Abstract
AIM: To investigate the combined chemotherapeutic effects of celecoxib when used with 5-FU in vitro.
METHODS: Two human colon cancer cell lines (HCT-15 and HT-29) were treated with 5-FU and celecoxib, alone and in combination. The effects of each drug were evaluated using the MTT [3- (4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay, flow cytometry, and western blotting.
RESULTS: 5-FU and celecoxib showed a dose-dependent cytotoxic effect. When treated with 10-3 mol/L 5-FU (IC50) and celecoxib with its concentration ranging from 10-8 mol/L to 10-4 mol/L of celecoxib, cells showed reduced cytotoxic effect than 5-FU (10-3 mol/L) alone. Flow cytometry showed that celecoxib attenuated 5-FU induced accumulation of cells at subG1 phase. Western blot analyses for caspase-3 and poly (ADP-ribose) polymerase (PARP) cleavage showed that celecoxib attenuated 5-FU induced apoptosis. Western blot analyses for cell cycle molecules showed that G2/M arrest might be possible cause of 5-FU induced apoptosis and celecoxib attenuated 5-FU induced apoptosis via blocking of cell cycle progression to the G2/M phase, causing an accumulation of cells at the G1/S phase.
CONCLUSION: We found that celecoxib attenuated cytotoxic effect of 5-FU. Celecoxib might act via inhibition of cell cycle progression, thus preventing apoptosis induced by 5-FU.
Keywords: Antagonism, Celecoxib, Colorectal neoplasms, Fluorouracil
INTRODUCTION
Colorectal cancer is the second leading cause of cancer death in the United States[1]. Despite advances in medical practices and chemotherapeutic protocols, survival rates in cases of colorectal cancer have changed little over the last 20 years. In the treatment of colorectal cancer, 5-fluorouracil (5-FU), a potent inhibitor of thymidylate synthesis during DNA synthesis, is one of the most commonly used chemotherapeutic agents, but the overall response rate is only around 15% when used in adjuvant chemotherapy[2]. 5-FU is also used to treat various cancers, but results are not satisfactory. To increase the response rate, 5-FU is usually used in combination with other drugs, such as cyclophosphamide and methotrexate (breast cancer)[3], cisplatin (head and neck cancer)[4], or leucovorin (colorectal cancer)[5,6]. 5-FU has also been tested in combination chemotherapy with other drugs, which act via different mechanisms[7-9].
Thun et al[10] suggested that NSAIDs reduce the risk of colorectal cancer, and several investigators tested the application of NSAIDs as a new chemopreventive agent[11,12]. Celecoxib, a cyclooxygenase-2 (COX-2) specific inhibitor known to have antiproliferative effects on colorectal cancer[13,14], was also tested as a chemotherapeutic agent[15]. Milas et al[16] combined radiation therapy with a COX-2 inhibitor in a mouse cancer model, and observed an enhanced therapeutic response. In a recent clinical trial that incorporated the use of celecoxib as a combined chemotherapeutic drug, Altorki et al[17] tested celecoxib as a combined chemotherapeutic agent with paclitaxel and carboplatin on the early-stage non-small cell lung cancer. In their report, celecoxib showed additive or synergistic effect.
In this paper, we tested celecoxib as a possible candidate for combined chemotherapeutic agent to be used with 5-FU in the treatment of colorectal cancer. Thus, we treated HCT-15 and HT-29 human colon cancer cell lines with 5-FU and celecoxib and assessed their effects by MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay, flow cytometry and western blotting.
MATERIALS AND METHODS
Cell culture
The HCT-15 and HT-29 human colon cancer cell lines were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere. To examine the effects of 5-FU and celecoxib alone, the cells were treated with 10-6 mol/L, 10-5 mol/L, 10-4 mol/L, 10-3 mol/L, and 10-2 mol/L 5-FU and celecoxib for 24 h, respectively. To address the effects of 5-FU and celecoxib co-treatment, various concentrations of celecoxib were added immediately after treating cells with 10-3 mol/L 5-FU.
MTT assay
The MTT assay was performed as previously described[18]. In brief, HCT-15 and HT-29 human colon cancer cells were cultured in a 24-well plate (Corning Inc., Corning, NY) at a density of 5 × 104 cells per well. The cells were then treated with varying concentrations of 5-FU, or celecoxib, or both drugs. After 48 h, the cells were washed and treated with MTT. Plates were incubated in the dark for 4 h, and the absorbances were measured at 570 nm using a microtiter plate reader (Bio-Tek, Winooski, VT). To determine cell viability, percent viability was calculated as [(absorbance of drug-treated) sample/(control absorbance)] × 100.
Flow cytometry
Apoptosis detection and analysis of cell cycle distribution were performed by flow cytometry, as described previously[19,20]. Briefly, cells were incubated for 24 h in a medium without FBS to synchronize the cell cycle. Cells were then treated for 48 h in the medium containing 10% FBS with celecoxib, 5-FU or both, respectively. Cells were harvested by trypsinization, washed twice with PBS, incubated with 0.125% Triton X-100, and stained with propidium iodide (PI) in PBS containing 0.2 mg/mL RNase A. Stained cells were analyzed using a FACS calibur (Becton Dickinson, San Jose, CA, USA). For each sample, cells were counted until the count reached 10 000 cells in a predefined G1-gate. The percentages of cells in the subG1, G0/G1, S, and G2/M phases were determined using the CELLQUEST software.
Western blotting
Cells were incubated for 48 h with 10-5 mol/L celecoxib, 10-3 mol/L 5-FU or both drugs. Cells were harvested in cold phosphate-buffered saline (PBS), collected by centrifugation, resuspended in a homogenizing buffer (50 mmol/L Tris-HCl, pH 7.5; 150 mmol/L NaCl; 1 mmol/L EDTA; 0.1 mmol/L phenylmethylsulfonyl fluoride (PMSF); and 10 μg/ml of each of aprotinin, leupeptin, and pepstatin), and sonicated on ice. Protein concentrations of the homogenates were determined using the bicinochoninic acid (BCA) method (Pierce). Homogenates were diluted to a final concentration of 2 mg/ml with 2X reducing stop buffer (0.25 mol/L Tris-HCl, pH 6.8, 5 mmol/L EDTA, 5 mmol/L EGTA, 25 mmol/L dithiothreitol, 2% SDS, and 10% glycerol with bromophenol blue as the tracking dye). Samples (50 μg of protein) were resolved on SDS-polyacrylamide gels, and transferred to nitrocellulose. Blots were blocked in 5% nonfat dried milk in TBST (20 mmol/L Tris-HCl, pH 7.6, 137 mmol/L NaCl, 0.1% Tween 20) for 1 h at room temperature. Blots were then incubated with the respective primary antibodies directed against poly(ADP-ribose) polymerase (PARP) (1:1000, Cell Signaling Technology), active caspase-3 (1:1000, Cell Signaling Technology), cdk1 (1:1000, Neomarkers), cdk2 (1:1000, Neomarkers), cyclin A (1:1000, Neomarkers), and cyclin B (1:1000, Neomarkers), in the same buffer overnight at 4°C. The membranes were then washed three times with TBST and incubated with HRP-conjugated goat anti-mouse IgG (1:2000) monoclonal antibody or with HRP- conjugated goat anti-rabbit IgG (1:2000) polyclonal antibody for 2 h at room temperature. The membranes were then rinsed three times for 30 min with TBST, four times quickly with distilled water, and developed using the enhanced chemiluminescence method (ECL) (Amersham).
Reagents
Cell culture reagents were purchased from Gibco (Carlsbad, CA). Celecoxib was obtained as a generous gift from Dr. JH Chung (Kyung Hee Univ) and 5-FU was obtained from Sigma (St. Louis, MO). The stock solution of celecoxib was prepared by dissolving it in DMSO (Sigma). All other chemicals were purchased from Sigma unless otherwise stated.
RESULTS
MTT assay
To investigate the effects of 5-FU and celecoxib in isolation, various concentrations of 5-FU or celecoxib were added to HCT-15 or HT-29 cells. When treated with 10-6 mol/L, 10-5 mol/L, 10-4 mol/L, 10-3 mol/L or 10-2 mol/L 5-FU, the viabilities of HCT-15 cells were 83.6% ± 2.9%, 65.0% ± 4.1%, 69.9% ± 1.3%, 53.6% ± 3.6%, and 21.6% ± 3.7%, respectively. In HT-29 cells, the viabilities of cells for the same concentrations were 89.0% ± 2.0%, 83.8% ± 1.8%, 65.3% ± 4.7%, 43.1% ± 2.7%, and 24.5% ± 1.2%, respectively (Figure 1).
Figure 1.
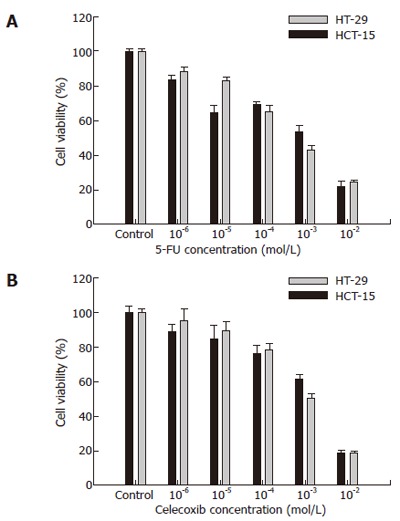
Viability of colon cancer cells treated with 5-FU and celecoxib. Cell viability was determined by MTT assay. Cell viabilities at various concentrations of 5-FU (A) and celecoxib (B) were evaluated using a cell viability index (%), which was defined as: (mean absorbance in the test group/mean absorbance in the control group) x 100. Black bars represent HCT-15 cells and white bars HT-29 cells. Results are mean ± SE.
When treated with 10-7 mol/L, 10-6 mol/L, 10-5 mol/L, 10-4 mol/L, or 10-3 mol/L celecoxib, the viabilities of HCT-15 cells were 89.37% ± 3.7%, 85.0% ± 7.8%, 76.4% ± 5.2%, 61.2% ± 3.3%, and 19.1% ± 2.0%, respectively, and of HT-29 cells were 95.6% ± 6.7%, 90.2% ± 4.6%, 78.9% ± 3.3%, 51.1% ± 2.7%, and 19.1% ± 0.8%, respectively (Figure 2).
Figure 2.
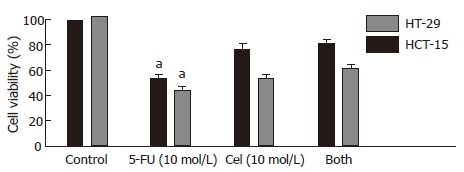
Combinatorial chemotherapy of HCT-15 or HT-29 human colon cancer cells with 5-FU and/or celecoxib. Cells were treated with 10-3 mol/L 5-FU, 10-5 mol/L celecoxib, or both. Cell viability was determined by the MTT assay, and expressed as the cell viability index (%) defined as: (mean absorbance in the test group/mean absorbance in the control group) × 100. Results are mean ± SE. aP < 0.05 vs Both.
Based on the MTT results, we opted for 10-3 mol/L 5-FU to be used as the drug-treated standard. Therefore, cells were treated with 10-3 mol/L 5-FU and various concentrations of celecoxib. Cells treated with celecoxib ranging in the concentration of 10-8 mol/L to 10-4 mol/L showed antagonistic effects on the 10-3 mol/L 5-FU-treated cells. But 10-3 mol/L celecoxib showed synergistic effects (data not shown) (Figure 3).
Figure 3.
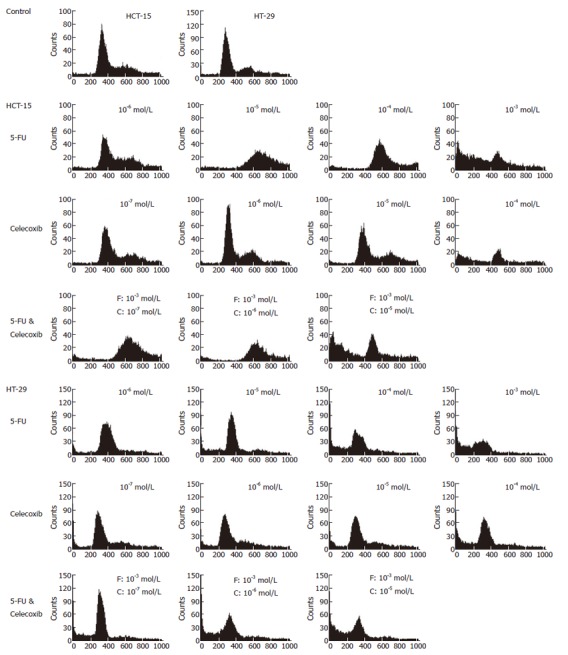
Flow cytometry of human colon cancer cells. Cells were treated with various concentrations of 5-FU, celecoxib, and both drugs. The cells were washed once with PBS, centrifuged and stained with propidium iodide, and then analyzed using a FACSCalibur. F represents 5-FU, and C celecoxib.
Flow cytometry
We analyzed cell cycle distributions by flow cytometry to investigate the effects of 5-FU, celecoxib, or co-treatment of both drugs. In HCT-15 cells, 5-FU induced accumulation of cells at subG1 phase with 10-3 mol/L 5-FU. When treated with 10-3 mol/L 5-FU and 10-7 mol/L or 10-6 mol/L celecoxib, the cell population in the subG1 phase was reduced (Figure 4). These results show that celecoxib attenuates the cell death induced by 5-FU (SubG1 phase fraction; 43.33% in 10-3 mol/L 5-FU, and 3.88 and 4.18% in 5-FU with 10-7 mol/L or 10-6 mol/L celecoxib, respectively). However, at higher celecoxib concentrations cell death was increased by 5-FU treatment (data not shown). In HT-29 cells, co-treatment of celecoxib reduced the accumulation of cells at subG1 phase (SubG1 phase fraction; 68.27% in 10-3 mol/L 5-FU, and 8.29, 9.66, and 9.68% in 5-FU with 10-7 mol/L, 10-6 mol/L, or 10-5 mol/L celecoxib, respectively) (Figure 4, Table 1).
Figure 4.
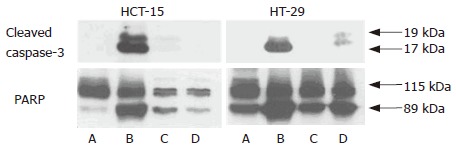
Western blotting of apoptosis molecules. A: Control; B: 10-3 mol/L 5-FU; C: 10-5 mol/L Celecoxib; D: 5-FU (10-3 mol/L) and celecoxib (10-5 mol/L). HCT-15 and HT-29 human colon cancer cell lines were treated with 10-3 mol/L 5-FU, 10-5 mol/L celecoxib, and 5-FU (10-3 mol/L) and celecoxib (10-5 mol/L). Western blotting for apoptosis-related molecules was performed as described in ‘Materials and Methods’. Co-treatment with celecoxib attenuated the caspase-3 expression and PARP cleavage.
Table 1.
Cell cycle distribution of HCT-15 and HT-29 cells after treatment with various concentrations of 5-FU, celecoxib and 5-FU and celecoxib
| HCT-15 | SubG1 (%) | G0/G1 (%) | S (%) | G2/M (%) | |
| Control | 2.75 | 51.94 | 14.61 | 30.90 | |
| 5-FU | 10-6 mol/L | 2.49 | 37.35 | 18.93 | 41.40 |
| 10-5 mol/L | 2.97 | 0.91 | 11.25 | 84.96 | |
| 10-4 mol/L | 2.77 | 0.26 | 27.09 | 70.13 | |
| 10-3 mol/L | 43.33 | 15.93 | 20.54 | 20.55 | |
| Celecoxib | 10-7 mol/L | 1.95 | 42.00 | 17.54 | 38.87 |
| 10-6 mol/L | 4.14 | 58.80 | 15.85 | 21.60 | |
| 10-5 mol/L | 1.70 | 37.45 | 15.96 | 45.32 | |
| 10-4 mol/L | 31.99 | 7.45 | 33.10 | 27.67 | |
| 5-FU (10-3 mol/L) & Celecoxib | 10-7 mol/L | 3.88 | 0.46 | 14.48 | 81.43 |
| 10-6 mol/L | 4.18 | 0.56 | 32.86 | 62.73 | |
| 10-5 mol/L | 40.46 | 6.95 | 28.35 | 24.55 | |
| HT-29 | SubG1 (%) | G0/G1 (%) | S (%) | G2/M (%) | |
| Control | 2.12 | 60.49 | 4.62 | 32.84 | |
| 5-FU | 10-6 mol/L | 8.92 | 73.77 | 3.73 | 13.85 |
| 10-5 mol/L | 17.51 | 62.56 | 3.13 | 17.13 | |
| 10-4 mol/L | 37.70 | 47.87 | 3.63 | 10.87 | |
| 10-3 mol/L | 68.27 | 25.69 | 1.68 | 4.61 | |
| Celecoxib | 10-7 mol/L | 8.29 | 62.37 | 6.11 | 23.53 |
| 10-6 mol/L | 9.66 | 60.02 | 7.24 | 23.29 | |
| 10-5 mol/L | 9.68 | 60.69 | 6.39 | 23.44 | |
| 10-4 mol/L | 26.10 | 54.30 | 4.02 | 15.76 | |
| 5-FU (10-3 mol/L) & Celecoxib | 10-7 mol/L | 18.78 | 69.39 | 1.89 | 9.99 |
| 10-6 mol/L | 36.15 | 46.46 | 3.16 | 14.35 | |
| 10-5 mol/L | 43.34 | 41.34 | 2.74 | 12.61 |
Western blotting
MTT-based cytotoxicity assays and flow cytometry showed that treatment with celecoxib attenuated 5-FU-induced cytotoxicity. So we examined caspase-3 expression and PARP cleavage, as markers of apoptosis. PARP is a 112-kDa nuclear protein that is cleaved specifically by activated caspase-3 and -6 into 89-kDa and 29-kDa apoptotic fragments. 5-FU induced caspase-3 expression and PARP cleavage but celecoxib attenuated these changes and thus prevented the apoptosis of colon cancer cells (Figure 5).
Figure 5.
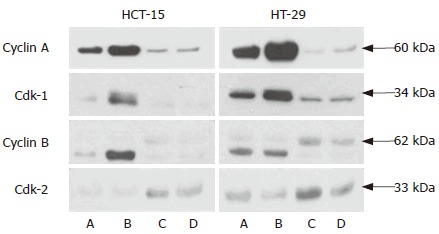
Western blotting of cell cycle-regulatory molecules. A: Control; B: 10-3 mol/L 5-FU; C: 10-5 mol/L Celecoxib; D: 5-FU (10-3 mol/L ) and celecoxib (10-5 mol/L ). HCT-15 and HT-29 human colon cancer cells were treated with 10-3 mol/L 5-FU, 10-5 mol/L celecoxib, and the two in combination. Western blotting for cdk1, cyclin A, cyclin B, and cdk2 were performed as described in ‘Materials and Methods’. Celecoxib reduced G2/M phase accumulation, and increased the G1/S phase protein, cdk-2.
We also investigated the expressions of several cell cycle-regulatory molecules. Cell cycle progression is mediated by cyclins and Cdks. Cdk1 with cyclin A and B modulates the G2/M transition and cdk2 with cyclin E controls the G0/G1 transition. We found that the expressions of cdk1, cyclin A and cyclin B were increased in the 5-FU only treated group and celecoxib attenuated the expressions of these molecules. Cdk2 was found to be elevated in all celecoxib treated groups. These results indicated that cell cycle progression is inhibited by celecoxib, and that this might be a cause of its antagonistic effect. We also performed another set of experiments at a 5-FU concentration of 10-4 mol/L (data not shown). The results were similar to those above results.
DISCUSSION
Although 5-FU is currently the first-line agent for colorectal cancer, the overall response rate to 5-FU in adjuvant treatment is less than 15%[2]. This lack of an acceptable response has stimulated intensive development effort to develop new cancer drugs and upon new combined regimens in colorectal cancer treatment. Many efforts have been made to combine 5-FU with second-line agents such as, cisplatin, interferon, leucovorin, methotrexate, N-phosphonoacetyl-L-aspartate (PALA) and uridine[21]. However, results have not been satisfactory. Recently, a combined celecoxib/radiation therapy showed an improved response in a mouse tumor model[16]. The first combined celecoxib with paclitaxel and carboplatin clinical chemotherapeutic trial was successful[17]. In their study, celecoxib was given concomitantly with paclitaxel and carboplatin to non-small-cell lung cancer patients preoperatively. The results obtained were very promising.
In the present study, we examined the effect of celecoxib on 5-FU-induced cell death in two human colon cancer lines. Interestingly, the MTT assay showed that celecoxib antagonized the cytotoxic effect of 10-3 mol/L 5-FU. So we investigated the mechanism underlying this antagonism by flow cytometry and western blot. First, we focused apoptosis signal and cell cycle regulation. By flow cytometry, 5-FU treatment increased cell accumulation at subG1 phase, which indicate apoptosis in these cells. Celecoxib decreased apoptotic cells at subG1 phase. In western blot analyses, antagonistic effect of celecoxib was proved. Co-treatment of celecoxib with 5-FU attenuated the caspase-3 expression and PARP cleavage. These results well correspondence with flow cytometry data.
It is reported that down-regulation of cyclin B1 expression is observed after treatment of celecoxib and p21 is up-regulated[22]. These changes were not dependent on p53. HCT-15 and HT-29 cells have mutant p53. Mutated p53 is often expressed in variety of human tumors and contribute to malignant process by loss of tumor suppressor function and gain of novel functions that enhance transformed properties of cells[23]. Swamy et al[24] reported that celecoxib increases nuclear localization of active p53. The treatment of celecoxib in HCT-15 and HT-29 cells might involve inhibition of function of mutated p53.
Although we examined the cell cycle distribution by flow cytometry, these results cannot fully explain involvement of cell cycle arrest as a mechanism of antagonistic action of celecoxib. So we also studied the cell cycle regulatory protein expressions. In western blot analyses, cyclin A, cyclin B and cdk1 expressions were increased, which indicates that cell cycle progression was arrested at the G2/M phase. Celecoxib treatment inhibited cell cycle progression at the G2/M phase, and reduced the expressions of cyclin A, cyclin B and cdk1, but the expression of cdk2, which regulates the G1/S transition, was increased. Our results are similar to study by Peng et al[25]. According to the studies by Peng et al[25], celecoxib down-regulated the expression of CDK2 and CDK4 expression in HT-29 cells. Although we do not conduct on the experiment of CDK4, the results from CDK2 was similar to their study.
In this study, we investigated whether celecoxib has the potential to be used with 5-FU as a combined chemotherapeutic agent for the treatment of colorectal cancer. However, unfortunately, celecoxib was found to antagonize the effect of 5-FU in human colon cancer cells. Although our results are based on an in vitro study and sometimes in vitro data does not correspond with results in vivo results, the results of the present study indicate that celecoxib cannot be used with a cell cycle-arresting agents like 5-FU. Further studies are needed to clarify our results and studies in clinical setting focusing on the usefulness of celecoxib as a combined chemotherapeutic agent are also needed.
Footnotes
S-Editor Wang J L- Editor BS Anand E- Editor Chin GJ
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa R, Kusunoki M, Yanagi H, Noda M, Furuyama JI, Yamamura T, Hashimoto-Tamaoki T. Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy. Cancer Res. 2001;61:1029–1037. [PubMed] [Google Scholar]
- 3.Norum J. Adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF) in breast cancer--is it cost-effective. Acta Oncol. 2000;39:33–39. doi: 10.1080/028418600430941. [DOI] [PubMed] [Google Scholar]
- 4.de Mulder PH. The chemotherapy of head and neck cancer. Anticancer Drugs. 1999;10 Suppl 1:S33–S37. doi: 10.1097/00001813-199911001-00007. [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A, Louvet C, André T, Tournigand C, Krulik M. A review of GERCOD trials of bimonthly leucovorin plus 5-fluorouracil 48-h continuous infusion in advanced colorectal cancer: evolution of a regimen. Groupe d'Etude et de Recherche sur les Cancers de l'Ovaire et Digestifs (GERCOD) Eur J Cancer. 1998;34:619–626. doi: 10.1016/s0959-8049(97)00364-x. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 7.Mełeń-Mucha G. Effects of short term treatment with pentagastrin, proglumide, tamoxifen given separately or together with 5-fluorouracil on the growth in the murine transplantable Colon 38 cancer. Neoplasma. 2001;48:133–138. [PubMed] [Google Scholar]
- 8.Johnson KR, Young KK, Fan W. Antagonistic interplay between antimitotic and G1-S arresting agents observed in experimental combination therapy. Clin Cancer Res. 1999;5:2559–2565. [PubMed] [Google Scholar]
- 9.Chen XX, Lai MD, Zhang YL, Huang Q. Less cytotoxicity to combination therapy of 5-fluorouracil and cisplatin than 5-fluorouracil alone in human colon cancer cell lines. World J Gastroenterol. 2002;8:841–846. doi: 10.3748/wjg.v8.i5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thun MJ, Namboodiri MM, Heath CW. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, Speizer FE. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 12.Kubba AK. Non steroidal anti-inflammatory drugs and colorectal cancer: is there a way forward. Eur J Cancer. 1999;35:892–901. doi: 10.1016/s0959-8049(99)00054-4. [DOI] [PubMed] [Google Scholar]
- 13.Arico S, Pattingre S, Bauvy C, Gane P, Barbat A, Codogno P, Ogier-Denis E. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277:27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 14.Koki AT, Masferrer JL. Celecoxib: a specific COX-2 inhibitor with anticancer properties. Cancer Control. 2002;9:28–35. doi: 10.1177/107327480200902S04. [DOI] [PubMed] [Google Scholar]
- 15.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 16.Milas L, Kishi K, Hunter N, Mason K, Masferrer JL, Tofilon PJ. Enhancement of tumor response to gamma-radiation by an inhibitor of cyclooxygenase-2 enzyme. J Natl Cancer Inst. 1999;91:1501–1504. doi: 10.1093/jnci/91.17.1501. [DOI] [PubMed] [Google Scholar]
- 17.Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah K, Pasmantier MW, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 18.Yoo YM, Yim SV, Kim SS, Jang HY, Lea HZ, Hwang GC, Kim JW, Kim SA, Lee HJ, Kim CJ, et al. Melatonin suppresses NO-induced apoptosis via induction of Bcl-2 expression in PGT-beta immortalized pineal cells. J Pineal Res. 2002;33:146–150. doi: 10.1034/j.1600-079x.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 19.Darzynkiewicz Z, Bruno S, Del Bino G, Gorczyca W, Hotz MA, Lassota P, Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13:795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- 20.Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 21.Calabresi P, Chabner BA. Chemotherapy of neoplastic diseases. In Goodman&Gilman's The pharmacological basis of therapeutics, 10th ed, New York: McGraw-Hill Companies; 2001. [Google Scholar]
- 22.Dvory-Sobol H, Cohen-Noyman E, Kazanov D, Figer A, Birkenfeld S, Madar-Shapiro L, Benamouzig R, Arber N. Celecoxib leads to G2/M arrest by induction of p21 and down-regulation of cyclin B1 expression in a p53-independent manner. Eur J Cancer. 2006;42:422–426. doi: 10.1016/j.ejca.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Pugacheva EN, Ivanov AV, Kravchenko JE, Kopnin BP, Levine AJ, Chumakov PM. Novel gain of function activity of p53 mutants: activation of the dUTPase gene expression leading to resistance to 5-fluorouracil. Oncogene. 2002;21:4595–4600. doi: 10.1038/sj.onc.1205704. [DOI] [PubMed] [Google Scholar]
- 24.Swamy MV, Herzog CR, Rao CV. Inhibition of COX-2 in colon cancer cell lines by celecoxib increases the nuclear localization of active p53. Cancer Res. 2003;63:5239–5242. [PubMed] [Google Scholar]
- 25.Peng J, Zhang GY, Xiao ZQ. Effects of celecoxib on the proliferation and apoptosis of human colorectal cancer cell line HT-29. ZhongNan DaXue XueBao YiXueBan. 2004;29:261–265. [PubMed] [Google Scholar]


