 5. The fusion fascia is composed of loose connective tissue. While it appears to be fragile to the naked eye during an operation, it is actually quite resistant. A large number of arteries, arterioles, veins and nerves exist between the fascia and pancreatic parenchyma.
Figure 5.
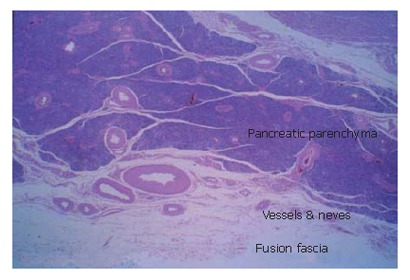
Histological findings of the "fusion fascia" of the pancreas.
Figure 6 displays shows a posterior view of the head of the pancreas in an autopsy case. This is the surface that is ablated by Kocher mobilization. The posterior surface of the pancreas is covered with fusion fascia of Treitz.
Figure 6.
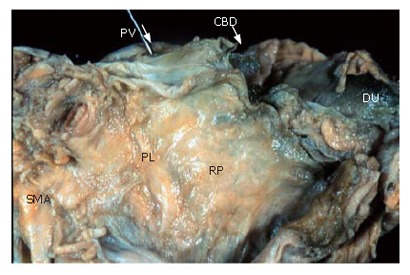
A posterior view of the head of the pancreas in an autopsy case. The posterior surface of the pancreas is covered with fusion fascia of Treitz.
The cut end of the portal vein and the superior mesenteric artery shown at the top of the figure are also covered with fusion fascia and exist on the abdominal side.
Thus, the extrapancreatic nerve plexuses and the portal vein, superior mesenteric artery and parenchyma of the pancreatic head are situated in the same area, and are surrounded by the fusion fascia of Treitz[10].
A similar anatomical configuration is displayed in the body and tail of the pancreas. The splenic vein and artery and parenchyma of the pancreas are situated in the same area, and are surrounded by the fusion fascia of Toldt.
When the fusion fascia of Treitz is ablated from the parenchyma of the pancreas, an important pancreatoduodenal vessel, the PSPDV, is revealed as it is shown in Figure 7.
Figure 7.
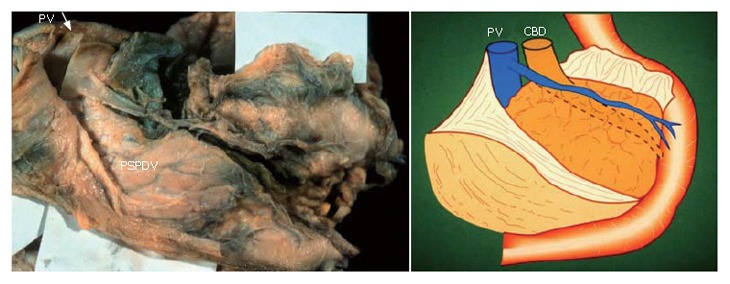
When the fusion fascia of Treitz is ablated from the parenchyma of the pancreas, an important pancreatoduodenal vessel, for example the PSPDV, is revealed. A posterior view of the head of the pancreas after ablation of the fusion fascia of Treitz.
A Sagittal section as it can be seen through the neck of the pancreas is shown in Figure 8. The fusion fascia is indicated by the dotted line. The superior mesenteric artery (SMA) penetrates the fusion fascia, just after its origin from the abdominal aorta.
Figure 8.
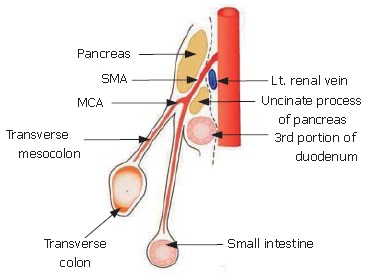
Sagittal section through the neck of the pancreas. The fusion fascia is indicated by the dotted line.
SURGICAL TECHNIQUES BASED ON THE ANATOMY
When the pancreas is ablated from the retro-peritoneum, the pancreas and splenic vein are covered with the fusion fascia of Toldt (Figure 9). In the first step of this operation, the fascia of Toldt is cut to reveal the splenic vein (Figure 10). X-ray ductography of the resected specimen of IPMN in the body of the pancreas is shown in the lower part of the Figure 10.
Figure 9.
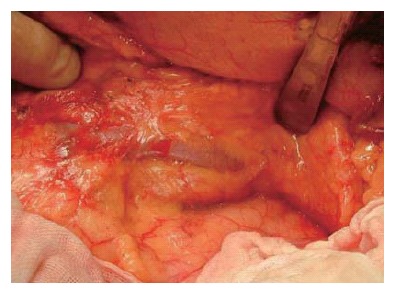
When the pancreas was ablated from the retroperitoneum, the pancreas and splenic vein are covered with the fusion fascia of Toldt.
Figure 10.
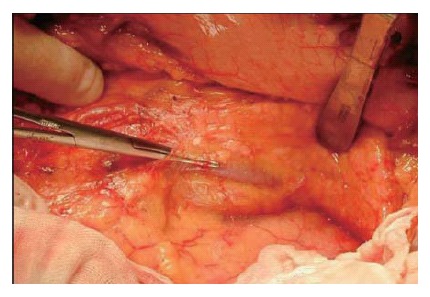
The fusion fascia of Toldt is longitudinally cut above the splenic vein.
The branches of the splenic vein should be gently ligated and divided (Figure 11). An important technique is to remove the splenic vein from the body of the pancreas toward the spleen. It is very difficult to remove the splenic vein in the other direction because (1) it is difficult to discriminate the distal end of the pancreas from the fatty tissue in the hilum of the spleen, and (2) in this area the splenic artery and vein are already divided into small vessels that can be easily injured.
Figure 11.
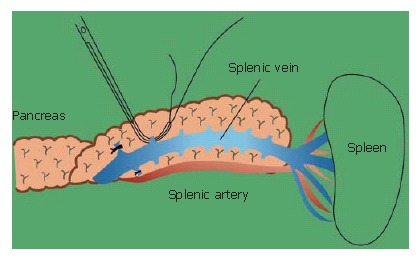
The branches from the splenic vein on both sides should be carefully ligated and divided.
The splenic vein in the body of the pancreas is removed from the pancreatic body (Figure 12).
Figure 12.
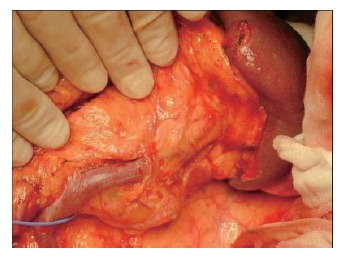
The splenic vein in the body of the pancreas is removed from the pancreatic body.
The splenic artery is removed from the spleen toward the head of the pancreas (Figure 13). Then, the distal pancreas can be confidently removed.
Figure 13.
The splenic artery is removed from the spleen toward the head of the pancreas. Then, the distal pancreas can be removed.
Wesafely performed this procedure for benign lesions and Intraductal papillary-mucinous neoplasm (IPMN) in eighteen cases (Table 1). This procedure was also performed for IPMN in 10 cases in the last five years[11,12]. All of the operation were successfully performed. No procedure related or disease recurrence casualties were reported.
Table 1.
The spleen preserving procedure for benign lesions and IPMN in eighteen cases
| Case No. | Age, Gendar | Diagnosis | Location | Size (cm) | Pathological findings |
| 1 | 54, F | Endocrine tumor | Body | 1.0 | Glucagonoma |
| 2 | 51, F | Cystic tumor | Body | 1.2 | Mucinous cystadenoma |
| 3 | 54, F | Insulinoma | Tail | 1.5 | Insulinoma |
| 4 | 54, F | Cystic tumor | Tail | 2.0 | Serous cystadenoma |
| 5 | 22, M | Chr. pancreatitis | Total | — | Chronic pancreatitis |
| 6 | 26, M | Cystic tumor | Tail | 2.0 | Edidermoid cyst |
| 7 | 42, M | Cystic tumor | Tail | 3.0 | Edidermoid cyst |
| 8 | 66, M | IPMN | Body | 3.0 | Pseudocyst, dysplasia |
| 9 | 73, M | IPMN | Body & tail | 3.0 | In situ carcinoma |
| 10 | 54, M | IPMN | Tail | 3.0 | Hyperplasia and adenoma |
| 11 | 78, M | IPMN | Body & tail | 7.5 × 5.5 | Benign-Borderline |
| 12 | 79, M | IPMN | Body & tail | 2.7 | Adenoma |
| 13 | 70, M | IPMN | Body | 2.5 × 1.8 | In situ carcinoma |
| 14 | 46, F | IPMN | Body | 2.5 | In situ carcinoma |
| 15 | 60, F | IPMN | Body & tail | 2.5 | In situ carcinoma |
| 16 | 57, M | IPMN | Body | 3.5 | Adenoma |
| 17 | 61, M | IPMN | Body | 4.0 | Adenoma |
| 18 | 70, M | Endocrine tumor | Tail | 0.5 | Endocrine tumor |
Mean age 56.5; M:F = 12:6; IPMN 9; Cystic tumor 4, Endocrine tumor 3, Chr. pancreatitis 2.
The mostly feared potential complication was postoperative bleeding from the splenic vein caused by digestion of the ablated wall of the vein by pancreatic juice originating from the end of the pancreas[1]. Another feared complication was tortion of the splenic vessels. However, these complications did not arise in any case.
IMPORTANT AND INTERESTING CASES
Case No. 1
A 79 year-old patient with simultaneous gastric carcinoma and IPMN in the body and tail of the pancreas: Endoscopic examination showed early gastric carcinoma of the body of the stomach (Figure 14). MRCP revealed the extremely dilated main pancreatic duct, as well as cystic dilatation of the branch duct in the body of the pancreas. In combination with other findings, we made a diagnosis of main and branch duct IPMN .
Figure 14.
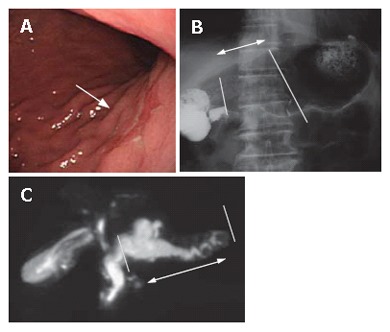
Case No.1: A 79 yr-old patient with simultaneous gastric carcinoma and IPMN in the body and tail of the pancreas. Endoscopic examination and MRCP. A: Stomach: Early carcinoma; B: Pylorus-preservingdistal gastrectomy; C: Spleen-preserving distal pancreatectomy.
At surgery , the pancreas was not hard and tumor did not seem to invade the parenchyma of the pancreas by both inspection and (Figure 15) intraoperative ultrasonography. No apparent lymphnode metastasis around the pancreas was detected. MRI did not show any malignant findings preoperatively. Therefore, we decided to do pylorus-preserving distal gastrectomy and spleen preserving distal pancreatectomy. Re-construction of stomach was done by end-to-end gastro-gastrostomy.
Figure 15.
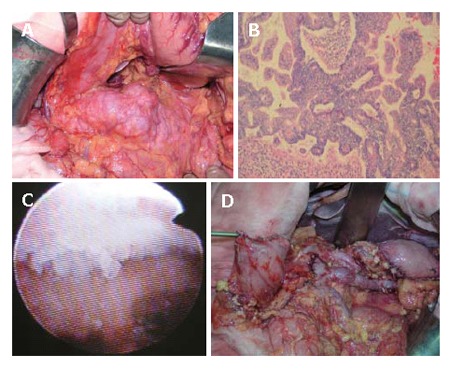
Case No.1 The operative and histological findings, and egg like appearance by intraoperative pancreatoscopy. A: IPMN of the pancreas; B: Body: Carcinoma, noninvasive; C: Body: Egg like appearance; D: After stomach resection and spleen-preserving DP.
Figure 15 show the intra-operative view of the pancreas, and egg-like appearance of the main pancreatic duct in the tail of the pancreas by intra-operative pancreatoscope in this case. Histological investigation revealed carcinoma in situ in the main pancreatic duct in the body, and adenoma in the branch duct of the body of the pancreas.
The middle part of the stomach was segmentaly resected and distal pancreas was also resected with conservation of the splenic artery and vein and the spleen. The gastro-gastro-anastomosis was done after this operation. This patient is still alive with no recurrence of carcinoma in the last three years.
If distal pancreatectomy with splenectomy had been performed for the lesions of the distal pancreas at this operation, the residual stomach should also have been resected because of no blood supply from the short gastric arteries originated from the splenic artery. That is, total gastrectomy with esophago-jejunostomy should have been performed.
However, total gastrectomy would have been excess surgery for early gastric carcinoma of this patient. In addition, the damage done to the patient by reconstruction of the gastrointestinal tract in combination with distal pancreatectomy and splenectomy would have been much greater than with only distal pancreatectomy with preservation of the spleen and residual stomach with end to end anastomosis.
Case No. 2
A 54-year-old patient with a diagnosis of IPMN in the body of the pancreas (13): CT scan revealed two cystic lesions in the body of the pancreas (Figure 16). Endoscopic examination revealed the slight dilatation of the papilla of Vater. Endoscopic ultrasound revealed the cystic dilatation of the branch duct with protrusive lesions in the body of the pancreas (Figure 17). In combination with other findings, we made a diagnosis of branch type of IPMN in this case.
Figure 16.
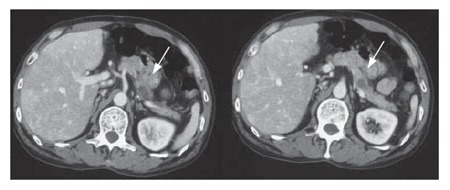
Case No. 2: A 54-yr-old patient with a diagnosis of IPMN in the body of the pancreas. CT scan. The cystic dilatation of the branch duct with protrusive lesions in the body of the pancreas. The main pancreatic duct is not dilated.
Figure 17.
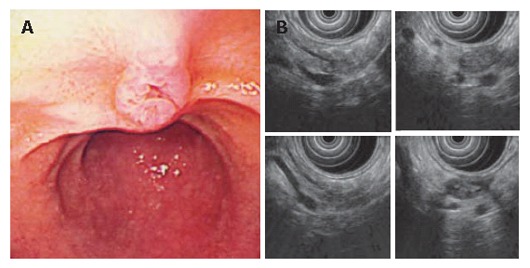
Endoscopic examination revealed the slight dilatation of the papilla of Vater. Endoscopic ultrasound revealed the cystic dilatation of the branch duct with protrusive lesions in the body of the pancreas. A: Slight dilatation of the orifice of the papilla of Vater; B: The cystic dilatation of the branch duct with protrusive lesions in the body of the pancreas. The main pancreatic duct is not dilated.
Thirty-two years before the operation, the patient had undergone distal gastrectomy for duodenal ulcer, with reconstruction by the Billroth II method. If distal pancreatectomy with splenectomy had been performed for the lesion of the distal pancreas at this operation, the residual stomach should also have been resected because of no blood supply from the short gastric arteries originated from the splenic artery. Furthermore, the adhesion on the left side of the intraabdominal cavity should have been ablated for use of the jejunum for esophago-jejunostomy. The damage done to the patient by reconstruction of the gastrointestinal tract in combination with distal pancreatectomy and splenectomy would have been much greater than with only distal pancreatectomy with preservation of the spleen and residual stomach.
In this case, although the preoperative diagnosis of the cystic lesion was a branch-type IPMN, this was histologically revealed to be a pseudocyst. Dysplasia was found in the branch duct epithelium of the tail of the pancreas.
Even with advances in imaging techniques, diagnosis of a cystic lesion of the pancreas is still very difficult. Ordinary distal pancreatectomy with splenectomy would have been over surgery in this case, and this should be avoided and could be avoided by the spleen preserving method.
Case No. 3
A 25 year old male: The imaging procedures such as abdominal CT scan, EUS and MRCP suggested a branch type of IPMN (Figure 18). The presence of invasion was unclear both preoperatively and intraoperatively. The patient was young that we decided to perform spleen preserving distal pancreatectomy.
Figure 18.
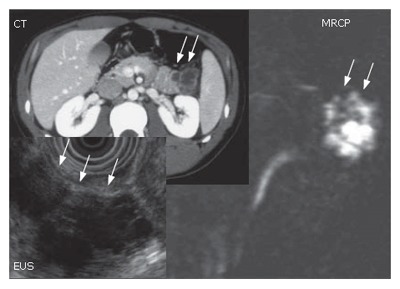
Case No. 3. A 25 yr old male. The imaging procedures such as abdominal CT scan, EUS and MRCP suggested a branch type of IPMN.
Figure 19 top-left shows the distal part of the pancreas with tumors. The spleen, splenic artery and vein were preserved. However, intraoperative histologic examination revealed that the tumor was malignant with invasive parts. Therefore, we converted the procedure to the distal pancreatectomy with splenectomy in combination with radical lymphnode dissection. This is a very rare case of carcinoma derived from IPMN, developed in a very young man.
Figure 19.
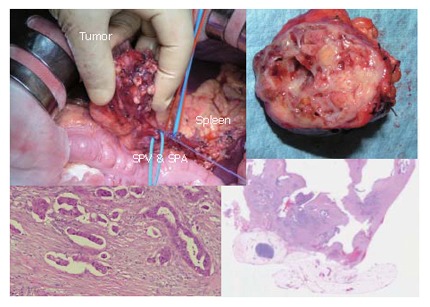
The operative and histological findings, and cut surfice of the tumor.
In conclusion, spleen-preserving distal pancreas-tectomy with preservation of the splenic vessels can be performed easily and safely. Our experience suggests that this procedure should be conducted for IPMN of the distal pancreas as well as chronic pancreatitis.
Footnotes
S- Editor Liu Y L- Editor Chiarioni G E- Editor Zhou T
References
- 1.Kimura W, Inoue T, Futakawa N, Shinkai H, Han I, Muto T. Spleen-preserving distal pancreatectomy with conservation of the splenic artery and vein. Surgery. 1996;120:885–890. doi: 10.1016/s0039-6060(96)80099-7. [DOI] [PubMed] [Google Scholar]
- 2.White SA, Sutton CD, Weymss-Holden S, Berry DP, Pollard C, Rees Y, Dennison AR. The feasibility of spleen-preserving pancreatectomy for end-stage chronic pancreatitis. Am J Surg. 2000;179:294–297. doi: 10.1016/s0002-9610(00)00333-0. [DOI] [PubMed] [Google Scholar]
- 3.Govil S, Imrie CW. Value of splenic preservation during distal pancreatectomy for chronic pancreatitis. Br J Surg. 1999;86:895–898. doi: 10.1046/j.1365-2168.1999.01179.x. [DOI] [PubMed] [Google Scholar]
- 4.Kimura W, Kuroda A, Makuuchi M. Problems in the diagnosis and treatment of a so-called mucin-producing tumor of the pancreas. Pancreas. 1998;16:363–369. doi: 10.1097/00006676-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Leonard AS, Giebink GS, Baesl TJ, Krivit W. The overwhelming postsplenectomy sepsis problem. World J Surg. 1980;4:423–432. doi: 10.1007/BF02393164. [DOI] [PubMed] [Google Scholar]
- 6.Francke EL, Neu HC. Postsplenectomy infection. Surg Clin North Am. 1981;61:135–155. doi: 10.1016/s0039-6109(16)42339-x. [DOI] [PubMed] [Google Scholar]
- 7.Malangoni MA, Dillon LD, Klamer TW, Condon RE. Factors influencing the risk of early and late serious infection in adults after splenectomy for trauma. Surgery. 1984;96:775–783. [PubMed] [Google Scholar]
- 8.Sugimachi K, Kodama Y, Kumashiro R, Kanematsu T, Noda S, Inokuchi K. Critical evaluation of prophylactic splenectomy in total gastrectomy for the stomach cancer. Gann. 1980;71:704–709. [PubMed] [Google Scholar]
- 9.Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7:473–479. doi: 10.1007/s005340070017. [DOI] [PubMed] [Google Scholar]
- 10.Kimura W. Theoretical basis and techniques for resection of extrapancreatic nerve plexus in the head of the pancreas during Whipple procedurefor carcinoma of the pancreas: Suggestion of the perspective of surgical anatomy and pathology. JJPS. 2006;19:463–470. [PubMed] [Google Scholar]
- 11.Kimura W. IHPBA in Tokyo, 2002: surgical treatment of IPMT vs MCT: a Japanese experience. J Hepatobiliary Pancreat Surg. 2003;10:156–162. doi: 10.1007/s00534-002-0799-2. [DOI] [PubMed] [Google Scholar]
- 12.Kimura W, Fuse A, Hirai I, Suto K. Spleen-preserving distal pancreatectomy for intraductal papillary-mucinous tumor (IPMT) Hepatogastroenterology. 2004;51:86–90. [PubMed] [Google Scholar]
- 13.Kimura W, Fuse A, Hirai I, Suto K, Suzuki A, Moriya T, Sakurai F. Spleen-preserving distal pancreatectomy with preservation of the splenic artery and vein for intraductal papillary-mucinous tumor (IPMT): three interesting cases. Hepatogastroenterology. 2003;50:2242–2245. [PubMed] [Google Scholar]


