Abstract
AIM: To study whether H pylori locate in the gallbladder mucosa of patients with chronic cholecystitis.
METHODS: Using Warthy-Starry (W-S) silver stain and immunohistochemistry stain with anti-H pylori antibodies, we screened paraffin specimens in 524 cases of cholecystitis. H pylori urease gene A (HPUA) and H pylori urease gene B (HPUB) were analyzed by polymerase chain reaction (PCR) in the fresh tissue specimens from 81 cases of cholecystitis.
RESULTS: H pylori-like bacteria were found in 13.55% of the gallbladders of the cholecystitis patients using W-S stain. Meanwhile, bacteria positive for H pylori antibodies were also found in 7.1% of the gallbladders of patients with cholecystitis by immunohistochemistry. Of 81 gallbladders, 11 were positive for both HPUA and HPUB, 4 were positive for HPUA only and 7 were positive for HPUB only.
CONCLUSION: H pylori exist in the gallbladders of patients with chronic cholecystitis.
Keywords: H pylori, Gallbladder mucosa, Chronic cholecystitis, Polymerase chain reaction, H pylori urease gene
INTRODUCTION
H pylori closely correlate with chronic gastritis[1-5], peptic ulcer[6-8], gastric carcinoma and malignant lymphoma of gastric mucosa-related lymphoid tissues (MALToma)[9-11]. Recently, it has been found that H pylori have certain relationship to some diseases in the organs besides the stomach and duodenum[12]. However, it is still unclear whether H pylori have any correlation with adjacent structures of the stomach[13], the liver and the gallbladder[14,15]. In the present study, we compared the detection rate of H pylori in the stomachs of patients with chronic cholecystitis using Warthy-Starry (W-S) silver stain, immunohistochemistry and polymerase chain reaction (PCR) and showed that H pylori exist in the gallbladders.
MATERIALS AND METHODS
Subjects
A total of 142 patients who had chronic cholecystitis confirmed by pathologic examination since 1995 in our hospital and received gastroscopy and H pylori urease test were included. Randomly chosen patients without diseases of the gallbladder, who received gastroscopy due to symptoms of digestive tract were used as control group. The paraffin sections of 524 cholecystitis specimens were stained with W-S to observe H pylori-like bacteria. From fresh gallbladder specimens of 81 patients receiving cholecystectomy due to cholelithiasis and chronic cholecystitis in our hospital, DNA was extracted for PCR amplification.
Methods
Paraffin specimens of cholecystitis were sliced into 4 µm sections, stained with W-S and sealed with D.P.X for an optic microscopic observation for the existence of H pylori-like bacteria. Immunohistochemistry stain of H pylori was made using streptovitacin peroxidase (SP). Anti-H pylori antibody (Dako Corporation) was diluted to 1:10. At the same time, PBS was used as blank control, normal blood serum as negative control and the sections of gastric mucosa with positive H pylori as positive control. PCR amplification of H pylori urease genes A and B was carried out on 81 specimens. The gallbladder was placed in a prepared bottle for DNA extraction from gallbladder mucosa and frozen at -20˚C. The procedures of PCR amplification were as follows: (1) Primer synthesis of urease gene A (HPUA) 1 and HPUA2 was based on nucleic acids 304-714 of urease gene A, with an amplification portion of 411 bp. While the primers of urease gene B (HPUB) 1 and HPUB2 were designed according to nucleic acids 1971-2102 of urease gene B, with amplification part of 132 bp. These two primers maintained a high specificity and primer arrays that were HPUA1: 5'-GCCAATGGTAAATTAGTT-3'; HPUA2: 5'-CTCCTTAATTGTTTAC-3'; HPUB1: 5'-TGGGATTAGCGAGTATGT-3'; HPUB2: 5'-CCCATTTGACTCAATG-3', respectively, the primers of which were synthesized by Shanghai Bioengineering Corporation. (2) Reaction systems and parameters were as described previously[16]; (3) Gel electrophoresis analysis of the products of PCR amplification was performed under ultraviolet light and compared with the marker.
Statistical analysis
The data were processed with Chi-square test and P < 0.05 was considered a significant difference.
RESULTS
H pylori in the stomach of patients with chronic cholecystitis
There were 142 patients with chronic cholecystitis including 13 with gastric ulcer detected by gastroscopy, 8 with duodenal ulcer and 121 with chronic gastritis, of which 76 had bile reflux. A total of 123 randomly chosen patients with digestive symptoms who were examined by gastroscopy and without gallbladder diseases were used as control, of which 6 cases were with gastric ulcer, 7 with duodenal ulcer, 2 with gastric carcinoma and 108 with chronic gastritis. Bile reflux occurred in 36 cases in the control group. In both groups, the detection rates (61/123 vs 81/142) of H pylori in the stomach were similar, but the detection rate of the bile reflux in the cholecystitis group was significantly higher than that in the control group (36/123 vs 76/142 P < 0.01, Table 1). There was no significant difference with respect of detection rate of H pylori in both groups (16/36 vs 35/76, P > 0.05).
Table 1.
Results of gastroscopy in both groups
| Group | n |
Bile reflux |
Duodenal ulcer |
Gastric ulcer |
H pylori (+) |
| n (%) | n (%) | n (%) | n (%) | ||
| Control | 123 | 36 (29.3) | 7 (5.7) | 6 (4.9) | 61 (49.6) |
| Chronic cholecystitis | 142 | 76 (53.5)b | 8 (5.6) | 13 (9.2)b | 81 (57.0) |
P < 0.01 vs control group.
W-S silver stain of the filed cholecystitis paraffin
Under optic microscopes, in contrast with the yellow staining of the gastric mucosa, there could be seen bended, curved and spiral brown bacteria in the epithelial cells of the gastric mucosa that were used as positive control. Using W-S silver stain, H pylori-like bacteria could be seen in 71 (13.6%) out of 524 cholecystitis specimens under optic microscopes, showing curved, pole-like, bended, spiral and fusiform bacteria, and also some spherical shaped bacteria. In 34 specimens, besides positive H pylori-like bacteria, other bacteria could be seen (Figure 1).
Figure 1.
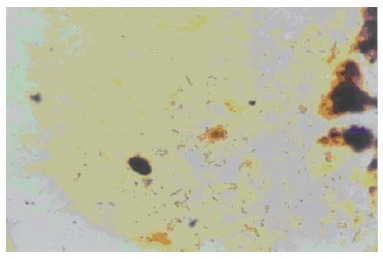
Curved positive stain bacteria in the surface mucus of gallbladder mucosa (WS × 200).
Immunohistochemical observation of anti-H pylori antibody
After 71 specimens positive for H pylori-like bacteria were stained immunohistochemically with anti-H pylori antibody, bacteria positive for H pylori antibody could be seen in only 37 cases (52.1%) under optic microscopes. That is to say, bacteria positively stained with anti-H pylori antibody only accounted for 7.1% (37/524) of 524 cholecystitis specimens under optic microscopes. These positive bacteria were brown in color, bended with curved-poles, and spiral in shape. They were mainly located on the epithelial cell surface and within the mucosal glands, scattered or aggregated (Figure 2).
Figure 2.

Positive stain bacteria of H pylori antibody in the surface mucus of gallbladder mucosa (SP × 200).
PCR amplification of HPUA and HPUB
Of 81 cholecystectomy specimens, positive amplification zone of HPUA or HPUB was seen in 22 (27.2%), including 11 (13.6%) with positive amplification zones of HPUA and HPUB, 4 with positive HPUA only and 7 with positive HPUB only. However, in 6 gastric mucosa specimens as positive control, the two primers had positive amplification zones of HPUA and HPUB (Figure 3).
Figure 3.
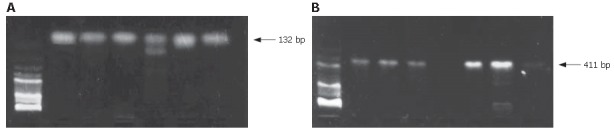
A: PCR amplification of primers of gallbladder mucosa DNA urease gene B (HPUB) and the positive amplification zone of 132 bp; B: PCR amplification of primers of gallbladder mucosa DNA urease gene A (HPUA) and the positive amplification zone of 411 bp.
DISCUSSION
By means of gastroscopy, we have found that the incidence rate of bile reflux in patients with chronic cholecystitis was significantly higher than that in the control group. It indicates that patients with chronic cholecystitis are apt to bile regurgitation, which occurs in 80% of patients with chronic cholecystitis while in only 32% of normal persons[17,18]. H pylori are sensitive to bile salts, especially the unconjugated bile salts that exert poisonous effect on H pylori. The latter cannot live in an environment with bile salts[19]. We also found that in the stomachs of patients with chronic lithic cholecystitis and a bile reflux, H pylori were present in up to 46.1% (35/76), higher than 44.4% (16/36) in patients without bile reflux. It indicates that a high incidence rate of H pylori infection still existed in the stomach although there was a bile reflux. The results of our study are consistent with that of Caldwell et al[20], who studied the infection rate of gastritis and H pylori in patients with chronic calculus cholecystitis before and after cholecystectomy and found that the duodenogastric reflux increased after cholecystectomy. The gastritis was aggravated and the infection rate of H pylori in the stomach increased from preoperative 32% to postoperative 68%, with a significant difference (P < 0.05). Therefore, they concluded that H pylori could live in the basic condition and even aggravate gastritis. Our study indicates that the patients with chronic lithic cholecystitis were apt to bile regurgitation; however, there was still a high infection rate of H pylori in their stomachs, suggesting that a kind of H pylori that can resist bile salts exists. Bile regurgitation may play a role in selecting H pylori so that H pylori resistant to bile salts can survive and, in combination with the bile, aggravate the injury of gastric mucosa[21]. Thus, H pylori that resist the bile salts and survive under basic conditions can enter the gallbladder via an inverse infection route through the common passage.
In 524 chronic cholecystitis specimens, 71 showed positive bacteria by W-S silver stain, while only 37 had positive bacteria by immunohistochemistry stain using anti-H pylori antibodies. It suggests that W-S silver stain to screen H pylori in gallbladder specimens is suitable for an initial selection. In patients with chronic calculus cholecystitis, the infection rate of H pylori was 7.1% (37/524), a rather low level, which was confirmed by immunohistochemistry using anti-H pylori antibodies. H pylori were mainly present in the surface of epithelial cells of the gallbladder mucosa, sometimes in intercellular zone or within the mucous gland. The H pylori, spiral, U- and S-typed in morphology, are distributed in scattered or aggregated fashion. All these characteristics are similar to that of H pylori in the stomach.
After the fresh specimens of 81 patients with chronic cholecystitis treated with cholecystectomy were amplified by PCR with specific primers of HPUA and HPUB, the positive amplification zone existed in 27.2% (22/81), significantly higher than the positive rate (13.6%) detected by W-S silver stain and that (7.1%) by immunohistochemistry stain using H pylori antibodies. This is partly because the template DNA amplified by PCR was extracted from tissues (0.5 cm × 0.5 cm) of gallbladder mucosa and the PCR with a high sensitivity had a high detection rate of H pylori. In the meantime, the PCR was conducted to detect DNA of H pylori; therefore, under many conditions, the positive amplification zone would appear only if H pylori DNA existed in the gallbladder mucosa, whether the H pylori were dead or the remains from previous infections. In the present study, in specimens with positive PCR amplification, 11 were positive for HPUA and HPUB; while in the control group, 6 gastric mucosa specimens infected by H pylori were positive for HPUA and HPUB. It proved that H pylori exist in the gallbladder. However, it still remains unclear whether H pylori are present in the gallbladder of patients with positive HPUA or HPUB, whether the gallbladder is infected by H pylori and whether the genes of H pylori change in the gallbladder[22]. We conclude that H pylori are present in the gallbladder and may relate to the incidence of cholecystitis, which requires further research.
Footnotes
Supported by the National Natural Science Foundation of China, No. 39970039
S- Editor Liu Y L- Editor Zhu LH E- Editor Che YB
References
- 1.Apostolov E, Al-Soud WA, Nilsson I, Kornilovska I, Usenko V, Lyzogubov V, Gaydar Y, Wadström T, Ljungh A. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol. 2005;40:96–102. doi: 10.1080/00365520410009546. [DOI] [PubMed] [Google Scholar]
- 2.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 3.Tsukanov VV, Grishchenko NN. Association of Helicobacter pylori with chronic cholecystitis. Eksp Klin Gastroenterol. 2003;(6):80–82. [PubMed] [Google Scholar]
- 4.Osadchuk MA, Geras'kina TB. Chronic cholecystitis--some lithogenic aspects. Ter Arkh. 1997;69:27–30. [PubMed] [Google Scholar]
- 5.Pradhan SB, Dali S. Relation between gallbladder neoplasm and Helicobacter hepaticus infection. Kathmandu Univ Med J (KUMJ) 2004;2:331–335. [PubMed] [Google Scholar]
- 6.Vere CC, Cazacu S, Comănescu V, Mogoantă L, Rogoveanu I, Ciurea T. Endoscopical and histological features in bile reflux gastritis. Rom J Morphol Embryol. 2005;46:269–274. [PubMed] [Google Scholar]
- 7.Andersen LP. New Helicobacter species in humans. Dig Dis. 2001;19:112–115. doi: 10.1159/000050664. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi M, Saito T, Ohno H, Midorikawa S, Sanji T, Handa Y, Morita S, Yoshida H, Tsurui M, Misaka R, et al. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol. 1996;31:294–298. doi: 10.1007/BF02389534. [DOI] [PubMed] [Google Scholar]
- 9.Monstein HJ, Jonsson Y, Zdolsek J, Svanvik J. Identification of Helicobacter pylori DNA in human cholesterol gallstones. Scand J Gastroenterol. 2002;37:112–119. doi: 10.1080/003655202753387455. [DOI] [PubMed] [Google Scholar]
- 10.Farinati F, Cardin R, Russo VM, Busatto G, Franco M, Rugge M. Helicobacter pylori CagA status, mucosal oxidative damage and gastritis phenotype: a potential pathway to cancer? Helicobacter. 2003;8:227–234. doi: 10.1046/j.1523-5378.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 11.Arnaout AH, Abbas SH, Shousha S. Helicobacter pylori is not identified in areas of gastric metaplasia of gall bladder. J Pathol. 1990;160:333–334. doi: 10.1002/path.1711600410. [DOI] [PubMed] [Google Scholar]
- 12.Chu CL, Li YQ, Zhang Y, Li WJ, Zhao XC. Expression of extracellular-signal regulated proein kinase in gastric carcinoma tissues and its relation with Helicobacter pylori infection. Shijie Huaren Xiaohua Zazhi. 2003;11:481–482. [Google Scholar]
- 13.Li XH, Zhang GY, Luo FJ, Xu MH, Li Q. Influence of expression of matrix metalloproteinase induced by H. pylori infection in gastric cancer cell line. Shijie Huaren Xiaohua Zazhi. 2003;11:544–546. [Google Scholar]
- 14.Dore MP, Sepulveda AR, Bacciu PP, Blasi F, Simula L, Marras L, Piccolo D, Cherchi GB, Graham DY, Realdi G. Detection of Chlamydiae pneumoniae but not Helicobacter pylori DNA in atherosclerosis plaques. Dig Dis Sci. 2003;48:945–951. doi: 10.1023/a:1023059815117. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Fan XG, Chen YP, Li N, Tang LJ. Detection of Helicobacter species 16Sr RNA gene in paraffin-embedded hepatocellular carcinoma tissues. Shijie Huaren Xiaohua Zazhi. 2002;10:877–882. [Google Scholar]
- 16.Wen-qin Cai, Bo-Yun Wang. Practicable immunocy-tochemistry and nucleic acid molecular hybridization. Chengdu: Sichuan Science and Technology Publishing House; 1994. pp. 496–498. [Google Scholar]
- 17.Silva CP, Pereira-Lima JC, Oliveira AG, Guerra JB, Marques DL, Sarmanho L, Cabral MM, Queiroz DM. Association of the presence of Helicobacter in gallbladder tissue with cholelithiasis and cholecystitis. J Clin Microbiol. 2003;41:5615–5618. doi: 10.1128/JCM.41.12.5615-5618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallone CA, Tran S, Semret M, Discepola F, Behr M, Barkun AN. Helicobacter DNA in bile: correlation with hepato-biliary diseases. Aliment Pharmacol Ther. 2003;17:453–458. doi: 10.1046/j.1365-2036.2003.01424.x. [DOI] [PubMed] [Google Scholar]
- 19.Messini F. Helicobacter pylori and hepatobiliary diseases. Clin Ter. 2003;154:55–56. [PubMed] [Google Scholar]
- 20.Caldwell MT, McDermott M, Jazrawi S, O'Dowd G, Byrne PJ, Walsh TN, Hourihane DO, Hennessy TP. Helicobacter pylori infection increases following cholecystectomy. Ir J Med Sci. 1995;164:52–55. doi: 10.1007/BF02968117. [DOI] [PubMed] [Google Scholar]
- 21.Bulajic M, Maisonneuve P, Schneider-Brachert W, Müller P, Reischl U, Stimec B, Lehn N, Lowenfels AB, Löhr M. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer. 2002;95:1946–1953. doi: 10.1002/cncr.10893. [DOI] [PubMed] [Google Scholar]
- 22.Leong RW, Sung JJ. Review article: Helicobacter species and hepatobiliary diseases. Aliment Pharmacol Ther. 2002;16:1037–1045. doi: 10.1046/j.1365-2036.2002.01282.x. [DOI] [PubMed] [Google Scholar]


