Abstract
AIM: To investigate the presence and role of liver epithelial cells in the healthy human adult liver.
METHODS: Fifteen days after human hepatocyte primary culture, epithelial like cells emerged and started proliferating. Cell colonies were isolated and sub-cultured for more than 160 d under specific culture conditions. Cells were analyzed for each passage using immunofluorescence, flow cytometry and reverse transcription-polymerase chain reaction (RT-PCR).
RESULTS: Flow cytometry analysis demonstrated that liver epithelial cells expressed common markers for hepatic and stem cells such as CD90, CD44 and CD29 but were negative for CD34 and CD117. Using immunofluorescence we demonstrated that liver epithelial cells expressed not only immature (α-fetoprotein) but also differentiated hepatocyte (albumin and CK-18) and biliary markers (CK-7 and 19), whereas they were negative for OV-6. RT-PCR analysis confirmed immunofluorescence data and revealed that liver epithelial cells did not express mature hepatocyte markers such as CYP2B6, CYP3A4 and tyrosine amino-transferase. Purified liver epithelial cells were transplanted into SCID mice. One month after transplantation, albumin positive cell foci were detected in the recipient mouse parenchyma.
CONCLUSION: According to their immature and bipotential phenotype, liver epithelial cells might represent a pool of precursors in the healthy human adult liver other than oval cells.
Keywords: Epithelial-like cells, Liver stem cell, Hepatocyte, Differentiation, Cell therapy
INTRODUCTION
Mammalian adult liver shows a high capacity to regenerate under different physiopathological circumstances thanks to mature and progenitor cells with stem-like properties[1-4]. Following cellular loss, fully differentiated hepatocytes and biliary epithelial cells can proliferate and completely repair the liver[5-8]. When proliferation of these cells is impaired during chronic or extensive damage of the liver, non-parenchymal epithelial progenitor cells, expressing both biliary and hepatocytic phenotypes, are activated and participate in liver regeneration[9-11].
Studies using hepato-toxins or chemical carcinogens treated animal models, revealed the existence of oval cells in the canals of Hering[12-14]. These cells express both hepatic [albumin (Alb), alpha fetoprotein (AFP)] and biliary [cytokeratin (CK)-7, CK-19, OV-6, γ-glutamyltranspeptidase (GGT)] markers, and some antigens traditionally associated with hematopoietic cells, including c-kit, Thy-1 and CD34[14,15] as well. During liver regeneration, stimulated oval cells express high levels of AFP, proliferate in the periportal area and migrate into liver parenchyma. Liver epithelial cells (LECs) differ from oval cells, and are as well candidate hepatic progenitor cells. Such liver epithelial cells were obtained from the livers of both normal[16] and carcinogen treated rats[17], normal pigs[18], and human subacute hepatic failure patient[10], but so far not from healthy human adult livers.
In the current study, we demonstrated the presence of LECs in healthy human liver and isolated these cells after primary culture. We successfully maintained their proliferation more than 160 d and analyzed their characteristics by flow cytometry, immunofluorescence, and RT-PCR. Furthermore, LECs were transplanted into SCID mice to evaluate their engraftment capacity.
MATERIALS AND METHODS
Cell isolation and culture
Liver parenchymal cell fraction was isolated from livers of 13 healthy cadaveric donors (Table 1) using the two-step perfusion technique[19,20]. Ten to twenty million isolated cells were suspended in hepatocyte growth medium (HGM) [Williams’ E medium (Invitrogen) containing 10% fetal bovine serum (FBS) (Perbio, Hyclone), 1% penicillin-streptomycin (Invitrogen), 100 nmol/L insulin (Lilly), 1 μmol/L dexamethasone (Organon), and 25 ng/mL epidermal growth factor (Peprotech)] and plated on dishes coated with acid soluble typeIcollagen at a density of 105 cells/cm² in a fully humidified atmosphere containing 5% CO2. Cells were allowed to attach and the first medium change was performed after 24 h and then every 3 d.
Table 1.
Medical history of donors
| Age (yr) | Sex | Cause of death |
| 4 | Male | Cranial trauma |
| 9 | Male | Cranial trauma |
| 16 | Female | Trauma |
| 18 | Male | Trauma |
| 19 | Male | Cranial trauma |
| 36 | Male | Cardiac arrest |
| 43 | Male | Euthanasia |
| 43 | Female | Cranial trauma + Cerebral hemorrhage |
| 43 | Female | Cerebral hemorrhage |
| 44 | Female | Cerebral hemorrhage |
| 47 | Female | Pulmonary embolism |
| 51 | Male | Suicide |
| 61 | Male | Cerebral hemorrhage |
Fifteen days later, hepatocytes died and small colonies of LECs emerged from the culture dishes. The culture medium was then switched to DMEM containing 10% FBS and 1% penicillin/streptomycin in order to stimulate the growth of these cells. When colonies were evident, the other cell types were mechanically eliminated and LECs were lifted using trypsin (Invitrogen). Isolated LECs were replated on 6 well collagenI- coated plates and maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. For each passage, cells were analyzed using immunofluorescence, flow cytometry and RT-PCR.
Immunofluorescence
Isolated cells were cultured on collagen I-coated glass cover-slips and fixed with paraformaldehyde 4% (Merck). Then cells were permeabilised for 15 min using 1% Triton X-100 (Sigma) in TBS buffer (50 mmol/L Tris-HCl pH 7.4 and 150 mmol/L NaCl). Non-specific immuno-staining was prevented by 1 h incubation in TBS buffer containing 3% non-fat dry milk at 37°C. The primary antibodies [monoclonal mouse anti-human Alb (Sigma), polyclonal rabbit anti-human AFP (Dakocytomation), monoclonal mouse anti-human CK-7 (Chemicon), monoclonal mouse anti-human CK-19 (Dakocytomation) and monoclonal mouse anti-human oval cell (OV)-6 (donation from professor Tania Roskams-Katholic Universteit van Leuven)] were incubated with cells for 1 h at room temperature. The secondary antibodies used [Cy3 conjugated anti-mouse IgG (Jackson immunoresearch laboratories) and FITC-conjugated anti-rabbit IgG (Sigma)] were incubated for 1 h at room temperature. The nuclei were revealed by 4, 6-Diamidino-2-phénylindole (DAPI, Sigma) staining.
Periodic acid Schiff reaction
Cells were fixed with paraformaldehyde 4% (Merck) during 20 min at room temperature, incubated with 1% periodic acid solution for 10 min, rinsed in distilled water and placed in Schiff reagent for 15 min. After washing for 10 min with water, nuclei were revealed by hematoxylin staining.
Flow cytometry
Cells were harvested, suspended at a concentration of 500-1000/μL in PBS and incubated for 30 min at 4°C with the following antibodies: CD45-APC, CD34-APC (Becton Dickinson), CD90-PC5, CD73-PE, CD29-PE, HLA-DR-FITC (BD Pharmingen); CD44-FITC, CD117-PC5 (Immunotech); HLA-ABC-PE (Dako Systems), CD13-PE (Beckham Coulter) and CD105 Endoglin-FITC (Immunokontact). The corresponding isotypes were used for evaluation of non-specific binding of monoclonal antibodies. Cells were then washed and suspended in Isoton® (Beckham Coulter) for reading with a Beckham Coulter Flow Cytometer.
Reverse transcription-polymerase chain reaction
Total RNA was prepared using Tripure isolation Reagent (Roche) and cDNA was generated using the ThermoscriptTM RT kit (Invitrogen). PCR amplification was performed for 28 cycles using specific primers (Table 2) and ElongaseTM Enzyme Mix (Invitrogen). PCR products were subject to 1% agarose gel electrophoresis and visualized after ethidium bromide staining.
Table 2.
Primers used for RT- PCR analysis of LECs
| Gene name | Primers | PCR product size |
| AFP | 5’-TGAAATGACTCCAGTAAACCC-3’ | 199 pb |
| 5’-GATGAAGCAAGAGTTTCTCATT-3’ | ||
| Oct-4 | 5’-CGA CCA TCT GCC GCT TTG AG-3’ | 573 pb |
| 5’-CCC CCT GTC CCC CAT TCC TA-3’ | ||
| Human hepatocyte nuclear factor 4 (HNF4) | 5’-CCA AGT ACA TCC CAG CTT TC-3’ | 295 pb |
| 5’-TTG GCA TCT GGG TCA AAG-3’ | ||
| ALB | 5’-CCTTGGTGTTGATTGCCTTTGCTC-3’ | 308 pb |
| 5’-CATCACATCAACCTCTGGTCTCACC-3’ | ||
| CK-8 | 5’-AAG GGC TGA CCG ACGAGA TC-3’ | 537 pb |
| 5’-GCT TCC TGT AGG TGG CGA TC-3’ | ||
| Glucose 6 phosphatase (G6P) | 5’-TCA GCT CAG GTG GTC CTC TT-3’ | 291 pb |
| 5’-CCT CCT TAG GCA GCC TTC TT-3’ | ||
| α-antitrypsin (AAT) | 5’-TCGCTACAGCCTTTGCAATG-3’ | 142 pb |
| 5’-GGAACTCCTCCGTACCCTCAA-3’ | ||
| Tyrosine aminotran- sferase (TAT) | 5’-TGA GCA GTC TGT CCA CTG CCT-3’ | 359 pb |
| 5’-ATG TGA ATG AGG AGG ATC TGA G-3’ | ||
| Cytochrome P450 1B1 (CYP1B1) | 5’-GAGAACGTACCGGCCACTATCACT-3’ | 357 pb |
| 5’-GTTAGGCCACTTCACTGGGTCATGAT-3’ | ||
| CYP2B6 | 5’-CCT CTT CCA GTC CAT TAC CG-3’ | 551 pb |
| 5’-TGA CTG CCT CTG TGT ATG GC-3’ | ||
| CYP3A4 | 5’-TGC TGT CTC CAA CCT TCA CC-3’ | 802 pb |
| 5’-TAG CTT GGA ATC ATC ACC ACC-3’ | ||
| CK-19 | 5’-TTTGAGACGGAACAGGCTCT-3’ | 426 pb |
| 5’-CAGCTCAATCTCAAGACCCTG-3’ | ||
| GGT | 5’-GAC GAC TTC AGC TCT CCC AG-3’ | 489 pb |
| 5’-CTT GTC CCT GGA TTG CTT GT-3’ | ||
| Multidrug resistance- associated protein 2 (MRP2) | 5’-ACA CCA ACC AGA AAT GTG TC-3’ | 660 pb |
| 5’-CCA AGG CCT TCC AAA TCT C-3’ | ||
| Glyceraldehyde- 3-phosphate dehydrogenase (GAPDH) | 5’-CGGAGTCAACGGATTTGGTCGTAT-3’ | 307 pb |
| 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’ |
Mouse transplantation and immunohistochemistry
One million LECs were suspended in 100 μL PBS and injected into the spleen of 6-8 wk SCID mice, half of them being subject to 2/3 hepatectomy. Thirty days after transplantation, livers were harvested, formalin-fixed and embedded in paraffin. Five µm liver sections were deparaffinized and hydrated to distilled water. Endogenous peroxidase activity was blocked by incubation for 15 min in a 3% hydrogen peroxide methanol solution. Slides were incubated overnight with polyclonal rabbit anti-human Alb antibody (Calbiochem) diluted at 1/2500 in PBS containing 0.1% bovine serum albumin. Staining detection was visualized by Envision Dako Anti-Rabbit (Dakocytomation) using diaminobenzidine (Sigma) as chromogenic substrate. The nuclei were revealed by hematoxylin staining.
RESULTS
Characterization of LECs
Isolated parenchymal cell fraction contained majority of hepatocytes with viability estimated at 90% using trypan blue exclusion technique. After 15 d culture in collagen typeI- coated plates, hepatocytes died and small colonies of LECs emerged and proliferated (Figure 1A). The LECs appeared in polygonal shape with central nucleus, several nucleoli and a high cytoplasm/nucleus ratio (Figure 1B). LECs colonies grew up, increased in size and had contact inhibition after filling free spaces in the culture dishes. LECs were obtained both from freshly isolated and cryopreserved cell suspensions. Purified LECs resisted to trypsin application and maintained their proliferation potential of more than 5 passages in DMEM medium supplemented with 10% FBS (Figure 1C and D) that leads to purification of the cell population, indicating the self-renewing ability of LECs in vitro.
Figure 1.
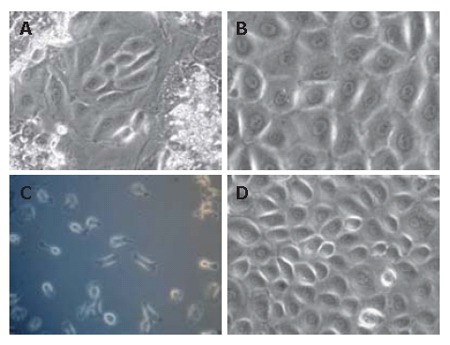
Morphology of LECs after liver cells primary culture (× 200). A: After 15 d primary culture, small colonies of LECs emerged; B: organized in monolayers; C: When colonies were evident, the other cells types were mechanically eliminated and LECs were lifted and replated; D: LEC population reaching 70% confluence analyzed for the other passages.
As shown in Figure 2, the flow cytometry analysis of LECs immuno-phenotype revealed that the cell population strongly expressed common markers of haematopoietic and mesenchymal stem cells. Indeed, LECs population was positive for CD90 (Thy-1) and CD44, moderately expressed CD73 and was negative for CD105 and CD13. LECs expressed CD29, an immature liver cell marker, but were negative for the hematopoietic markers CD34, and CD117 whereas expression of CD45 was weakly noted. Most of LECs expressed HLA-ABC but not HLA-DR.
Figure 2.
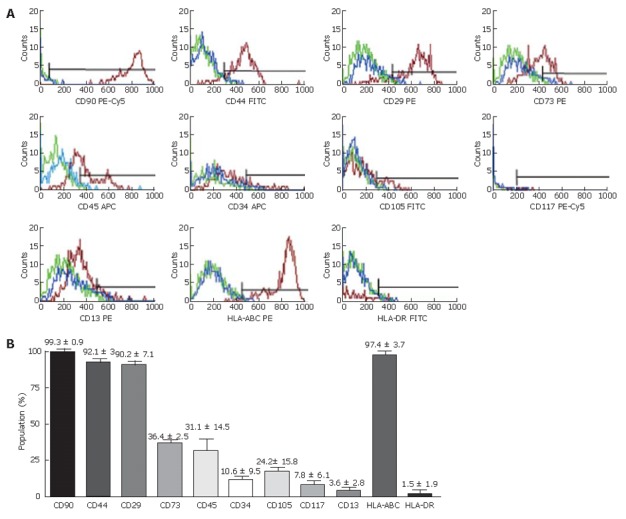
Flow cytometry analysis of purified LECs. A: Flow cytometry histograms showing three cell populations isolated from different livers. Antigens expression was detected with specific antibodies (red). Isotype-matched antibody controls (blue) and auto-fluorescence (green) showing the non-specific fluorescent labeling; B: Mean expression percentage of analyzed cell markers obtained from three different LEC populations after the third passage.
As demonstrated by immunofluorescence, LECs were homogenously positive for AFP, Alb and CK-18 (Figure 3A-C). Biliary markers such as CK-19 and CK-7 were also positive in all the cell population (Figure 3D and E). Up to now, oval cells are not unambiguously defined as precursors of hepatocyte and biliary cells in adult liver diseases. But morphology of LECs was distinguishable from oval cells and did not express oval cell marker (OV-6) (Figure 3F). Metabolic function analysis of LECs also demonstrated their immature status. Thus LECs did not secrete Alb and urea in the culture medium (data not shown) and periodic acid-Schiff staining showed the absence of glycogen storage (Figure 3G).
Figure 3.
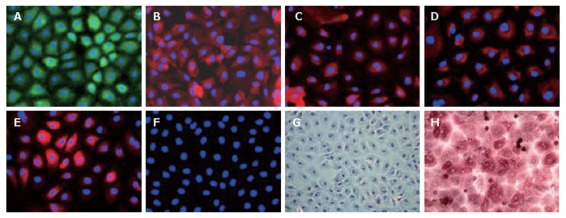
Immunofluorescence characterization of LECs in primary culture (× 200). Immunofluorescence was visualized using anti-rabbit FITC (green) and anti-mouse Cy3 (rouge). The nuclei, stained by DAPI, appeared in blue. A: The expression of AFP, fetal liver marker, reflected their immature status; LECs expressed both hepatocytic (B: ALB; C: CK-18) and biliary markers (D: CK-19; E: CK-7); F: LECs did not express oval cell marker OV-6; G, H: The representative photographs at phase contrast microscopy after PAS staining. LECs in the primary culture were not able to store glycogen (G) as compared to human hepatocytes (H). The nuclei were stained with hematoxylin.
The data were reproducible in 3 different liver cell supensions and the bi-potential phenotype of LECs was confirmed using RT-PCR analysis. These cells strongly expressed Alb, CK-8, CK-19 and CYP1B1 (Figure 4). Furthermore, they expressed immature markers such as AFP and POU transcription factor octamer-binding protein 4 (Oct-4) but not HNF-4 (transcription factor expressed early in endoderm formation). Expression of mature hepatocyte markers such as G6P and AAT was weakly noted whereas hepatocyte markers of final maturation phase (CYP2B6, CYP3A4 and TAT) were not found. Expression of genes associated with biliary phenotype was also noted as for GGT and MRP2.
Figure 4.
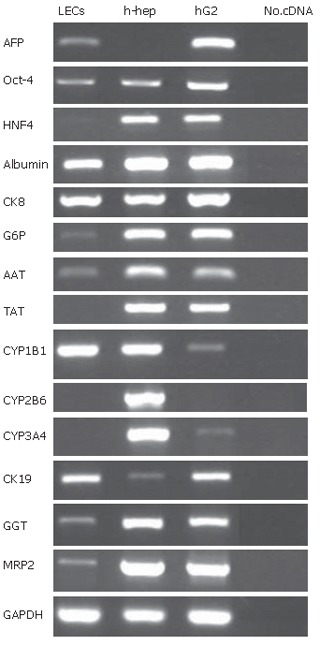
RT-PCR analysis of purified LECs. RT-PCR was assessed on mRNA extracted from purified LECs at the 3rd passage. Data shown are agarose gel electrophoresis of amplification products. Isolated human liver cells (h-hep) and HepG2 cells were used as positive controls. LECs expressed human fetal liver markers (α-fetoprotein (AFP) and transcription factor Oct-4) and adopted hepatocyte (positive for ALB, CK-8, G6P, AAT, and CYP1B1) and biliary (positive for CK-19, GGT and MRP2) phenotypes. These cells did not express HNF4, TAT, CYP2B6 and CYP3A4.
These parameters were maintained up to 5 passages. After that, LECs adopted a very large cytoplasm (Figure 5A and B). Whereas expression of Alb, CK-18 and CK-19 (Figure 5D-F) was maintained during all of cultured time, AFP (Figure 5C) and CK-7 (Figure 5G) staining was significantly decreased.
Figure 5.
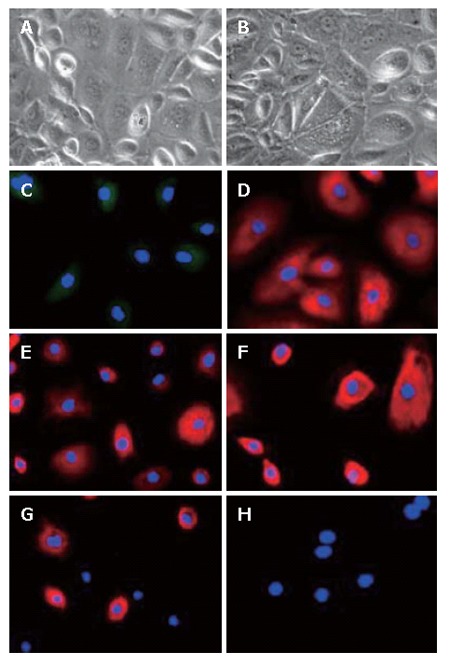
Characterization of LECs at the 5th passage (× 200). The phase contrast microscopy photographs of LECs at the 5th passage showing an increased size (A and B). Using immunofluorescence, in parallel to morphological changes, LECs lost the expression of AFP (C) and CK-7 (G) but maintained the expression of ALB (D), CK-18 (E) and CK-19 (F). LECs remained mostly negative for oval cell marker OV-6 (H).
Intrasplenic transplantation of LECs
To investigate the in vivo outcome of LECs, transplantation of these cells was performed in the spleen of SCID mice, half of them were hepatectomized. One month after transplantation, mice were sacrificed and plasma analyzed for the detection of human Alb. No presence of this marker was noted after transplantation. In parallel, liver tissues were analyzed using immunohistochemistry. Foci or isolated cells positively stained for human Alb were detected in the recipient liver tissue of 3 transplanted mice (Figure 6A and B) (2 of them were hepatectomized). The detected cells were mostly localized near vascular structures.
Figure 6.
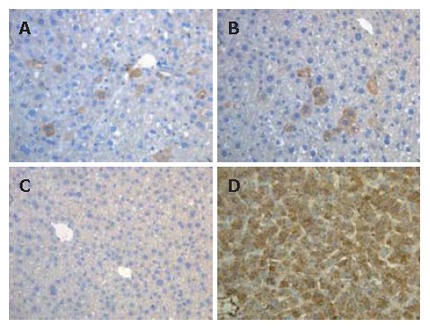
Immunohistochemical analysis of LECs in the liver of transplanted SCID mice. Foci or isolated cells stained for human Alb (brown) were detected around centrolobular vein (A) and portal area (B) of transplanted SCID mice (2 hepatectomized and 1 normal SCID mice; C: Human Alb cells were not detected in control mice; D: All of cells in human liver sections were stained for human Alb.
DISCUSSION
In the present study, we report the in vitro isolation of LECs from normal adult human liver. The culture of the purified cells was maintained in vitro for more than 160 d (seven passages) leading to their characterization at both the immuno-cytochemical and genetic levels. LECs injected into the spleen of SCID mice showed their ability to migrate and engraft into the recipient liver tissue.
Cell suspension obtained after collagenase liver disaggregation might contain all the cell types in the liver, the largest part being hepatocytes whereas the presence of minority of non-parenchymal cells is not excluded. When majority of hepatocytes undergoes cell death, LECs spontaneously emerge and proliferate. These cells were closely associated in a monolayer and formed several isolated colonies in the culture dishes. We hypothesized that these cells are resident in the normal adult liver and probably co-isolated with hepatocytes. Their detection in the intact liver may remain difficult because of absence of specific markers. Their proliferation may be stimulated due to chemical signals released in the culture medium after the death of mature hepatocytes (as proposed in vivo in liver injury animal models). Furthermore, LECs have been shown to survive in vitro after they were lifted in new culture dishes leading to the purification of cell population. LECs were evaluated both in the primary culture (heterogeneous environment) and after trypsin application, at the immunofluorescence level and showed that these cells homogenously expressed hepatocytic (Alb, CK-18) and biliary (CK-7 and CK-19) markers. The hepatobiliary phenotype was confirmed using RT-PCR analysis up to the 5th passage. LECs were also positive for AFP and expressed Oct-4 but not the final maturation phase markers CYP2B6, CYP3A4 and TAT, indicating their immature state. The expression of these markers was maintained while cells continue proliferating. The bi-potential phenotype of LECs has already been described for the oval cells that were presumed to be precursors of hepatocyte and biliary cells in adult liver[2,14,15,21]. LECs were morphologically different from oval cells (high cytoplasm/nuclear ratio) and did not express markers such as CD117, CD105 and OV-6[14,15]. The bi-potential phenotype of LECs may propose these cells as originating from another resident progenitor/stem cells pool within the normal adult liver. LECs were positive for CD90, a marker of hematopoietic and fetal liver stem cells[22-24]. They also expressed integrin β1 (CD29), a marker of hepatoblasts, which persists after maturation process of both hepatocyte and biliary epithelial cells[22,25].
The morphological and immunofluorescence analyses demonstrate that human LECs resemble those previously described in rats[2]. In young adult Fisher 344 rats, LECs were isolated from the non-parenchymal fraction after an incomplete collagenase-digestion of the liver. In this study, hepatocytes were eliminated by differential centrifugation[26]. In adult pig liver, epithelial cells have been described to emerge as clusters after the culture of parenchymal cell fraction. Nonetheless, a non-parenchymal cell fraction, rather than a parenchymal cell fraction, was recommended for obtaining such epithelial cells[18,27]. The LECs reported in these studies even positive for Alb, AFP and the oval cell marker OV-6 did not express biliary cell markers such as CK-19 and GGT. In human, Selden and colleagues have isolated epithelial cell population from the liver of a 6 wk subacute liver failure patient[10]. The authors hypothesized that this condition will enrich the recipient liver of progenitor liver cells. In our study, we demonstrate that LECs, although with low proportion, can be obtained from normal human liver tissue even when we plated a very limited initial cell suspension. Furthermore, we can observe that LECs isolated in our study express CK-8 in contrary to cells isolated from the subacute liver failure patient. The differences observed between these studies may be correlated to the use of liver isolation and cell culture techniques.
In our study, culture of purified LECs was maintained after incubation in DMEM medium supplemented with 10% FBS. The immunofluorescence analysis revealed that after the 5th passage, some LECs had a very large cytoplasm and lose CK-7 although expression of Alb and CK-18 was maintained. A decrease in AFP expression was also noted. These data could suggest spontaneous in vitro differentiation of these cells. Such phenomenon has been documented for porcine LECs[18,27]. Nevertheless, other studies demonstrated the ability of LECs to differentiate into hepatocytes and biliary cells after incubation with growth factors defined media[28,29]. In the rat model, normal LECs have also been shown to engraft and to differentiate into hepatocytes or biliary cells[30,31].
To evaluate this parameter, purified LECs were intrasplenically transplanted in 4 SCID mice, half of them being hepatectomized (70%). Evaluation of engraftment efficiency was analyzed 1 mo after transplantation. Although no human Alb was detected in serum, immunohistochemistry analysis demonstrated the presence of human cells within the recipient parenchyma mostly near vascular structures. These data suggest the ability of LECs to migrate and to engraft in the liver. In our study, hepatectomy did not stimulate the engraftment and proliferation potential of human LECs in SCID mouse liver suggesting a low ability of LECs to respond to murine differentiation signals. It is also possible that an inhibition of mouse liver cell regeneration induced by hepato-toxins is necessary for stimulating LECs proliferation.
In conclusion, we demonstrated the presence of LECs population within adult normal human liver. These cells expanded in vitro, presented bi-potential phenotype and were able to migrate and to engraft in the recipient parenchyma after intrasplenic transplantation. As being obtained after isolation of normal human liver, the pool of these progenitor/stem-like cells may reside in the liver, however, additional data regarding the progenitor’s biology of this organ are needed.
Footnotes
Supported by DGTRE, Région Wallonne (Grant Waleo HEPATERA) and by the Fondation saint-Luc. The first author is a recipient of fellowship from FNRS and the Université Catholique de Louvain
S- Editor Liu Y L- Editor Ma JY E- Editor Chin GJ
References
- 1.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 2.Coleman WB, Butz GM, Howell JA, Grisham JW. Transplantation and differentiation of rat liver epithelial stem-like cells. In: Gupta S, Jansen PLM, Klempnauer J, Manns MP editors. Hepatocyte transplantation. Proceedings of Falk symposium 126; 2002. pp. 38–50. [Google Scholar]
- 3.Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, Pani P, Laconi E. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069–1074. doi: 10.1016/s0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]
- 4.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 5.Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156:607–619. doi: 10.1016/S0002-9440(10)64765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon GJ, Butz GM, Grisham JW, Coleman WB. Isolation, short-term culture, and transplantation of small hepatocyte-like progenitor cells from retrorsine-exposed rats. Transplantation. 2002;73:1236–1243. doi: 10.1097/00007890-200204270-00008. [DOI] [PubMed] [Google Scholar]
- 8.Avril A, Pichard V, Bralet MP, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737–743. doi: 10.1016/j.jhep.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C. Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J Hepatol. 1997;26:343–352. doi: 10.1016/s0168-8278(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 10.Selden C, Chalmers SA, Jones C, Standish R, Quaglia A, Rolando N, Burroughs AK, Rolles K, Dhillon A, Hodgson HJ. Epithelial colonies cultured from human explanted liver in subacute hepatic failure exhibit hepatocyte, biliary epithelial, and stem cell phenotypic markers. Stem Cells. 2003;21:624–631. doi: 10.1634/stemcells.21-6-624. [DOI] [PubMed] [Google Scholar]
- 11.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 12.Golding M, Sarraf C, Lalani EN, Alison MR. Reactive biliary epithelium: the product of a pluripotential stem cell compartment? Hum Pathol. 1996;27:872–884. doi: 10.1016/s0046-8177(96)90212-9. [DOI] [PubMed] [Google Scholar]
- 13.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 14.Alison MR. Characterization of the differentiation capacity of rat-derived hepatic stem cells. Semin Liver Dis. 2003;23:325–336. doi: 10.1055/s-2004-815561. [DOI] [PubMed] [Google Scholar]
- 15.Alison MR, Vig P, Russo F, Bigger BW, Amofah E, Themis M, Forbes S. Hepatic stem cells: from inside and outside the liver? Cell Prolif. 2004;37:1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao MS, Smith JD, Nelson KG, Grisham JW. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 17.Hooth MJ, Coleman WB, Presnell SC, Borchert KM, Grisham JW, Smith GJ. Spontaneous neoplastic transformation of WB-F344 rat liver epithelial cells. Am J Pathol. 1998;153:1913–1921. doi: 10.1016/S0002-9440(10)65705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kano J, Tokiwa T, Zhou X, Kodama M. Colonial growth and differentiation of epithelial cells derived from abattoir adult porcine livers. J Gastroenterol Hepatol. 1998;13 Suppl:S62–S69. [PubMed] [Google Scholar]
- 19.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 20.Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Thorgeirsson SS, Grisham JW. Overview of recent experimental studies on liver stem cells. Semin Liver Dis. 2003;23:303–312. doi: 10.1055/s-2004-815559. [DOI] [PubMed] [Google Scholar]
- 22.Laurson J, Selden C, Hodgson HJ. Hepatocyte progenitors in man and in rodents--multiple pathways, multiple candidates. Int J Exp Pathol. 2005;86:1–18. doi: 10.1111/j.0959-9673.2005.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceafalan L, Vidulescu C, Radu E, Regalia T, Popescu I, Pana M, Serghei L, Voiculescu B, Popescu LM. Expression of stem cell markers on fetal and tumoral human liver cells in primary culture. Rev Med Chir Soc Med Nat Iasi. 2005;109:96–104. [PubMed] [Google Scholar]
- 24.Dan YY, Riehle KJ, Lazaro C, Teoh N, Haque J, Campbell JS, Fausto N. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci USA. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 26.Grisham JW, Coleman WB, Smith GJ. Isolation, culture, and transplantation of rat hepatocytic precursor (stem-like) cells. Proc Soc Exp Biol Med. 1993;204:270–279. doi: 10.3181/00379727-204-43663. [DOI] [PubMed] [Google Scholar]
- 27.Kano J, Noguchi M, Kodama M, Tokiwa T. The in vitro differentiating capacity of nonparenchymal epithelial cells derived from adult porcine livers. Am J Pathol. 2000;156:2033–2043. doi: 10.1016/S0002-9440(10)65075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman WB, Smith GJ, Grisham JW. Development of dexamethasone-inducible tyrosine aminotransferase activity in WB-F344 rat liver epithelial stemlike cells cultured in the presence of sodium butyrate. J Cell Physiol. 1994;161:463–469. doi: 10.1002/jcp.1041610309. [DOI] [PubMed] [Google Scholar]
- 29.Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y. In vitro differentiation of WB-F344 rat liver epithelial cells into the biliary lineage. Differentiation. 2002;69:209–215. doi: 10.1046/j.1432-0436.2002.690414.x. [DOI] [PubMed] [Google Scholar]
- 30.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, Grisham JW. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 31.Chapman L, Coleman W, Hixson D. Integration, survival and differentiation of liver epithelial cells in hepatic and pancreatic ducts. FASEB J. 1999;13:A160. [Google Scholar]


