Abstract
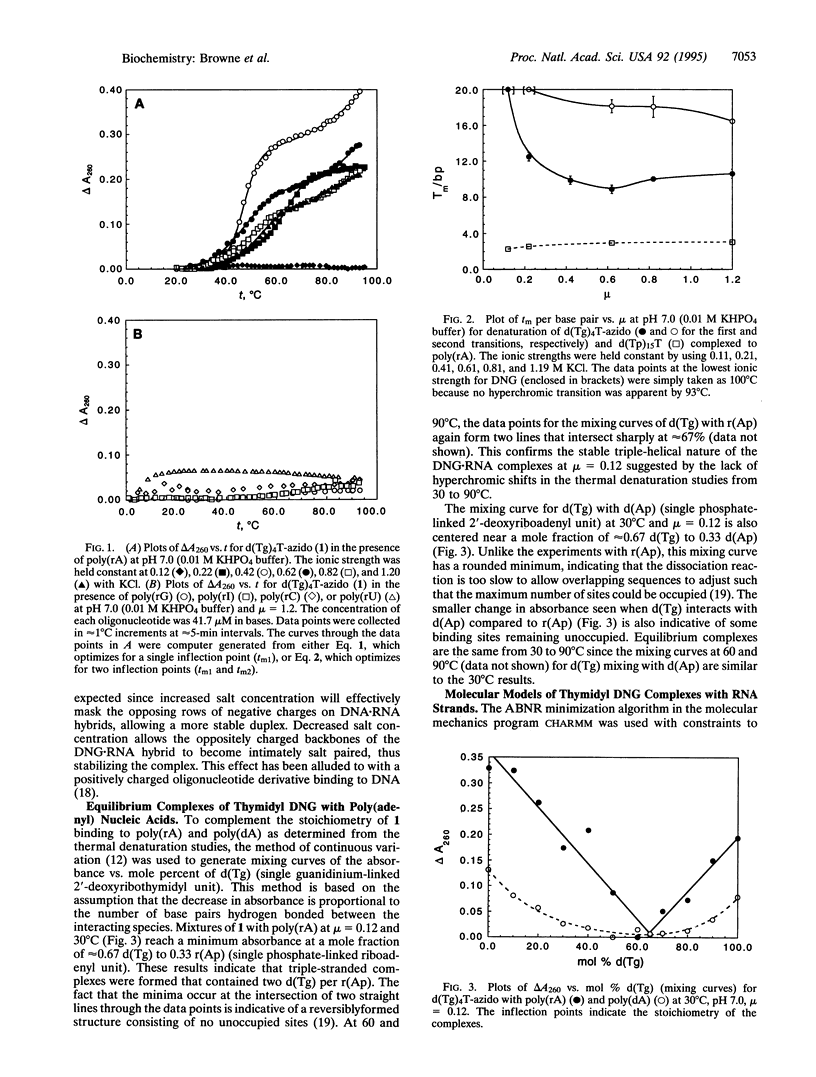
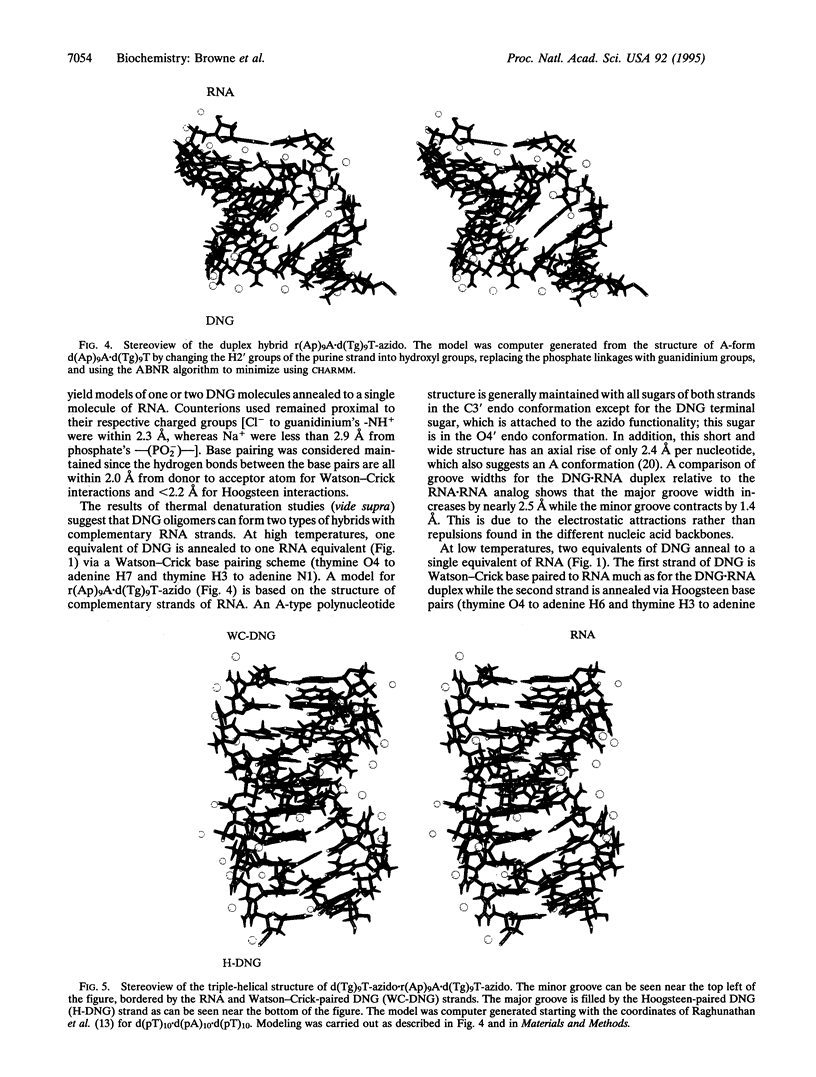
Deoxyribonucleic guanidine is a potential antisense agent that is generated via the replacement of the negative phosphodiester linkages of DNA [--O--(PO2-)--O--] with positively-charged guanidinium (g) linkages [--NH--C(==NH2+)--NH--]. A pentameric thymidyl deoxyribonucleic guanidine molecule [d(Tg)4T-azido] has been shown to base pair specifically to poly(rA) with an unprecedented affinity. Both double and triple strands consisting of one and two equivalents of d(Tg)4T-azido paired with one equivalent of poly(rA) are indicated by thermal denaturation experiments. At an ionic strength of 0.22, the five bases of d(Tg)4T-azido are estimated to dissociate from a double helix with poly(rA) at > 100 degrees C! The effect of ionic strength on thermal denaturation is very pronounced, with stability greatest at low ionic strengths. The method of continuous variation indicates that there is an equilibrium complex with a molar ratio of d(Tg) to r(Ap) or d(Ap) of 2:1. Based on this evidence, models of the structures of d(Tg)9T-azido bound to r(Ap)9A are proposed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almarsson O., Bruice T. C., Kerr J., Zuckermann R. N. Molecular mechanics calculations of the structures of polyamide nucleic acid DNA duplexes and triple helical hybrids. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7518–7522. doi: 10.1073/pnas.90.16.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S. T. Progress toward oligonucleotide therapeutics: pharmacodynamic properties. FASEB J. 1993 Apr 1;7(6):533–539. doi: 10.1096/fasebj.7.6.7682523. [DOI] [PubMed] [Google Scholar]
- Crooke S. T. Therapeutic applications of oligonucleotides. Annu Rev Pharmacol Toxicol. 1992;32:329–376. doi: 10.1146/annurev.pa.32.040192.001553. [DOI] [PubMed] [Google Scholar]
- Dempcy R. O., Almarsson O., Bruice T. C. Design and synthesis of deoxynucleic guanidine: a polycation analogue of DNA. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):7864–7868. doi: 10.1073/pnas.91.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempcy R. O., Browne K. A., Bruice T. C. Synthesis of a thymidyl pentamer of deoxyribonucleic guanidine and binding studies with DNA homopolynucleotides. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6097–6101. doi: 10.1073/pnas.92.13.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELSENFELD G. Theoretical studies on the interaction of synthetic polyribonucleotides. Biochim Biophys Acta. 1958 Jul;29(1):133–144. doi: 10.1016/0006-3002(58)90153-7. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E., Egholm M., Berg R. H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Privé G. G., Yanagi K., Dickerson R. E. Structure of the B-DNA decamer C-C-A-A-C-G-T-T-G-G and comparison with isomorphous decamers C-C-A-A-G-A-T-T-G-G and C-C-A-G-G-C-C-T-G-G. J Mol Biol. 1991 Jan 5;217(1):177–199. doi: 10.1016/0022-2836(91)90619-h. [DOI] [PubMed] [Google Scholar]
- Raghunathan G., Miles H. T., Sasisekharan V. Symmetry and molecular structure of a DNA triple helix: d(T)n.d(A)n.d(T)n. Biochemistry. 1993 Jan 19;32(2):455–462. doi: 10.1021/bi00053a009. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Young P. R., Kallenbach N. R. Secondary structure in polyuridylic acid. Non-classical hydrogen bonding and the function of the ribose 2'-hydroxyl group. J Mol Biol. 1978 Dec 15;126(3):467–479. doi: 10.1016/0022-2836(78)90053-0. [DOI] [PubMed] [Google Scholar]