Abstract
AIM: To establish a culture system of marrow mese-nchymal stem cells (MSCs)from hepatitis B patients and normal adults and to compare their biological characteristics.
METHODS: MSCs were isolated from bone marrow in 34 male hepatitis B patients and 15 male normal adults and cultivated in vitro. Their biological characteristics including surface markers, shapes and appearances, growth curves, first passage time and passage gene-rations were compared.
RESULTS: Cultivation achievement ratio of hepatitis B patients was lower than that of normal adults, no statistical significance (82.35% vs 100%, P > 0.05). Compared with MSCs of normal adults, MSCs of hepatitis B patients presented a statistical lower growth curve, longer first passage time (13.0 ± 1.6 d vs 11.4 ± 1.5 d, P < 0.05), fewer passaging generation numbers (10.5 ± 1.4 generations vs 12.3 ± 1.7 generations, P < 0.05), though both shared same appearances, shapes and surface markers. MSCs in hepatitis B patients would expand, spread out and age more easily and there were more refractive particles in the cytoplasm.
CONCLUSION: MSCs from hepatitis B patients can be cultured in vitro. Although their appearance, shape and surface marker are similar to those of MSCs from normal adults, there are differences in their biological characteristics.
Keywords: Marrow mesenchymal stem cells, Culture in vitro, Biological characters
INTRODUCTION
Marrow mesenchymal stem cells (MSCs) are characterized by self-renewal and multi-directional differentiation[1-6]. MSCs have the following merits: ease of isolation and cultivation, expansion potential, stable phenotype, com-patibility by different delivery methods, and slight adverse reaction after implantation in vivo, and playing an important role in cellular therapeutics and tissue engineering[7-10].
Autogenetic MSCs do not cause immunological rejec-tions and have been used in France, Germany, Japan, Italy and China[11,12]. We have used autogetic MSCs in autoplastic transplantation therapy for severe liver failure and gained satisfactory therapeutic effects (in press).
However, there is only one MSC among 104-105 mono-nuclear cells in the bone marrow, which limits its clinical application. Many researches have proved that MSCs can be incubated and amplified in vitro[13-15]. Up to now, no studies on the biological characteristics, cultivation and amplification in vitro of MSCs in hepatitis B patients are available.
We tried to establish a cultivation system of in vitro MSCs from hepatitis B patients to compare their biological characteristics with those from normal humans.
MATERIALS AND METHODS
Preparation of bone marrow-derived MSCs
MSCs from bone marrow of 34 hepatitis B patients and 15 normal adults were prepared. All the 34 patients at the age of 35-57 years were inpatients visiting our department from September 2005 to June 2006. Of the 15 normal adults at the age of 30-40 years, 5 were volunteers and 10 were patients with external injury after plastic operation in Department of Orthopaedics.
Cultivation of MSCs in vitro
Five mL bone marrow was aspirated from anterosuperior iliac spine, mixed with 5 mL PBS, and centrifuged at 1500 r/min for 5 min. The supernatant was removed and dispersed in 5 mL PBS. Cell suspension was added to 5 mL Percoll separating medium (Sigma Company, USA) and centrifuged at 2500 r/min for 30 min. Nucleated cells in the middle layer were drawn off and washed with 5 mL PBS, centrifuged at 1500 r/min for 5 min. The supernatant was removed. Dispersed by Cult-M culture solution (An-Pu Biochemistry Company, Beijing, China), the cell sediments were inoculated in a 25 cm2 culture flask and incubated at 37°C in an atmosphere containing 5% CO2. The culture medium was changed after 3 d and then every 2 d. MSCs were digested by 0.25% Trypsin, 0.1% EDTA and passaged (1:2) when they grew to 70%-80%.
Comparison of MSCs from hepatitis B patients and normal adults
MSCs from bone marrow of 34 hepatitis B patients and 15 normal adults were inoculated and cultivated. The cultivation ratios were compared.
The appearance of MSCs was observed under an inverted phase contrast microscope (M20-35DX, Olympus Company, Japan) and their differences were comp-ared on d 4 and 8 after inoculation and at generations 5 and 10, respectively. The 4th passage MSCs from hepatitis B patients were digested, washed with PBS and adjusted to the density of 1.0 × 106 cells/mL. After addition of FITC-CD44 antibody, PerCP-CD45 antibody, PE-CD34 antibody (BD Biosciences Company, USA), they were detected by flow cytometry using mouse IgG1 as the isotypism contrast. Results were compared with MSCs from normal adults. P3 MSCs from hepatitis B patients and normal adults were inoculated in a 24-shadow mask at the density of 103 cells/hole. The number of cells in two holes was counted everyday for 10 d, and the growth curve was plotted. The primary passage time of MSCs from hepatitis B patients and normal humans was compared after inoculation at the density of 1.0 × 106 cells/cm2. The number of passage generations of MSCs from 28 hepatitis B patients and 15 normal human was also compared.
Statistical analysis
Data were expressed as mean ± SD and analyzed by SPSS s13.0 software using Chi square test. P < 0.05 was considered statistically significant.
RESULTS
MSCs from bone marrows of hepatitis B patients and normal adults were cultivated, the ratio was 82.35% and 100%, respectively, without statistical significance (Table 1, P > 0.05). All MSCs had a fusiform shape with a high karyoplasmic ratio and were integrated into steady colonies, like collagenoblasts. After observation on differences of appearance between hepatitis B patients and normal adults on the 4th and 8th d after inoculation and at the 5th and 10th generations, MSCs grew slower and were much easier to expand, spread out and age in hepatitis B patients than in normal adults, and more refractive particles were found in cytoplasm (Figure 1). CD34-CD44+ and CD44+CD45- cells accounted for 97.83% and 99.38% separately of P4 MSCs from hepatitis B patients, demonstrating a high purity. CD34-CD44+CD45- was expressed on its surface molecules as on MSCs[1] in the normal adults (Figure 2). The growth curve of MSCs from hepatitis B patients was lower than that from normal humans (Figure 3). The primary passage time of MSCs from hepatitis B patients and normal human was 13.0 ± 1.6 d and 11.4 ± 1.5 d, respectively (P < 0.05). The MSCs passage amount of MSCs from hepatitis B patients and normal humans were 10.5 ± 1.4 and 12.3 ± 1.7 generations, respectively (P < 0.05).
Table 1.
Comparison of cultivation ratio of MSCs from hepatitis B patients and normal humans n (%)
| Success | Failure | Total | |
| MSCs from hepatitis B patients | 28 (82.35) | 6 (17.65) | 34 |
| MSCs from normal human | 15 (100) | 0 (0) | 15 |
| Total | 43 (87.76) | 6 (12.24) | 49 |
Figure 1.
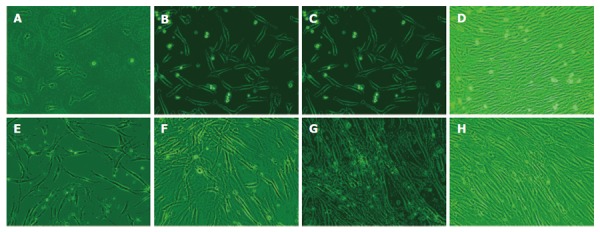
Morphology of MSCs from hepatitis B patients and normal humans after incubation for 4 d (A, B) and 8 d (C, D), generations 5 d and 10 d (E-H).
Figure 2.
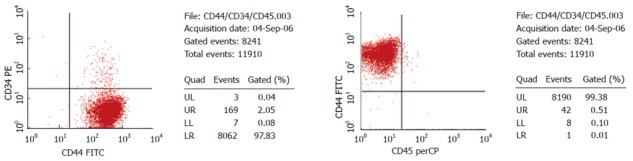
Expression of surface molecules CD34, CD44, CD45 of MSCs in hepatitis B patients.
Figure 3.
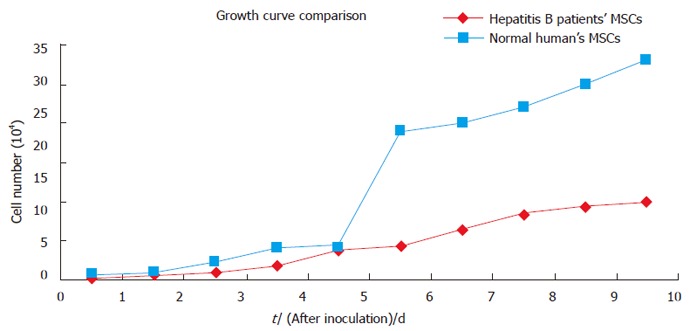
Growth curve of MSCs from hepatitis B patients and normal humans.
DISCUSSION
A total of 130 million Chinese are infected with HBV and every year a half million of patients die of terminal stage liver disease caused by HBV. There are no effective hepatitis B therapies. Internal medical therapy, anti-virus therapy and liver transplantation, cannot recover liver function, improve prognosis and quality of life of such patients. It is urgent to find effective therapies for HBV infection.
MSCs are adult stem cells, which can be differentiated in many tissues like bone, cartilage, muscle, tendon, myocardial cells, liver, lung, kidney, cranial nerve cells and spinal cord[16]. In recent years, xenogenic and autogeneic MSCs have been used in the treatment of hepatitis B patients[11,12]. Autogeneic MSCs in hepatitis B patients can avoid immunological rejection and tumorigenesis caused by cell fusion, although some scholars believe that MSCs have immunological regulation functions and do not lead to immunological rejection because MHC-I antigen is not expressed and MHC-II antigen has a low expression[17]. However, there is no evidence that MSCs do not express MHC-I and MHC-II antigens and exert immunological regulation after their differentiation into mature hepatocytes.
In the present study, we cultivated in vitro MSCs from hepatitis B patients, observed their bionomics and compared them with those from normal adults. Their surface molecules were similar but there were evident differences in culture ratio, growth curve, primary passage time, number of passage generations and appearance change after several passage times between them, sug-gesting that MSCs from hepatitis B patients are more difficult to cultivate in vitro, grow more slowly with worse activity, faster aging process, and less passage generations. HBV, chemicals and severe liver damage might influence their growth. It was reported that HBV can refrain the growth of stroma cells and hematopoietic stem cells in bone marrow[18,19]. Though MSCs in hepatitis B patients do not express HBsAg and HBcAg, it is still difficult to prove whether HBV-DNA is integrated into MSCs and affects their bionomics. Otherwise, influence of chemical medication on them cannot be ignored.
In conclusion, it is difficult to cultivate in vitro MSCs from hepatitis B patients. We should find a safe, quick and effective transplantation therapy to proliferate MSCs because of their limited number in the bone marrow.
ACKNOWLEDGMENTS
The authors thank all patients and volunteers for their participation in the study.
Footnotes
Supported by Technology Project Fund of Guangdong Province, No. 2003A3020303
S- Editor Liu Y L- Editor Wang XL E- Editor Zhou T
References
- 1.Pei XT. Stem cell biology. Beijing: Science Pub; 2003. [Google Scholar]
- 2.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 4.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 5.Bayes-Genis A, Salido M, Solé Ristol F, Puig M, Brossa V, Campreciós M, Corominas JM, Mariñoso ML, Baró T, Vela MC, et al. Host cell-derived cardiomyocytes in sex-mismatch cardiac allografts. Cardiovasc Res. 2002;56:404–410. doi: 10.1016/s0008-6363(02)00597-7. [DOI] [PubMed] [Google Scholar]
- 6.Mackenzie TC, Flake AW. Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis. 2001;27:601–604. doi: 10.1006/bcmd.2001.0424. [DOI] [PubMed] [Google Scholar]
- 7.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz EJ, Alexander GM, Prockop DJ, Azizi SA. Multipotential marrow stromal cells transduced to produce L-DOPA: engraftment in a rat model of Parkinson disease. Hum Gene Ther. 1999;10:2539–2549. doi: 10.1089/10430349950016870. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- 11.Yao P, Hu DR, Wang S, Wen W, Zhou YM, Gong LJ. Treatment of severe liver damage with autologous bone marrow stem cell transplantation in human patients. Zhonghua Gan Zang Bing Za Zhi. 2005;13:941–942. [PubMed] [Google Scholar]
- 12.Lee JW, Kim YH, Kim SH, Han SH, Hahn SB. Chondrogenic differentiation of mesenchymal stem cells and its clinical applications. Yonsei Med J. 2004;45 Suppl:41–47. doi: 10.3349/ymj.2004.45.Suppl.41. [DOI] [PubMed] [Google Scholar]
- 13.Soukup T, Mokrý J, Karbanová J, Pytlík R, Suchomel P, Kucerová L. Mesenchymal stem cells isolated from the human bone marrow: cultivation, phenotypic analysis and changes in proliferation kinetics. Acta Medica (Hradec Kralove) 2006;49:27–33. [PubMed] [Google Scholar]
- 14.Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;(174):249–282. [PubMed] [Google Scholar]
- 15.He NH, Zhao WL, Wang YM. Isolation, cultivation and biological identification of human fetal marrow mesenchymal stem cells. Zhonghua GanZangBing ZaZhi. 2005;13:213–217. [PubMed] [Google Scholar]
- 16.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao RC, Liao L, Han Q. Mechanisms of and perspectives on the mesenchymal stem cell in immunotherapy. J Lab Clin Med. 2004;143:284–291. doi: 10.1016/j.lab.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg HS, Bouffard P, Trépo C, Zeldis JB. In vitro inhibition of hemopoietic cell line growth by hepatitis B virus. J Virol. 1990;64:2577–2581. doi: 10.1128/jvi.64.6.2577-2581.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang ZW, Lin GW, Wu SM, Lin PD, Liang XH, Zhang WH. Virus hepatitis related bone marrow depression-27 cases reports. Shanghai Yixue. 1998;21:387–388. [Google Scholar]


