Abstract
AIM: To study the immunological protective effect of H pylori vaccine with chitosan as an adjuvant and its mechanism.
METHODS: Female BALB/c mice were randomly divided into seven groups and orally immunized respectively with PBS, chitosan solution, chitosan particles, H pylori antigen, H pylori antigen plus cholera toxin (CT), H pylori antigen plus chitosan solution, H pylori antigen plus chitosan particles once a week for four weeks. Four weeks after the last immunization, the mice were challenged twice by alive H pylori (1 × 109 CFU/mL) and sacrificed. Part of the gastric mucosa was embedded in paraffin, cut into sections and assayed with Giemsa staining. Part of the gastric mucosa was used to quantitatively culture H pylori. ELISA was used to detect cytokine level in gastric mucosa and anti- H pylori IgG1, IgG2a levels in serum.
RESULTS: In the groups with chitosan as an adjuvant, immunological protection was achieved in 60% mice, which was significantly higher than in groups with H pylori antigen alone and without H pylori antigen (P < 0.05 or 0.001). Before challenge, the level of IFN and IL-12 in gastric mucosa was significantly higher in the groups with chitosan as an adjuvant than in the control group and the group without adjuvant (P < 0.05 or 0.005). After challenge, the level of IFN and IL-12 was significantly higher in the groups with adjuvant than in the groups without adjuvant and antigen (P < 0.05 or 0.001). Before challenge, the level of IL-2 in gastric mucosa was not different among different groups. After challenge the level of IL-2 was significantly higher in the groups with adjuvant than in the control group (P < 0.05 or 0.001). Before challenge, the level of IL-10 in gastric mucosa was significantly higher in the groups with chitosan as an adjuvant than in other groups without adjuvant (P < 0.05 or 0.01). After challenge, the level of IL-10 was not different among different groups. Before challenge, the level of IL-4 in gastric mucosa was significantly higher in the groups with chitosan as an adjuvant than in other groups without adjuvant (P < 0.05). After challenge, the level of IL-4 was significantly higher in the groups with chitosan particles as an adjuvant than in the group with CT as an adjuvant (P < 0.05), and in the group with chitosan solution as an adjuvant, the level of IL-4 was significantly higher than that in control group, non-adjuvant group and the groups with CT (P < 0.05 or 0.001). The ratio of anti- H pylori IgG2a/IgG1 in serum was significantly lower in the groups with chitosan as an adjuvant than in the groups with CT as an adjuvant or without adjuvant (P < 0.01).
CONCLUSION: H pylori vaccine with chitosan as an adjuvant can protect against H pylori infection and induce both Th1 and Th2 type immune response.
Keywords: H pylori, Chitosan, Vaccine, Adjuvant, Th immune response
INTRODUCTION
Colonization of H pylori in the stomach is associated with the risk of developing different gastroduodenal diseases including chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma and gastric cancer. The current treatment against H pylori infection is a combination therapy with two different antibiotics plus a proton-pump inhibitor with or without colloidal bismuth, which can eradicate the bacteria in most cases. However, this therapy has some major drawbacks, including high cost and development of antibiotic resistance. So vaccination would be a suitable alternative or complement to antibiotic treatment to eradicate the bacteria. A large number of animal experiments have shown that immunization with H pylori antigen in combination with certain adjuvants can prevent and even eliminate H pylori infection[1-3]. At present, the effective adjuvants are cholera toxin (CT) and Escherichia coli heat-labile toxin (LT), but they cannot be used in humans due to their toxicity and side effects. There is no effective and non-toxic adjuvant for humans. Chitosan is a deacetylated product of chitin, which is non-toxic, non-irritable, non-antigenic, bioadhesive, biocompatible and biodegradable[4]. Some studies showed that chitosan as an immune adjuvant could effectively enhance the immune response of local mucosa[5-7] and antigen presentation[8]. But there is no report about chitosan as an adjuvant for H pylori vaccine. In the present study, mice were vaccinated with a bacterial whole-cell sonicate of H pylori plus chitosan and then challenged by H pylori, in order to delineate its effect and possible mechanisms against H pylori infection.
MATERIALS AND METHODS
Reagents and bacterial strains
Chitosan and 88.5% deacetylated chitosan powder were purchased from Qisheng Biological Products Limited Company, Shanghai. Campylobacter agar base and Brucella broth were purchased from Shanghai Reagent Providing and Research Centre for Diarrhea Disease Control, China. ELISA kits for IL-2, IFN, IL-12, IL-4 and IL-10 were purchased from Bender MedSystem (USA). Sheep-anti-mouse IgG1 and IgG2a peroxidase conjugate was purchased from Zymed-Laboratories INC (USA). H pylori Sydney strain 1 (SS1) was kindly provided by H pylori Strain Pool, China.
Animals
Female BALB/c mice, 6-8 wk of age and 22.5 g of mean weight, were purchased from Animal Centre of China Academy of Sciences, Shanghai (licensing number: SCXK (HU) 2002-0010). The mice were housed in a specific pathogen-free environment with free access to food and water.
Culture of H pylori
The Sydney strain of H pylori was used throughout the experiments. H pylori was grown in Campylobacter agar base containing 7.5% sheep blood under microaerobic conditions (5% O2, 10% CO2, 85% N2) at 37°C for 2-3 d, and harvested from the agar plates by eluting with broth culture. Bacterial density was detected at 660 nm. One OD was 108/mL.
Preparation of H pylori antigen
After cultured for 2-3 d, the Sydney strain of H pylori was eluted with phosphate buffer saline (PBS), and centrifuged at 1000 × g for 10 min. The pellet was washed three times with PBS, and cells were disrupted by sonication. After centrifugation at 8000 × g for 30 min at 4°C, supernatant was collected and stored at -85°C until use. Protein concentration was determined.
Preparation of chitosan particles
Ddeacetylated (88.5%) chitosan powders were suspended in saline to the final concentration of 10 mg/mL and sonicated at 80 HZ output power two times for 5 min each with a sonicator, at 1 min intervals. Following a light centrifugation (50 r/min, 10 min), small particles in the supernatants were removed, filtered through a 400/2800 stainless steel mesh, and further centrifuged to collect the particles at 1400 r/min for 10 min as previously described[9].
Preparation of chitosan solution
Deacetylated (88.5%) chitosan stock solution was prepared at 3% (w/w) in 0.8% (v/v) acetic acid 0.9% (w/v) saline and heated at 37°C to dissolve[10].
Vaccination and challenge of mice
BALB/c mice were randomly divided into 7 groups: (1) control (PBS alone), 15 mice; (2) chitosan solution alone, 12 mice; (3) chitosan particles alone, 13 mice; (4) H pylori antigen alone, 15 mice; (5) H pylori antigen plus chitosan solution, 15 mice; (6) H pylori antigen plus chitosan particles, 15 mice; (7) H pylori antigen plus CT, 12 mice.
BALB/c mice were orally immunized with H pylori antigen (1.2 mg/mouse), chitosan particles (500 μg/mouse), CT (5 μg/mouse), and 0.5% chitosan solution, once a week for four weeks. Chitosan particles were whipped into a stable emulsion by sonication with a sonifier at 20 HZ before immunization[9]. Four weeks after the last immunization, the mice were challenged twice by alive H pylori (1 × 109/mL, 0.5 mL/mouse).
Before challeng, five mice were randomly collected respectively from the control group, H pylori antigen group, H pylori antigen plus chitosan solution group and H pylori antigen plus chitosan particles group and killed. Samples were collected for further use. The other mice were killed 4 wk after the last challenge. Blood was collected by removing eyeballs immediately before the mice were killed. The stomach was isolated for histology, examination of H pylori, and determination of cytokine level. The stomach was washed in sterile 0.8% NaCl and cut longitudinally into two pieces. One was used for quantitative culture of H pylori, while the other was used for histology and determination of cytokine expression.
Assessment of bacterial load in stomach
The bacterial load in the stomach was determined by quantitatively culture of H pylori and improved Giemsa staining, when both of them negative was difined as H pylori negative and when anyone of them positive was difined as H pylori positive. For assessment of H pylori colonization, weighed stomachs were homogenized in 0.3 mL of Brucella broth, 1/4 and 1/8 serial dilutions were spread over the surfaces of serum plates containing 10 mg of vancomycin, 2500 IU of polymyxin and 5 mg of trimethoprim per liter. The plates were incubated for 3-7 d. Colonies were counted to determine the CFU per gram of stomach tissue. In Giemsa staining, the colonization was assessed by semiquantitative analysis of H pylori in gastric mucosa (nil = 0; 1-2 call/crypt = 1; 3-10 call/crypt = 2; 11-20 call/crypt = 3; > 21 call/crypt = 4)[11].
Determination of cytokines in gastric mucosa by ELISA
After weighed, the gastric mucosa was homogenized in 1.3 mL PBS and the homogenates were centrifuged at 3000 × g at 4°C for 20 min. Supernatant was harvested and diluted at 1:2. For quantification of IL-2, IFN, IL-12, IL-4 and IL-10 in the supernatants, commercial enzyme-linked immunosorbent assay (ELISA) systems were used. The limit of detection was 3 pg/mL for IL-4, 22 pg/mL for IL-10, and 6 pg/mL for IL-12, 8 pg/mL for IL-2, and 8 pg/mL for IFN. The results were represented as pg/mg wet weight of gastric mucosa.
Determination of H pylori-specific antibodies in serum
H pylori-specific antibodies (IgG1 and IgG2) in serum were detected by indirect ELISA. Each well of microtiter plates was coated with 100 μL of H pylori antigen solution at the concentration of 20 μg/mL in 0.01 mol/L sodium carbonate-bicarbonate buffer (pH 9.6) overnight at 4°C. After suction, 200 μL of 0.1% BSA solution in PBS-Tween-20 was added to each well and further incubated for 1 h at 37°C. After washed three times with PBS-Tween-20, 100 μL of diluted (1/100) serum samples was added to wells and incubated for 1 h at 37°C. After washed three times with PBS-Tween-20, 100 mL of sheep-anti-mouse IgG1 and IgG2a peroxidase conjugate was added to wells and incubated further for 1 h at 37°C. After washed three times with PBS-Tween-20, 100 μL of o-phenylendiamine solution containing 0.01% H2O2 was added to wells and incubated for 30 min at room temperature. The reaction was stopped by the addition of 50 μL of 2 mol/L sulfuric acid, and color development was measured by a plate reader at 492 nm. The results were represented as A value of samples/A value of control.
Statistical analysis
Differences in the protection rate against H pylori infection were analyzed by Fisher’s exact test. Differences in H pylori-specific antibody and cytokine level in gastric mucosa among experimental groups were detected for statistical significance by analysis of variance or Student’s t test. P < 0.05 was considered statistically significant.
RESULTS
Rates of immune protection against H pylori infection
Significant difference was found in the rates of immune protection of vaccines with different adjuvants against H pylori infection (P < 0.001), and the protection rates were significantly higher in the groups with adjuvant than in the groups without adjuvant and antigen (P < 0.05 or 0.001, Table 1).
Table 1.
Rates of immune protection of H pylori vaccine with different kinds of adjuvant
| Groups | n | H pylori positive n (%) | Rates of immune protection (%) |
| (1) Control | 10 | 10 (100) | 0 |
| (2) Chi-solution | 12 | 12 (100) | 0 |
| (3) Chi-particles | 13 | 13 (100) | 0 |
| (4) Hp antigen | 10 | 10 (100) | 0 |
| (5) Hp antigen + CT | 12 | 5 (41.67) | 58.33d |
| (6) Hp antigen + chi-solution | 10 | 4 (40) | 60ab |
| (7) Hp antigen + chi-particles | 10 | 4 (40) | 60ab |
| P < 0.001 |
P < 0.05 vs (1) and (4) groups;
P < 0.01 vs (2) and (3) groups;
P < 0.01 vs (1)-(4) groups; chi = chitoson.
H pylori colonization score in gastric mucosa
Significant difference was observed in density of H pylori colonization among different groups (P < 0.001), and the density of H pylori colonization was significantly lower in the groups with adjuvant than in the groups without adjuvant or antigen (P < 0.05 or 0.001, Table 2, Figure 1).
Table 2.
H pylori colonization score in gastric mucosa
| Groups | n |
H pylori colonization score |
|||
| 0 | 1 | 2 | 3 | ||
| (1) Control | 10 | 0 | 2 | 4 | 4 |
| (2) Chi-solutiond | 12 | 1 | 8 | 2 | 1 |
| (3) Chi-particlesd | 13 | 0 | 10 | 1 | 2 |
| (4) Hp antigenc | 10 | 1 | 5 | 2 | 2 |
| (5) Hp antigen + CTad | 12 | 7 | 2 | 1 | 2 |
| (6) Hp antigen + chi-solutionb | 10 | 6 | 4 | 0 | 0 |
| (7) Hp antigen + chi-particlesb | 10 | 7 | 3 | 0 | 0 |
| H = 57.181, P < 0.001 | |||||
P < 0.01 vs (1)-(4) groups;
P < 0.05 vs (4) group;
P < 0.05 vs (1) group;
P < 0.01 vs (1) group; chi = chitoson.
Figure 1.
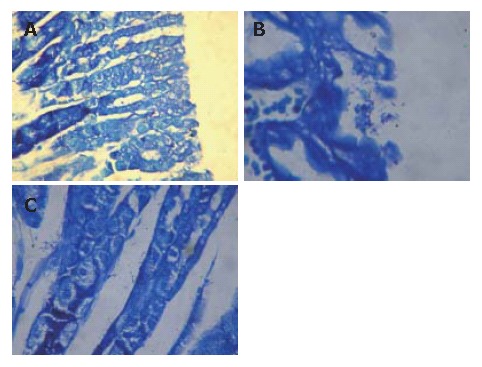
H pylori stained with Giemsa in gastric tissue of mice. No H pylori found in H pylori antigen + chi-particles group (A) , and lots of H pylori found on surface of gastric mucosa (B) and in gastric foveola (C) of control group (Giemsa dyeing × 400).
CFU/g of H pylori in gastric mucosa
Significant difference was found in H pylori colonization among different groups, as indicated by the number of CFU/g of H pylori in gastric mucosa (P = 0.001). The number of CFU/g of H pylori was significantly lower in the groups with chitosan as an adjuvant than in other groups without adjuvant (P < 0.05 or 0.01). There was no significant difference in H pylori colonization among the groups with CT as an adjuvant or without adjuvant (P > 0.05, Table 3).
Table 3.
Density of H pylori colonization in gastric mucosa
| Groups | n | Median of H pylori colony number (CFU/g) |
| (1) Control | 10 | 4.74 × 105 |
| (2) Chi-solution | 12 | 0.73 × 105 |
| (3) Chi-particles | 13 | 1.83 × 105 |
| (4) Hp antigen | 10 | 0.48 × 105 |
| (5) Hp antigen + CT | 12 | 0 |
| (6) Hp antigen + chi-solution | 10 | 0 |
| (7) Hp antigen + chi-particles | 10 | 0 |
| H = 27.43, P = 0.001 |
chi = chitoson.
Cytokine level in gastric mucosa
Before challenge, there was no significant difference in IL-2 level among different groups. But there was significant difference in levels of IFN, IL-12, IL-10 and IL-4 among different groups (P < 0.05 or 0.005), which were significantly higher in the groups with chitosan as an adjuvant than in other groups without adjuvant (P < 0.005, Table 4). After challenge, there was significant difference in levels of IFN, IL-12, IL-2 and IL-4 among different groups. The level of IL-2 was significantly higher in the groups with chitosan solution as an adjuvant than in the groups without antigen (P < 0.05 or 0.001). Moreover, the level of IL-2 was significantly higher in the groups with chitosan particles and H pylori antigen alone than in the control group and the group with chitosan solution alone (P < 0.05). The level of IFN and IL-12 was significantly higher in the groups with adjuvant than in the groups without antigen or adjuvant (P < 0.05 or 0.001). The level of IL-4 was significantly higher in the groups with chitosan particles than in the groups with CT (P < 0.05). Moreover, the level of IL-4 in the groups with chitosan solution as adjuvant was significantly higher than in other groups with chitosan solution alone, H pylori antigen alone and CT as adjuvant (P < 0.05). There was no significant difference in the level of IL-10 among different groups (P > 0.05, Table 5).
Table 4.
Levels of IL-2, IFN-γ, IL-12, IL-10 and IL-4 in gastric mucosa before challenge (mean ± SD)
| Groups | n | IL-2 | IFN-γ | IL-12 | IL-10 | IL-4 |
| (1) Control | 5 | 19.9 ± 12.6 | 28.6 ± 10.2 | 118.9 ± 46.2 | 67.2 ± 32.5 | 4.19 ± 2.95 |
| (2) Hp antigen | 5 | 20.6 ± 3.5 | 33.6 ± 13.7 | 165.3 ± 47.4 | 104.3 ± 19.1 | 6.49 ± 2.61 |
| (3) Hp antigen chi-solution | 5 | 28.6 ± 9.3 | 58.5 ± 12.2b | 283.5 ± 93.7cd | 255.3 ± 131.8cd | 14.70 ± 8.73a |
| (4) Hp antigen+ chi-particles | 5 | 27.6 ± 13.9 | 59.8 ± 15.2b | 283.4 ± 99.6cd | 237.1 ± 98.3cd | 14.48 ± 6.84a |
| F = 0.739 | F = 7.948 | F = 6.083 | F = 6.228 | F = 4.189 | ||
| P = 0.544 | P = 0.002 | P = 0.006 | P = 0.005 | P = 0.023 |
P < 0.05 vs (1) and (2) groups;
P < 0.01 vs (1) and (2) groups;
P < 0.05 vs (2) group;
P < 0.01 vs (1) group; chi = chitoson.
Table 5.
Levels of IL-2, IFN-γ, IL-12, IL-10 and IL-4 in gastric mucosa after challenge (mean ± SD)
| Groups | n | IL-2 | IFN-γ | IL-12 | IL-10 | IL-4 |
| (1) Control | 10 | 34.3 ± 11.8 | 48.7 ± 27.8 | 233.7 ± 125.7 | 86.4 ± 38.2 | 3.87 ± 1.99 |
| (2) Chi-solution | 11 | 31.8 ± 27.4 | 56.8 ± 26.1 | 308.5 ± 178.7 | 100.7 ± 52.6 | 4.47 ± 1.89 |
| (3) Chi-particles | 10 | 51.1 ± 42.6 | 58.8 ± 28.1 | 311.3 ± 129.6 | 88.4 ± 51.7 | 7.21 ± 4.02g |
| (4) Hp antigen | 9 | 97.9 ± 64.2a | 59.4 ± 15.0 | 319.4 ± 136.3 | 89.3 ± 29.01 | 4.89 ± 3.15 |
| (5) Hp antigen + CT | 10 | 80.9 ± 60.2c | 93.6 ± 23.5ef | 487.0 ± 289.3ef | 81.8 ± 49.5 | 3.67 ± 1.76 |
| (6) Hp antigen + chi-solution | 10 | 124.3 ± 75.2b | 107.5 ± 42.0d | 525.6 ± 112.2d | 108.5 ± 39.0 | 8.78 ± 4.96hi |
| (7) Hp antigen + chi-particles | 10 | 88.6 ± 57.0a | 105.9 ± 48.1d | 554.0 ± 164.4d | 93.1 ± 39.2 | 6.59 ± 1.38k |
| F = 3.370 | F = 6.346 | F = 5.448 | F = 0.745 | F = 3.214 | ||
| P = 0.002 | P < 0.001 | P < 0.001 | P > 0.05 | P = 0.003 |
P < 0.05 vs (1) and (2) groups;
P < 0.001 vs (1)-(3) groups;
P < 0.05 vs (1) group;
P < 0.01 vs (1)-(4) groups;
P < 0.05 vs (2)-(4) groups;
P < 0.01 vs (1) group;
P < 0.05 vs (1), (2) and (5) groups,
P < 0.01 vs (1), (2) and (5) groups;
P < 0.05 vs (4) group;
P < 0.05 vs (5) group; chi = chitoson.
There was significant difference in the levels of IL-2, IFN, IL-12, IL-10 and IL-4 before and after challenge by alive H pylori. The levels of IL-2, IFN and IL-12 were significantly higher in the groups with H pylori antigen after challenge than those before challenge (P < 0.05). The levels of IL-10 were significantly lower in the groups with adjuvant after than before challenge (P < 0.05). The level of IL-4 was significantly lower in the groups with chitosan particles as adjuvant after challenge than before challenge (P < 0.05). The level of cytokines before and after challenge was not significantly different in the other group (P > 0.05, Figure 2).
Figure 2.
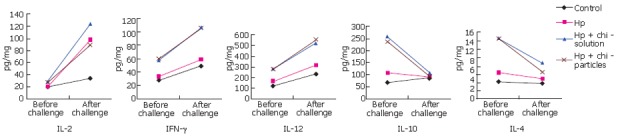
Levels of IL-2, IFN-γ, IL-12, IL-10 and IL-4 in gastric mucosa before and after challenge in different groups.
Level of anti-Hp IgG2a, IgG1 and ratio of IgG2a/IgG1 in serum
After challenge, there was significant difference in the levels of anti-Hp IgG2a, IgG1 and ratio of IgG2a/IgG1 in serum among different groups (P < 0.001). The level of anti-Hp IgG2a was significantly higher in the groups with chitosan particles than in other groups with H pylori antigen alone or chitosan alone and the control group (P < 0.05 or 0.001). The level of anti-Hp IgG2a was significantly higher in the groups with CT or chitosan solution than in the control group (P < 0.05). The level of anti-Hp IgG1 was significantly higher in the groups with adjuvant than in other groups with chitosan alone and in the control group (P < 0.05). Moreover, the level of anti-Hp IgG1 was significantly higher in the groups with H pylori antigen than in the control group (P < 0.05). The ratio of IgG2a/IgG1 was significantly lower in the groups with chitosan than in the groups with CT, H pylori antigen alone and in the control group (P < 0.01, Table 6).
Table 6.
Levels of anti-HpIgG2a, IgG1 and ratio of IgG2a/IgG1 in serum after challenge (mean ± SD)
| Groups | N | IgG2a | IgG1 | IgG2a/IgG1 |
| (1) Control | 10 | 4.44 ± 2.09 | 2.77 ± 1.18 | 2.13 ± 1.24 |
| (2) Chi-solution | 11 | 6.28 ± 3.62 | 3.96 ± 2.32 | 1.64 ± 0.85 |
| (3) Chi-particles | 10 | 5.56 ± 2.27 | 4.57 ± 2.09 | 1.43 ± 0.37gh |
| (4) Hp antigen | 9 | 9.21 ± 6.80a | 6.43 ± 4.88a | 2.356 ± 1 |
| (5) Hp antigen + CT | 10 | 11.22 ± 5.52a | 7.71 ± 5.11de | 2.19 ± 0.86 |
| (6) Hp antigen + chi-solution | 10 | 11.48 ± 5.55a | 9.04 ± 5.35b | 1.06 ± 0.4f |
| (7) Hp antigen + chi-particles | 10 | 16.13 ± 10.20b | 8.02 ± 5.29cd | 1 ± 0.37f |
| F = 4.572 | F = 10.308 | F = 3.780 | ||
| P < 0.001 | P < 0.001 | P < 0.001 |
P < 0.05 vs (1) group;
P < 0.01 vs (1)-(3) groups;
P < 0.05 vs (2) and (3) groups,
P < 0.01 vs (1) group;
P < 0.05 vs (2) group;
P < 0.01 vs (1), (4) and (5) groups;
P < 0.05 vs (5) group;
P < 0.01 vs (4) group.
DISCUSSION
All effective vaccines need a suitable antigen-presenting system that depends on adjuvant or vehicle[12]. H pylori antigen alone cannot induce protective immune response. Antigen-presenting system can introduce exogenous antigen into cells, and can enhance the immune response to antigen and even change the type of immune response. Many studies showed that chitosan can effectively promote local immune response and enhance antigen presentation[5-8]. In this study, we evaluated a vaccine delivery system with chitosan, the rate of immune protection of vaccine with chitosan as adjuvant against H pylori infection was 60%, which was significantly higher than with H pylori antigen alone or chitosan alone, indicating that chitosan can be used as a mucosa adjuvant of H pylori instead of CT.
CD4+ helper T cells (Th) in mice can be divided into Th1 and Th2 subtypes. Th1 cells can synthesize and secrete IL-2, IL-12, IFN-γ, take part in cell-mediated immune response and promote the production of IgG2a by B cells. Th2 cells can also secrete cytokines such as L-4, IL-5, and IL-10, help B cells produce antibody, take part in humoral immune response and promote the production of antibodies such as IgG1, IgE and IgA. Negative feedback exists in the two types. Th can regulate immune response. Recently, different Th response types induced by H pylori vaccine and their effects in immune response are the main point in the mechanism of H pylori vaccine, indicating that the balance of Th1 and Th2 response is involved in the protection mechanism of H pylori vaccine. In natural H pylori infection, the presence of Th1 is the primary immune response. Th1-mediated cell immunity cannot protect against H pylori infection and is related to the severity of H pylori infection[13-15]. At the same time, CD4+ Th2 secreting IL-4 and IL-10 is depressed, thus IgA secreted by B cells is reduced, leading to persistent H pylori infection. After immunization, the type of immune response has changed from Th1 to Th2. Hatzifoti et al[16] reported that immunization of mice with DNA vaccine encoding urease B genes could up-regulate the expression of Th2 cytokine IL-10. Mohammadi et al[17] found that stimulating immune response to Th2 could reduce the number of H pylori and the intensity of inflammation of gastric mucosa, indicating that if the type of immune response induced by immunization has changed from Th1 to Th2, H pylori colonization in gastric mucosa can be inhibited by producing Th2 cytokines such as IL-4. Saldinger et al[18] immunized H. felis -infected mice with oral rUreB and CT, and found that infected mice were cured 3 wk after the 4th immunization. they also found that immunization could lead to the proliferation of CD4+ T cells in the spleen of mice accompanying gradual decrease in IFN-γ and increase in IL-4, indicating that immunization of mice with rUreB and CT could induce gradual Th2 immune response , thus eliminating H. felis. However, some studies showed that Th1 and Th2 response together is better than Th2 response only in preventing H pylori infection[19]. Gottweln et al reported that the two kinds of H pylori vaccine with complete Freund's or aluminum as adjuvant, which induce Th1 and Th2 immune response respectively, could induce protective immune response in vivo in mice, indicating that Th1 and Th2 immune response have the effect of immune protection. Eisenberg et al[21] immunized neonatal and adult mice with H pylori antigen and complete or incomplete Freund’s adjuvant and found that the number of T cells producing Th1 cytokines like IFN-γ, IL-2 and Th2 cytokines like IL-4 IL-5 increased, have the effect of immune protection. Sommer et al[22] used CpG oligonucleotide as adjuvant to induce Th1 immune response, and found that it could not protect against H pylori infection but lead to more serious gastritis. Thus in the protective immune response to H pylori, Th1 and Th2 are needed. It must have a balance between Th1 and Th2 to achieve immune protection and to prevent tissue from damaging by serious inflammation[23].
In our study, before challenge by alive H pylori, the levels of IFN, IL-12, IL-4 and IL-10 were significantly higher in the groups with chitosan than in other groups without adjuvant, indicating that in the early stage of immune, they induce immune response to both Th1 and Th2. But 4 wk after challenge the levels of IL-2, IFN and IL-12 were significantly higher in the groups with adjuvant than in groups without adjuvant or in control group, indicating that after challenge they could promote the production of Th1 cytokines. The levels of IL-4 and IL-10 were significantly lower after challenge than before challenge. Chen et al[24] found that in the early stage of H pylori challenge (5 wk), the level of Th2 cytokines was significantly lower, even undetectable. Yu et al[25] found that after oral immunization with H pylori antigen plus LT, Th1 and Th2 immune response are induced in the early and advanced stage respectively, indicating that oral immunization can induce Th1 as well as Th2 immune response, which is in accordance to our study. In our study, after H pylori challenge the level of IL-4 was significantly higher in the group with chitosan particles as adjuvant than in the group with CT as adjuvant, and the level of IL-4 was significantly higher in the group with chitosan solution as adjuvant than in the groups with CT as adjuvant, chitosan solution alone and H pylori antigen along, indicating that H pylori vaccine with chitosan is better than that with CT in inducing Th2 cytokines especially IL-4. In addition, after H pylori challenge the levels of anti-Hp IgG2a and IgG1 were significantly higher in the groups with adjuvant than in the control group. IgG2a and IgG1 were induced by Th1 and Th2 immune response respectively, indicating H pylori vaccine can up-regulate Th1 and Th2 immune response. But the ratio of IgG2a/IgG1 in serum was significantly lower in the groups with chitosan as adjuvant than in other groups with CT as adjuvant or H pylori antigen alone, indicating that chitosan as an adjuvant can reverse the inhibition of Th2 induced by H pylori infection and return to the balance between Th1 and Th2, thus contributing to the immune protection against H pylori infection.
Chitosan can regulate immune response. Studies showed that chitosan as a mucosa adjuvant of the vaccine against meningococci and Bordetella bronchiseptica could successfully induce protective immune response[26,27]. Seferian et al[28] inoculated BALB/c mice with chitosan plus β- human chorionic gonadotropin, and found that the mixed immune response to IgG1, IgG2a and IgG2b antibodies could be observed in the groups with chitosan emulsion as adjuvant. Bivas-Benita et al[29] immunized mice with oral Toxoplasma gondii GRA1 protein and DNA vaccine-loaded chitosan particles, and successfully induced specialized anti- GRA1 IgG1 and IgG2a, indicating that it can enhance immune respons to Th1 and Th2. McNeela et al[30] found that immunization with chitosan plus diphtheria toxin could induce both systemic and local specific humoral immune response. At the same time, it could enhance immune response to Th1 and Th2.
In conclusion, H pylori vaccine with chitosan as adjuvant could induce Th1 and Th2 immune response, and partially reverse the inhibition of Th2 induced by H pylori infection and recover the balance between Th1 and Th2. As an adjuvant of H pylori vaccine, chitosan is better than CT in immune protection against H pylori infection.
Footnotes
Supported by Natural Science Foundation of Jiangxi Province (No.30460052), Program of Jiangxi Provincial Leaders in Their Chosen Field of Learning, No. K010501
S- Editor Liu Y L- Editor Wang XL E- Editor Chin GJ
References
- 1.Jeremy AH, Du Y, Dixon MF, Robinson PA, Crabtree JE. Protection against Helicobacter pylori infection in the Mongolian gerbil after prophylactic vaccination. Microbes Infect. 2006;8:340–346. doi: 10.1016/j.micinf.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Jiao Zhi-yong, Chen Min-hu, Zhu Sen-lin, Li Guo-qing, Hu Pin-jin. Prevention of the Helicobacter pylori infection in mice with recombinant attenuated Salmonella typhimurium vaccine expressing the ureB/hlyE Fusion protein. Shijie Huaren Xiaohua Zazhi. 2005;13:787–789. [Google Scholar]
- 3.Nyström J, Raghavan S, Svennerholm AM. Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect. 2006;8:442–449. doi: 10.1016/j.micinf.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Synowiecki J, Al-Khateeb NA. Production, properties, and some new applications of chitin and its derivatives. Crit Rev Food Sci Nutr. 2003;43:145–171. doi: 10.1080/10408690390826473. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Bd, Qie Yq, Wang Jl, Zhang Y, Wang Qz, Xu Y, Wang Hh. Chitosan microspheres enhance the immunogenicity of an Ag85B-based fusion protein containing multiple T-cell epitopes of Mycobacterium tuberculosis. Eur J Pharm Biopharm. 2007;66:318–326. doi: 10.1016/j.ejpb.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Huo Z, Sinha R, McNeela EA, Borrow R, Giemza R, Cosgrove C, Heath PT, Mills KH, Rappuoli R, Griffin GE, et al. Induction of protective serum meningococcal bactericidal and diphtheria-neutralizing antibodies and mucosal immunoglobulin A in volunteers by nasal insufflations of the Neisseria meningitidis serogroup C polysaccharide-CRM197 conjugate vaccine mixed with chitosan. Infect Immun. 2005;73:8256–8265. doi: 10.1128/IAI.73.12.8256-8265.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borges O, Borchard G, Verhoef JC, de Sousa A, Junginger HE. Preparation of coated nanoparticles for a new mucosal vaccine delivery system. Int J Pharm. 2005;299:155–166. doi: 10.1016/j.ijpharm.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 8.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci. 2001;14:201–207. doi: 10.1016/s0928-0987(01)00172-5. [DOI] [PubMed] [Google Scholar]
- 9.Shibata Y, Foster LA, Metzger WJ, Myrvik QN. Alveolar macrophage priming by intravenous administration of chitin particles, polymers of N-acetyl-D-glucosamine, in mice. Infect Immun. 1997;65:1734–1741. doi: 10.1128/iai.65.5.1734-1741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerink MA, Smithson SL, Srivastava N, Blonder J, Coeshott C, Rosenthal GJ. ProJuvant (Pluronic F127/chitosan) enhances the immune response to intranasally administered tetanus toxoid. Vaccine. 2001;20:711–723. doi: 10.1016/s0264-410x(01)00423-6. [DOI] [PubMed] [Google Scholar]
- 11.Sakagami T, Dixon M, O'Rourke J, Howlett R, Alderuccio F, Vella J, Shimoyama T, Lee A. Atrophic gastric changes in both Helicobacter felis and Helicobacter pylori infected mice are host dependent and separate from antral gastritis. Gut. 1996;39:639–648. doi: 10.1136/gut.39.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyle PM, McGeary RP, Blanchfield JT, Toth I. Mucosal immunisation: adjuvants and delivery systems. Curr Drug Deliv. 2004;1:385–396. doi: 10.2174/1567201043334588. [DOI] [PubMed] [Google Scholar]
- 13.Sommer F, Faller G, Röllinghoff M, Kirchner T, Mak TW, Lohoff M. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur J Immunol. 2001;31:396–402. doi: 10.1002/1521-4141(200102)31:2<396::aid-immu396>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 15.Eaton KA, Benson LH, Haeger J, Gray BM. Role of transcription factor T-bet expression by CD4+ cells in gastritis due to Helicobacter pylori in mice. Infect Immun. 2006;74:4673–4684. doi: 10.1128/IAI.01887-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzifoti C, Bajaj-Elliott M, Dorrell N, Anyim M, Prentice MB, Nye KE, Wren B, Morrow WJ. A plasmid immunization construct encoding urease B of Helicobacter pylori induces an antigen-specific antibody response and upregulates the expression of beta-defensins and IL-10 in the stomachs of immunized mice. Vaccine. 2004;22:2651–2659. doi: 10.1016/j.vaccine.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–1857. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 18.Saldinger PF, Porta N, Launois P, Louis JA, Waanders GA, Bouzouréne H, Michetti P, Blum AL, Corthésy-Theulaz IE. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–897. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 19.Akhiani AA, Pappo J, Kabok Z, Schön K, Gao W, Franzén LE, Lycke N. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J Immunol. 2002;169:6977–6984. doi: 10.4049/jimmunol.169.12.6977. [DOI] [PubMed] [Google Scholar]
- 20.Gottwein JM, Blanchard TG, Targoni OS, Eisenberg JC, Zagorski BM, Redline RW, Nedrud JG, Tary-Lehmann M, Lehmann PV, Czinn SJ. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Infect Dis. 2001;184:308–314. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg JC, Czinn SJ, Garhart CA, Redline RW, Bartholomae WC, Gottwein JM, Nedrud JG, Emancipator SE, Boehm BB, Lehmann PV, et al. Protective efficacy of anti-Helicobacter pylori immunity following systemic immunization of neonatal mice. Infect Immun. 2003;71:1820–1827. doi: 10.1128/IAI.71.4.1820-1827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer F, Wilken H, Faller G, Lohoff M. Systemic Th1 immunization of mice against Helicobacter pylori infection with CpG oligodeoxynucleotides as adjuvants does not protect from infection but enhances gastritis. Infect Immun. 2004;72:1029–1035. doi: 10.1128/IAI.72.2.1029-1035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren Z, Pang G, Lee R, Batey R, Dunkley M, Borody T, Clancy R. Circulating T-cell response to Helicobacter pylori infection in chronic gastritis. Helicobacter. 2000;5:135–141. doi: 10.1046/j.1523-5378.2000.00021.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Chen MH, Li GQ, Jiao ZY, Liang WQ, Hu PJ, Shen ZY. Th type immune response induced by Helicobacter pylori vaccine in mice. Zhonghua Xiaohua Zazhi. 2003;23:739–743. [Google Scholar]
- 25.Yu CQ, Zou QM, Wang FQ, zhang WJ. Regula tive role of Th1 and Th2 cells on mucosal immune respon ses in BALB/c mice induced by oral immun iza tion with Helicobacter pylori vaccine. Mianyixue Zazhi. 2001;17:158. [Google Scholar]
- 26.Kang ML, Kang SG, Jiang HL, Shin SW, Lee DY, Ahn JM, Rayamahji N, Park IK, Shin SJ, Cho CS, et al. In vivo induction of mucosal immune responses by intranasal administration of chitosan microspheres containing Bordetella bronchiseptica DNT. Eur J Pharm Biopharm. 2006;63:215–220. doi: 10.1016/j.ejpb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Baudner BC, Verhoef JC, Giuliani MM, Peppoloni S, Rappuoli R, Del Giudice G, Junginger HE. Protective immune responses to meningococcal C conjugate vaccine after intranasal immunization of mice with the LTK63 mutant plus chitosan or trimethyl chitosan chloride as novel delivery platform. J Drug Target. 2005;13:489–498. doi: 10.1080/10611860500353195. [DOI] [PubMed] [Google Scholar]
- 28.Seferian PG, Martinez ML. Immune stimulating activity of two new chitosan containing adjuvant formulations. Vaccine. 2000;19:661–668. doi: 10.1016/s0264-410x(00)00248-6. [DOI] [PubMed] [Google Scholar]
- 29.Bivas-Benita M, Laloup M, Versteyhe S, Dewit J, De Braekeleer J, Jongert E, Borchard G. Generation of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: preparation, characterization, and preliminary in vivo studies. Int J Pharm. 2003;266:17–27. doi: 10.1016/s0378-5173(03)00377-6. [DOI] [PubMed] [Google Scholar]
- 30.McNeela EA, O'Connor D, Jabbal-Gill I, Illum L, Davis SS, Pizza M, Peppoloni S, Rappuoli R, Mills KH. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19:1188–1198. doi: 10.1016/s0264-410x(00)00309-1. [DOI] [PubMed] [Google Scholar]


