Abstract
AIM: To investigate the mutation of p53 immuno-histochemically in non-tumorous gastric mucosa with H pylori infection before and after H pylori eradication therapy.
METHODS: 53 subjects (36 male, 17 female, mean age ± SEM, 57.1 ± 12.1) undergoing endoscopic examination were included in this study. 42 of 53 patients were H pylori-positive, and 11 were H pylori-negative. All H pylori-positive patients had successful eradication therapy. Biopsy specimens were taken from five points of the stomach, as recommended by the updated Sydney system. Immunohistochemical studies were performed by using primary antibodies against p53 (DO-7 and PAb240).
RESULTS: p53 (DO-7 and PAb240) immunoreactivity was shown in the neck region of the gastric pits, however, quite a few cells were found to be immunopositive for p53 (PAb240) in the H pylori-infected gastric mucosa. The proportion of patients immunopositive for p53 (PAb240) was significantly reduced 6 mo after eradication [28/42 (66.7%) to 6/42 (14.3%)] (P < 0.05), while the biopsies taken from H pylori-negative patients showed no immunoreactivity for p53 (PAb240). p53 (PAb240)-positive patients were divided into two groups by the number of positive cells detected: one with more than six positive cells per 10 gastric pits (group A, n = 12), and the other with less than five positive cells per 10 gastric pits (group B, n = 30). Atrophy scores in group A were significant higher than those in group B at the greater curvature of the antrum (group A: 2.00 ± 0.14 vs group B: 1.40 ± 0.15, P = 0.012), the lesser curvature of the corpus (group A: 2.00 ± 0.21 vs group B: 1.07 ± 0.23, P = 0.017), and the greater curvature of the corpus (group A: 1.20 ± 0.30 vs group B: 0.47 ± 0.21, P = 0.031). Group A showed significant higher intestinal metaplasia scores than group B only at the lesser curvature of the antrum (group A: 2.10 ± 0.41 vs group B: 1.12 ± 0.29, P = 0.035).
CONCLUSION: H pylori-associated chronic gastritis expressed the mutant-type p53, which was significantly associated with more severe atrophic and metaplastic changes. H pylori eradication led to a significant reduction in the expression of the mutant-type p53. It is considered that H pylori-infected chronic gastritis is associated with a genetic instability that leads to gastric carcinogenesis, and H pylori eradication may prevent gastric cancer.
Keywords: H pylori, H pylori eradication, Atrophic gastritis, Mutant-type p53, Gastric cancer, Updated Sydney system
INTRODUCTION
Helicobacter pylori is one of the main pathogens that cause many kinds of gastroduodenal diseases. These include acute gastritis[1-3], chronic atrophic gastritis[3,4], intestinal metaplasia[5], peptic ulcer[6,7], mucosal associated lymphoid tissue (MALT) lymphoma[8], and other disorders[9,10]. Although many epidemiological studies and animal models revealed close association between gastric cancer and H pylori infection[11-13], there have been few studies that report on genetic alterations suggestive of gastric carcinogenesis associated with chronic H pylori infection[14-17]. Previously, we reported the expression of p53, a product of oncosupressor-gene and cell cycle regulator, in the H pylori-infected human gastric mucosa[18] as well as in a Japanese monkey model[19]. p53 expression was observed in the neck region of the gastric pit, and was reported to be reduced after H pylori eradication[18]. p53 mutation is among the major episodes in the multi-step process of gastric carcinogenesis, while it has also been reported in pre-malignant lesions of the stomach, such as chronic gastritis, intestinal metaplasia, and dysplasia[20-22]. However, it remains unclear whether p53 expression in the gastric pit is mutant-type or wild-type. In this study, we used the primary antibodies reacting with both wild and mutant type-p53, and those reacting only with the mutant-type p53 in our immunohistochemical studies to clarify the p53 alterations in the H pylori-infected gastric mucosa.
MATERIALS AND METHODS
Subjects
A total of 53 subjects (36 male, 17 female, mean age SEM, 57.1 ± 12.1) who underwent endoscopic examination were included in this study. 42 out of 53 patients were H pylori-positive and 11 were H pylori-negative. In 42 H pylori-positive patients, 14 patients had gastric ulcers, 5 had duodenal ulcers, 5 had gastro-duodenal ulcers, and 15 had chronic gastritis. All H pylori-positive patients had successful eradication therapy, and underwent biopsies before and six months after eradication. Biopsies for examination were taken at the same biopsy sites in H pylori-negative subjects as in the H pylori positive patients.
Detection of H pylori in gastric biopsy specimens
H pylori in the stomach was detected by the rapid urease test, culture, and histological examination. For the urease test, biopsy specimens were immediately inserted into the rapid urease test solution. For culture detection, biopsy material was cultured on 7% sheep’s blood agar plates under micro-aerobic conditions and at high humidity and at 37°C for four days. H pylori was histologically detected by May-Giemsa stain. H pylori eradication was considered successful when the results of all three tests were found negative.
Histological evaluation
Biopsy specimens were taken from five points of the stomach, as recommended by the updated Sydney system[23], i.e. the lesser curvature of the antrum (A1), and the greater curvature of the antrum (A2), the smaller curvature of the angle (IA), and the lesser curvature of the middle corpus (B1), and the greater curvature of the upper corpus (B2). All biopsy materials were fixed in buffered formalin for 24 h and embedded in paraffin. Serial sections were stained with haematoxylin-eosin and with May-Giemsa stain. The status of the gastric mucosa was evaluated according to the updated Sydney system. The degree of inflammation, neutrophil activity, atrophy, and intestinal metaplasia were classed by four grades, with 0 being for ‘normal’, 1 for ‘mild’, 2 for ‘moderate’, and 3 for ‘marked’, respectively.
Immunohistochemical detection of p53
Serial paraffin sections were washed in 1/15 mol/L phosphate buffered saline (PBS, pH 7.4) three times for five minutes, and pre-incubated in normal rabbit serum (1:10 in PBS) for 20 min. Next, these sections were incubated with primary antibodies for 16 h at 4°C, followed by the avidin-biotin complex method. The sections were immersed in 0.05 mol/L Tris-HCl buffer containing 0.02% 3, 3’-diaminobenzidine tetrahydrochloride and 0.005% H2O2, and the nuclei were counterstained with hematoxylin. Control sections incubated with normal mouse IgG instead of the primary antibody showed no non-specific staining. The primary antibodies used in this study were mouse monoclonal anti-p53 protein (clone DO-7 and PAb240, Dako, Carpinteria, CA, USA), and were diluted to 1:50. Control sections incubated with normal mouse IgG instead of the primary antibody showed no non-specific staining.
Evaluation of p53 (DO-7 and PAb240)
Cells immunopositive for p53 (DO-7) were counted in longitudinal sectioned foveolar pits and were found visible along their whole length. Labeling indices for p53 (DO-7) are indicated as the proportion of positive cells among the cell total of the gastric mucosa. At least 500 cells in the gastric epithelial cells were calculated in each specimen. Cells immunopositive for p53 (PAb240) were counted in per 10 longitudinal gastric pits.
Statistical analysis
Statistical analysis (Wilcoxon signed-rank test and Mann-Whitney’s U test) was performed to analyze the updated Sydney system score and labeling indices.
RESULTS
Immunohistochemical detection of p53 (DO-7)
Immunoexpression of p53 (DO-7) was observed in the neck region of the H pylori-infected gastric pits from all biopsy sites (Figure 1). These p53-positive cells were significantly reduced 6 mo after H pylori eradication. In contrast, the gastric mucosa without H pylori infection showed very few positive cells in the gastric pits. The labeling index for p53 (DO-7) in the H pylori-infected group before eradication was significantly higher than that in the non-H pylori-infected group (P < 0.001) (Figure 2). After eradication, the labeling index for p53 was significantly reduced at all biopsy sites (A2; from 14.98% to 6.80%; P < 0.001, A1; from 12.63% to 4.96%; P < 0.001, IA; from 14.24% to 4.26%; P < 0.001, B1; from 17.49% to 6.41%; P < 0.001, B2; from 14.45% to 4.48%; P < 0.001) (Figure 2).
Figure 1.
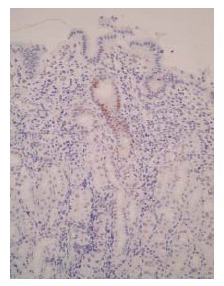
Immunohistochemistry for p53 (DO-7) in H pylori-infected gastric mucosa. Positive staining for p53 (DO-7) was observed in the gastric pit, especially in the neck region before eradication (× 200).
Figure 2.
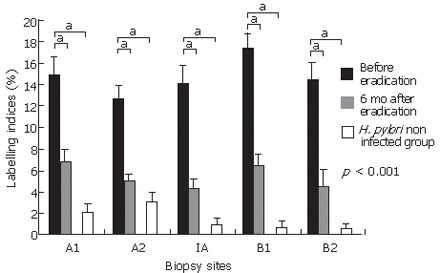
Labeling indices for p53 (DO-7) in 42 patients with H pylori infection, 6 mo after eradication, and 11 patients without H pylori infection. Results were shown as mean ± SEM. p53 (DO-7) indices were significantly reduced 6 mo after eradication at all biopsy sites. p53 indices for the H pylori-infected mucosa were significantly higher than those for the gastric mucosa without H pylori infection at all biopsy sites. bP < 0.001, vs H pylori non infected group.
Immunohistochemical detection of p53 (PAb240)
Immunoreactivity of p53 (PAb240) was also shown in the neck region of the gastric pits; however, quite a few cells were found to be immunopositive for p53 (PAb240) in the H pylori-infected gastric mucosa (Figure 3A and 3B). Indices for p53 (PAb240) in all examined patients are indicated in Table 1. Immunopositive cells for p53 (PAb240) were found in 28 out of 42 (66.7%) H pylori-positive patients, while H pylori-negative patients showed no immunoreactivity for p53 (PAb240) in all five biopsy specimens. Six months after eradication, the ratio of cells immunopositive for p53 (PAb240) was significantly reduced [28/42 (66.7%) to 6/42 (14.3%)] (P < 0.05) (Table 2). In patients immunoreactive for p53 (PAb240), the total number of positive cells from all five biopsy specimens ranged from 0 to 19.1 per 10 gastric pits. When divided by the number of positive cells, 30 H pylori-positive patients had less than five cells, 8 patients had six to 10 cells, 1 patient had 11 to 15 cells, and 3 patients had more than 16 positive cells, respectively, with the majority of patients found to have less than five positive cells. Thus the patients were divided into two groups by the number of positive cells: one with more than six positive cells per 10 gastric pits (group A, n = 12); and the other with less than five positive cells per 10 gastric pits (group B, n = 30).
Figure 3.
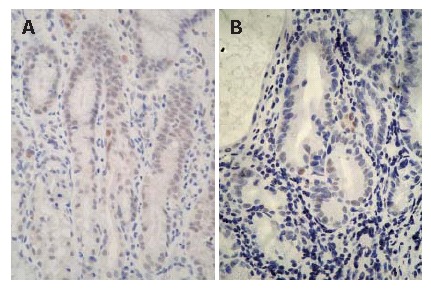
Immunohistochemistry for p53 (PAb240) in the H pylori-infected gastric mucosa. A small number of cells clearly showing the expression of p53 (PAb240) were detected in the neck region before eradication. A: a 53-yr-old male with gastric ulcer; B: a 51-yr-old male with chronic gastritis; × 400).
Table 1.
Immunohistochemical detection agaisnt p53 (PAb 240) in gastric mucosa
|
Number of positive cells per
ten crypts against p53 (PAb240)
before eradication |
Number of positive cells per
ten crypts against p53 (PAb240)
6 mo after eradication |
|||||||||||||
| Case | Sex | Age (yr) | Clinical diagnosis | A2 | A1 | IA | B1 | B2 | A2 | A1 | IA | B1 | B2 | |
| H pylori positive | 1 | F | 42 | Gstric ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | F | 26 | Duodenal ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | M | 64 | Gastric ulcer | 0 | 0 | 11.4 | 0 | 0 | 0.7 | 0 | 0 | 0 | 0 | |
| 4 | M | 64 | Gastric ulcer | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | M | 56 | Gastric ulcer | 0 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | M | 50 | Gastric ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 7 | M | 45 | Gastroduodenal ulcer | 0 | 0 | 0 | 0 | 2.3 | 0 | 1.1 | 0 | 0 | 0 | |
| 8 | M | 52 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 9 | M | 60 | Gastric ulcer | 0 | 0 | 0 | 0.8 | 0 | 0.5 | 0 | 0 | 2.2 | 0 | |
| 10 | F | 71 | Chronic gastritis | 1.7 | 0 | 0.8 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | M | 60 | Gastric ulcer | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | M | 55 | Gastric ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 13 | M | 42 | Gastroduodenal ulcer | 2.4 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 14 | M | 68 | Gastric ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 15 | M | 67 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 16 | M | 57 | Chronic gastritis | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 17 | F | 77 | Gastric ulcer | 0 | 0 | 0 | 1.7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 18 | F | 51 | Chronic gastritis | 0 | 0 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 19 | M | 64 | Gastric ulcer | 0 | 0 | 0 | 0 | 2.3 | 0 | 0 | 0 | 0 | 0 | |
| 20 | F | 57 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 21 | M | 51 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 22 | F | 75 | Chronic gastritis | 5.7 | 0 | 3.3 | 0 | 0.8 | 0 | 0 | 0 | 0 | 0 | |
| 23 | F | 65 | Chronic gastritis | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 | F | 51 | Chronic gastritis | 0.6 | 0 | 2.7 | 0 | 4.2 | 0 | 0 | 0 | 0 | 0 | |
| 25 | M | 53 | Gastric ulcer | 7.5 | 2.7 | 5 | 2.1 | 0 | 6 | 0 | 0 | 0 | 0 | |
| 26 | M | 68 | Chronic gastritis | 0.9 | 2.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 27 | M | 50 | Chronic gastritis | 0 | 1.3 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 28 | F | 78 | Chronic gastritis | 0 | 0 | 0 | 2.2 | 1.8 | 0 | 0 | 0 | 0 | 0 | |
| 29 | M | 58 | Gastric ulcer | 0 | 0 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 30 | M | 74 | Chronic gastritis | 0 | 0 | 0 | 1.7 | 4.5 | 0 | 0 | 0 | 0 | 0 | |
| 33 | M | 48 | Chronic gastritis | 1.1 | 0 | 3.8 | 0 | 3.8 | 0 | 0 | 0 | 1.7 | 0 | |
| 34 | F | 68 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 35 | F | 46 | Gastric ulcer | 3.3 | 0 | 7.5 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 36 | M | 64 | Duodenal ulcer | 0 | 0 | 0 | 6.7 | 0 | 2 | 1.7 | 0 | 0 | 5 | |
| 37 | M | 42 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 38 | F | 71 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 39 | M | 58 | Chronic gastritis | 0 | 1 | 0.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 40 | F | 46 | Chronic gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 41 | M | 76 | Chronic gastritis | 0.9 | 3 | 2.2 | 1.7 | 1.7 | 0 | 0 | 0 | 0 | 0 | |
| 42 | M | 51 | Chronic gastritis | 2.9 | 0 | 3.1 | 5.4 | 7.7 | 0 | 0 | 0 | 0 | 0 | |
| H pylori negative | 43 | M | 25 | 0 | 0 | 0 | 0 | 0 | ||||||
| 44 | M | 49 | 0 | 0 | 0 | 0 | 0 | |||||||
| 45 | M | 51 | 0 | 0 | 0 | 0 | 0 | |||||||
| 46 | M | 68 | 0 | 0 | 0 | 0 | 0 | |||||||
| 47 | M | 40 | 0 | 0 | 0 | 0 | 0 | |||||||
| 48 | F | 64 | 0 | 0 | 0 | 0 | 0 | |||||||
| 49 | F | 52 | 0 | 0 | 0 | 0 | 0 | |||||||
| 50 | M | 59 | 0 | 0 | 0 | 0 | 0 | |||||||
| 51 | M | 73 | 0 | 0 | 0 | 0 | 0 | |||||||
| 52 | F | 59 | 0 | 0 | 0 | 0 | 0 | |||||||
| 53 | M | 38 | 0 | 0 | 0 | 0 | 0 | |||||||
A1: Lesser curvature of the antrum; A2: Greater curvature of the antrum; IA: Lesser curvature of the angle; B1: Lesser curvature of the lower body; B2: Greater curvature of the upper body.
Table 2.
Positive ratio of Immunohistochemical detection against p53 (PAb 240)
| Before eradication | 6 mo after eradication | |
| H pylori positive | 66.7% (28 out of 42) | 14.3% (6 out of 42)b |
| H pylori negative | 0% (0 out of 11) |
P < 0.01 vs before eradication in H pylori positive group.
Before eradication, labeling indices of p53 (DO-7) were significantly higher in group A than in group B at all biopsy sites except the lesser curvature of the antrum (Figure 4). H pylori density, inflammation, and activity scores in the updated Sydney system showed no significant difference between the groups (Table 3). However, atrophy scores in group A were significantly higher than in group B at biopsy site A2 (group A: 2.00 ± 0.14 vs group B: 1.40 ± 0.15, P = 0.012), B1 (group A: 2.00 ± 0.21 vs group B: 1.07 ± 0.23, P = 0.017), and B2 (group A: 1.20 ± 0.30 vs group B: 0.47 ± 0.21, P = 0.031) (Table 3). There was no significant difference in atrophy scores between the two groups at biopsy site A1 and IA. group A showed significantly higher intestinal metaplasia scores than group B only at A1 (group A: 2.10 ± 0.41 vs group B: 1.12 ± 0.29, P = 0.035), with no significant difference in intestinal metaplasia scores seen at all the other biopsy sites.
Figure 4.
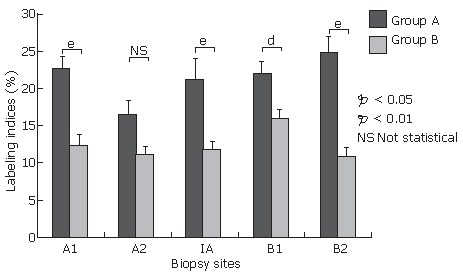
Labeling indices for p53 (DO-7) in group A with more than 6 positive cells for p53 (PAb240) per 10 gastric pits (n = 12) and group B with less than 5 positive cells for p53 (PAb240) per 10 gastric pits (n = 30) before eradication. Results are shown as mean ± SEM. Labeling indices for p53 (DO-7) were significantly higher for group A than for group B at all biopsy sites except for the lesser curvature of the antrum. aP < 0.05, bP < 0.01, NS, not statistical.
Table 3.
The differences of updated Sydney system score between the group showed more than six positive cells for p53 (PAb240) per 10 gastric pits (group A) and the group showed less than five cells for p53 (PAb240) per 10 gastric pits (Group B)
| Contents of updated Sydney system | Group |
Biopsy points |
||||
| A2 | A1 | IA | B1 | B2 | ||
| H pylori | Group A | 1.25 ± 0.25 | 0.50 ± 0.21 | 1.00 ± 0.21 | 0.88 ± 0.24 | 1.38 ± 0.21 |
| Group B | 0.71 ± 0.18 | 0.88 ± 0.22 | 0.88 ± 0.21 | 1.13 ± 0.19 | 1.41 ± 0.17 | |
| Inflammation | Group A | 2.20 ± 0.12 | 2.20 ± 0.12 | 2.10 ± 0.16 | 2.30 ± 0.13 | 1.90 ± 0.16 |
| Group B | 2.05 ± 0.18 | 1.99 ± 0.17 | 2.00 ± 0.17 | 2.00 ± 0.12 | 1.82 ± 0.12 | |
| Activity | Group A | 1.40 ± 0.15 | 1.00 ± 0.23 | 1.20 ± 0.23 | 1.20 ± 0.23 | 1.30 ± 0.23 |
| Group B | 1.06 ± 0.20 | 0.94 ± 0.18 | 1.00 ± 0.18 | 1.05 ± 0.16 | 0.94 ± 0.17 | |
| Atrophy | Group A | 2.00 ± 0.14 | 1.75 ± 0.13 | 2.13 ± 0.28 | 2.00 ± 0.21 | 1.20 ± 0.30 |
| Group B | 1.40 ± 0.15a | 1.57 ± 0.15 | 1.67 ± 0.18 | 1.07 ± 0.23a | 0.47 ± 0.21a | |
| Intestinal etaplasia | Group A | 0.90 ± 0.31 | 2.10 ± 0.41 | 1.80 ± 0.40 | 1.30 ± 0.36 | 0.20 ± 0.12 |
| Group B | 0.82 ± 0.27 | 1.12 ± 0.29c | 1.47 ± 0.32 | 1.11 ± 0.29 | 0.12 ± 0.12 | |
A1: the lesser curvature of the antrum; A2: the greater curvature of the antrum; IA: smaller curvature of the angle; B1: and the lesser curvature of the middle corpus; B2: the greater curvature of the upper corpus.
P < 0.05 vs atrophy scores in group A,
P < 0.05 vs intestinal metaplasia scores in group A.
DISCUSSION
Previously, we reported the significant over-expression of p53 in the H pylori-infected gastric pits, as well as its significant reduction after H pylori eradication[18]. Other researchers also reported that p53 was immunohistochemically detected in the non-neoplastic H pylori-infected gastric mucosa[24-28], and that H pylori eradication therapy also diminished the expression of p53[18,25,27].
p53 is thought to be one major player in the regulation of cell cycle[29] in which DNA damage activates p53 proliferation, and p53 binds to MDM2 and other molecules. This binding protein arrests cell proliferation and maintains the integrity of the cell by DNA repairing or cell apoptosis. In contrast, mutant p53 loses these abilities. In a previous report[18], we considered that immunodetection of p53 (DO-7) signified whether accelerated wild type-p53 or occurrence of mutant type-p53. Teh et al[28] reported that p53 detection in the non-neoplastic epithelium was considered to reflect an accumulation of wild-type p53 due to the sensitivity of the currently available antigens. Our study also suggested an accumulation of wild-type p53, especially in the H pylori-infected mucosa probably because of the DNA damage that occurred to H pylori infection. In addition, the present study showed that quite a few cells were found positive for mutant-type p53 (PAb240), instead of p53 (DO-7). There has been no study reported to date demonstrating the positive immunohistochemical staining for mutant-type of p53 in the non-neoplastic gastric mucosa. Our results indicated that while the majority of cells that reacted with p53 (DO-7) account for an accumulation of wild-type p53, mutant type p53 might also be expressed in some cells of the gastric pit. Murakami et al[14] and Morgan et al[31] reported the presence of point mutations in exon 5 to 8 of p53 in H pylori-positive gastritis, and several other studies have shown that gastric precancerous lesions, such as atrophic gastritis and intestinal metaplasia, are associated with p53 abnormalities[20-22,26,32]. Murakami et al[14] reported that the point mutations are present in 52.4% of the gastritis, with Morgan et al[31] reporting the presence of p53 mutations in 35% of the gastritis and 45% of the intestinal metaplasia. Unger et al[32] demonstrated that the expression of p53 and apoptotic indices in gastritis in the absence of intestinal metaplasia are significantly higher in H pylori-positive patients than in H pylori-negative patients. These studies are in agreement with our results indicating that in some p53 mutations are found mutant type-p53 products in the H pylori-infected gastric pits.
In a comparison between group A with more than six positive cells per 10 gastric pits and group B with less than five cells per 10 gastric pits, group A showed significantly higher labeling indices for p53 (DO-7) in all biopsy sites than group B. This result suggested an increase in wild-type p53 as it was caused by the greater DNA damage due to the gene instability in place that might increase mutant-type p53. Moreover, group A showed significantly higher atrophy and intestinal metaplasia scores in the stomach, suggesting that the gastric mucosa with more severe atrophic changes or extensive intestinal metaplasia has a greater number of mutant-type p53. Thus, it is likely that the development of atrophic changes and intestinal metaplasia gradually increased the occurrence of p53 mutations. Gastric cancer of the intestinal type follows a multi-step process of carcinogenesis, such as atrophic gastritis, intestinal metaplasia, and dysplasia[33]. Therefore, the present results indicate that H pylori infection in the gastric mucosa may be implicated in the pathway of gastric carcinogenesis. Nardone et al[26] reported that DNA aneuploidy was seen in 11 patients with H pylori infection and atrophy, with 8 of these found to show c-Myc expression, and 6 of these to have p53 expression, and they concluded that chronic H pylori infection may be responsible for genomic instability in a subset of H pylori-positive chronic atrophic gastritis. Unger et al[32] also reported that H pylori-positive gastritis accompanied with intestinal metaplasia showed a lack of increased apoptosis with a higher p53 expression, which suggests an increased genetic instability, thus concluding that p53 mutation is an early step in the multi-step process of gastric carcinogenesis. Our results confirm the relationship between atrophic and/or metaplastic gastric mucosa with H pylori infection and genetic instability, which lead to gastric carcinogenesis.
In the present study, H pylori eradication therapy led to a significant reduction in the expression of both p53 (DO-7) and p53 (PAb240). The number of mutant-type p53-positive patients was significantly decreased 6 mo after eradication. In our previous study, we exhibited that eradication reduces both p53 and MDM2 expression[18]. Hibi et al[25] and Satoh et al[27] also described a reduction in the expression of p53 after eradication. Nardone et al[26] reported that H pylori eradication reduced gastritis activity, atrophy, and complete metaplasia, accompanied by the disappearance of markers of genomic instability, thereby concluding that eradication can reverse inflammation, associated atrophy, metaplasia, and genomic instability. The present study also indicated that H pylori eradication diminished p53 abnormality, and this result accounted for the improvement of genetic instability.
Many researchers reported morphologic alterations of gastric mucosa, especially those of atrophic and metaplastic status after H pylori eradication[34], while their results are conflicting, with some of them reporting that eradication did not improve atrophy and/or intestinal metaplasia, and some concluding that eradication could improve the gastric atrophy and/or intestinal metaplasia. However, after the year 2000, the majority of papers reported improvements in the gastric mucosa with H pylori eradication.
The present study confirmed the hypothesis that H pylori eradication reverses the atrophic changes in the gastric mucosa and the genetic instability, thus preventing the development of gastric cancer.
In conclusion, in chronic gastritis associated with H pylori infection, the expression of mutant-type p53 was seen significantly greater in more severe atrophic and metaplastic changes. H pylori eradication led to a significant reduction in the expression of mutant-type p53. It is suggested that chronic gastritis associated with H pylori infection has genetic instability, which leads to gastric carcinogenesis, and that H pylori eradication may prevent gastric cancer.
Footnotes
Supported by Grants-in-Aid (C-14) from the Ministry of Health, Labor and Welfare, Japan
S- Editor Liu Y L- Editor Alpini GD E- Editor Chin GJ
References
- 1.Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust. 1985;142:436–439. doi: 10.5694/j.1326-5377.1985.tb113443.x. [DOI] [PubMed] [Google Scholar]
- 2.Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192–199. [PubMed] [Google Scholar]
- 3.Fujioka T, Kubota T, Shuto R, Kodama R, Murakami K, Perparim K, Nasu M. Establishment of an animal model for chronic gastritis with Helicobacter pylori: potential model for long-term observations. Eur J Gastroenterol Hepatol. 1994;6 Suppl 1:S73–S78. [PubMed] [Google Scholar]
- 4.Kuipers EJ, Uyterlinde AM, Peña AS, Roosendaal R, Pals G, Nelis GF, Festen HP, Meuwissen SG. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995;345:1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 5.Sakaki N, Momma K, Egawa N, Yamada Y, Kan T, Ishiwata J. The influence of Helicobacter pylori infection on the progression of gastric mucosal atrophy and occurrence of gastric cancer. Eur J Gastroenterol Hepatol. 1995;7 Suppl 1:S59–S62. [PubMed] [Google Scholar]
- 6.Schubert TT, Bologna SD, Nensey Y, Schubert AB, Mascha EJ, Ma CK. Ulcer risk factors: interactions between Helicobacter pylori infection, nonsteroidal use, and age. Am J Med. 1993;94:413–418. doi: 10.1016/0002-9343(93)90153-g. [DOI] [PubMed] [Google Scholar]
- 7.Maaroos HI, Kekki M, Vorobjova T, Salupere V, Sipponen P. Risk of recurrence of gastric ulcer, chronic gastritis, and grade of Helicobacter pylori colonization. A long-term follow-up study of 25 patients. Scand J Gastroenterol. 1994;29:532–536. doi: 10.3109/00365529409092468. [DOI] [PubMed] [Google Scholar]
- 8.Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 9.Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352:878. doi: 10.1016/S0140-6736(05)60004-9. [DOI] [PubMed] [Google Scholar]
- 10.Sato R, Murakami K, Watanabe K, Okimoto T, Miyajima H, Ogata M, Ohtsuka E, Kodama M, Saburi Y, Fujioka T, et al. Effect of Helicobacter pylori eradication on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura. Arch Intern Med. 2004;164:1904–1907. doi: 10.1001/archinte.164.17.1904. [DOI] [PubMed] [Google Scholar]
- 11.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 13.Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 14.Murakami K, Fujioka T, Okimoto T, Mitsuishi Y, Oda T, Nishizono A, Nasu M. Analysis of p53 gene mutations in Helicobacter pylori-associated gastritis mucosa in endoscopic biopsy specimens. Scand J Gastroenterol. 1999;34:474–477. doi: 10.1080/003655299750026191. [DOI] [PubMed] [Google Scholar]
- 15.Chiou CC, Chan CC, Sheu DL, Chen KT, Li YS, Chan EC. Helicobacter pylori infection induced alteration of gene expression in human gastric cells. Gut. 2001;48:598–604. doi: 10.1136/gut.48.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backert S, Gressmann H, Kwok T, Zimny-Arndt U, König W, Jungblut PR, Meyer TF. Gene expression and protein profiling of AGS gastric epithelial cells upon infection with Helicobacter pylori. Proteomics. 2005;5:3902–3918. doi: 10.1002/pmic.200401240. [DOI] [PubMed] [Google Scholar]
- 17.Mannick EE, Schurr JR, Zapata A, Lentz JJ, Gastanaduy M, Cote RL, Delgado A, Correa P, Correa H. Gene expression in gastric biopsies from patients infected with Helicobacter pylori. Scand J Gastroenterol. 2004;39:1192–1200. doi: 10.1080/00365520410003588. [DOI] [PubMed] [Google Scholar]
- 18.Kodama M, Fujioka T, Murakami K, Okimoto T, Sato R, Watanabe K, Nasu M. Eradication of Helicobacter pylori reduced the immunohistochemical detection of p53 and MDM2 in gastric mucosa. J Gastroenterol Hepatol. 2005;20:941–946. doi: 10.1111/j.1440-1746.2005.03880.x. [DOI] [PubMed] [Google Scholar]
- 19.Kodama M, Fujioka T, Kodama R, Takahashi K, Kubota T, Murakami K, Nasu M. p53 expression in gastric mucosa with Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:215–219. doi: 10.1111/j.1440-1746.1998.tb00640.x. [DOI] [PubMed] [Google Scholar]
- 20.Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s–1943s. [PubMed] [Google Scholar]
- 21.Tahara E, Kuniyasu H, Yasui W, Yokozaki H. Gene alterations in intestinal metaplasia and gastric cancer. Eur J Gastroenterol Hepatol. 1994;6 Suppl 1:S97–S102. [PubMed] [Google Scholar]
- 22.Shiao YH, Rugge M, Correa P, Lehmann HP, Scheer WD. p53 alteration in gastric precancerous lesions. Am J Pathol. 1994;144:511–517. [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Jones NL, Shannon PT, Cutz E, Yeger H, Sherman PM. Increase in proliferation and apoptosis of gastric epithelial cells early in the natural history of Helicobacter pylori infection. Am J Pathol. 1997;151:1695–1703. [PMC free article] [PubMed] [Google Scholar]
- 25.Hibi K, Mitomi H, Koizumi W, Tanabe S, Saigenji K, Okayasu I. Enhanced cellular proliferation and p53 accumulation in gastric mucosa chronically infected with Helicobacter pylori. Am J Clin Pathol. 1997;108:26–34. [PubMed] [Google Scholar]
- 26.Nardone G, Staibano S, Rocco A, Mezza E, D'armiento FP, Insabato L, Coppola A, Salvatore G, Lucariello A, Figura N, et al. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789–799. doi: 10.1136/gut.44.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh K, Kihira K, Kawata H, Tokumaru K, Kumakura Y, Ishino Y, Kawakami S, Inoue K, Kojima T, Satoh Y, et al. p53 expression in the gastric mucosa before and after eradication of Helicobacter pylori. Helicobacter. 2001;6:31–36. doi: 10.1046/j.1523-5378.2001.00003.x. [DOI] [PubMed] [Google Scholar]
- 28.Teh M, Tan KB, Seet BL, Yeoh KG. Study of p53 immunostaining in the gastric epithelium of cagA-positive and cagA-negative Helicobacter pylori gastritis. Cancer. 2002;95:499–505. doi: 10.1002/cncr.10697. [DOI] [PubMed] [Google Scholar]
- 29.Levine AJ, Finlay CA, Hinds PW. p53 is a tumor suppressor gene. Cell. 2004;116:S67–S69, 1 p following S69. doi: 10.1016/s0092-8674(04)00036-4. [DOI] [PubMed] [Google Scholar]
- 30.Momand J, Zambetti GP. Mdm-2: "big brother" of p53. J Cell Biochem. 1997;64:343–352. [PubMed] [Google Scholar]
- 31.Morgan C, Jenkins GJ, Ashton T, Griffiths AP, Baxter JN, Parry EM, Parry JM. Detection of p53 mutations in precancerous gastric tissue. Br J Cancer. 2003;89:1314–1319. doi: 10.1038/sj.bjc.6601302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger Z, Molnár B, Prónai L, Szaleczky E, Zágoni T, Tulassay Z. Mutant p53 expression and apoptotic activity of Helicobacter pylori positive and negative gastritis in correlation with the presence of intestinal metaplasia. Eur J Gastroenterol Hepatol. 2003;15:389–393. doi: 10.1097/00042737-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 34.Murakami K, Kodama M, Sato R, Okimoto T, Watanabe K, Fujioka T. Helicobacter pylori eradication and associated changes in the gastric mucosa. Expert Rev Anti Infect Ther. 2005;3:757–764. doi: 10.1586/14787210.3.5.757. [DOI] [PubMed] [Google Scholar]


