Abstract
AIM: To determine the effect of pioglitazone, a specific peroxisome proliferator-activated receptor-γ (PPARγ) ligand, on development of severe acute pancreatitis (SAP) and expression of nuclear factor-kappa B (NF-κB) and intercellular adhesion molecule-1 (ICAM-1) in the pancreas.
METHODS: Male Sprague-Dawley (SD) rats (160-200 g) were randomly allocated into three groups (n = 18 in each group): severe acute pancreatitis group, pioglitazone group, sham group. SAP was induced by retrograde infusion of 1 mL/kg body weight 5% sodium taurocholate (STC) into the biliopancreatic duct of male SD rats. Pioglitazone was injected intraperitoneally two hours piror to STC infusion. Blood and ascites were obtained for detecting amylase and ascitic capacity. Pancreatic wet/dry weight ratio, expression of NF-κB and ICAM-1 in pancreatic tissues were detected by immunohistochemical staining. Pancreatic tissue samples were stained with hematoxylin and eosin (HE) for routine optic microscopy.
RESULTS: Sham group displayed normal pancreatic structure. SAP group showed diffuse hemorrhage, necrosis and severe edema in focal areas of pancreas. There was obvious adipo-saponification in abdominal cavity. Characteristics such as pancreatic hemorrhage, necrosis, severe edema and adipo-saponification were found in pioglitazone group, but the levels of those injuries were lower in pioglitazone group than those in SAP group. The wet/dry pancreatic weight ratio, ascetic capacity, serum and ascitic activities of anylase in the SAP group were significantly higher than those in the sham group and pioglitazone group respectively (6969.50 ± 1368.99 vs 2104.67 ± 377.16, 3.99 ± 1.22 vs 2.48 ± 0.74, P < 0.01 or P < 0.05). According to Kusske criteria, the pancreatic histologic score showed that interstitial edema, inflammatory infiltration, parenchyma necrosis and parenchyma hommorrhage in SAP group significantly differed from those in the sham group and pioglitazone group (7.17 ± 1.83 vs 0.50 ± 0.55, 7.67 ± 0.82 vs 6.83 ± 0.75, P < 0.01, P < 0.05. The expression of NF-κB and ICAM-1 in sham group was lower than that in SAP group and pioglitazone group (0.50 ± 0.55 vs 33 ± 1.21, P < 0.01). There was a significant difference in the expression of NF-κB and ICAM-1 between SAP group and pioglitazone group (7.50 ± 1.05 vs 11.33 ± 1.75, 0.80 ± 0.53 vs 1.36 ± 0.54, P < 0.01 or P < 0.05) at 12 h after the induction of pancreatitis.
CONCLUSION: Pioglitazone attenuates the severity of SAP. The beneficial effect of pioglitazone is multifactorial due to its anti-inflammatory activities, most likely through the inhibition of ICAM-1 expression and NF-κB activation. Specific ligands of PPARγ may represent the novel and effective means of clinical therapy for SAP.
Keywords: Sodium taurocholate, Severe acute pancreatitis, Peroxisome proliferators-activated receptor- γ ligand, Nuclear transcription factor-κB, Intercellular adhesion molecule-1
INTRODUCTION
The morbidity and mortality of acute pancreatitis (AP) are high and the clinical course AP is unpredictable. Approximately 25% of AP patients develop severe inflammation with pancreatic and peripancreatic fat necrosis that requires intensive care. Effective treatment strategies are lack due to a relatively poor understanding of its exact pathogenesis[1].
The pathophysiology of acute pancreatitis involves a number of inflammatory mediators, which contribute to the initiation and progression of both local pancreatic destruction and systemic manifestations. Various proinflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), appear to play a major role in acute pancreatitis[2,3]. The inhibition of cytokine production may decrease the severity of pancreatitis[4,5].
It was recently reported that peroxisome proliferator-activated receptors (PPARs) play a modulatory role in the inflammatory response of different organs[6]. The PPAR family consists of at least three different isoforms; PPARα, PPARδ, and PPARγ[7]. PPARγ is predominantly detectable in adipose tissue, but it is also expressed in other tissues, including pancreatic tissue. When activated by a specific ligand, PPARs heterodimerize with retinoid X receptor and then bind to peroxisome proliferator response element leading to changes in the transcription of target genes involved in lipid metabolism, glucose homeostasis, cell proliferation and differentiation, and inflammatory response. PPARs are activated by natural ligands such as fatty acids, eicosanoids, oxidized fatty acids, and pharmacological compounds such as glitazones. Pioglitazone is a member of glitazones and has been used to increase the sensitivity to insulin in clinical practice[8].
Recent experimental studies appeared to have shed some light on the intracellular signaling pathway in the inflammatory cascade of AP[9,10]. For example, there is evidence that NF-κB plays a crucial role in the initiation of AP, not only in pancreatic acinar cells and monocytes/macrophages but also in specific distant organs, such as the lung. NF-κB is able to mediate a variety of inflammatory mediators involved in AP, including cytokines and adhesion molecules[11-13].
In addition, intercellular adhesion molecule-1 (ICAM-1) has been reported to be up-regulated and involved in the evolution of acute pancreatitis by recruiting leukocytes into the area of inflammation[14,15]. NF-κB activity increases with pancreatitis. The inhibition of NF-κB has been shown to ameliorate the inflammatory effects of pancreatitis[16-19].
PPARγ plays a critical role in adipogenesis and glucose metabolism[20,21]. In addition to these effects, it has been reported that PPARγ ligands possess in vitro anti-inflammatory properties. For instance, PPARγ ligands reduce the secretion of IL-1β, IL-6, and TNF-α in monocytes and macrophages[22], and the expression of ICAM-1 in endothelial cells[23], in part by antagonizing the activities of transcription factors such as AP-1 and NF-κB[22,24].
Several reports have demonstrated an anti-inflammatory action of the specific ligands of PPARγ. Activators of PPARγ inhibit the generation of pro-inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-6[22] and this effect seems to be dependent on the inhibition of NF-κB pathway[25].
The aim of the present study was to determine the effect of pioglitazone, a specific PPARγ ligand, on development of sodium taurocholate (STC)-induced SAP, to assess the effect of pretreatment with pioglitazone on expression of ICAM-1 inpanreas, and to evaluate the effect of pretreatment with pioglitazone on expression of NF-κB in pancreas.
In this study, we made a well-characterized secre-tagogue-induced murine model of pancreatitis to investigate the anti-inflammatory effects of pioglitazone, a PPARγ ligand. Pretreatment with pioglitazone markedly decreased the severity of pancreatitis, most likely through the inhibition of NF-κB activation and ICAM-1 expression.
Our results suggest that PPARγ can be used in the treatment of acute pancreatitis. Moreover, these findings further demonstrate NF-κB and ICAM-1 play a role in the pathogenesis of pancreatitis.
MATERIALS AND METHODS
Experimental model of pancreatitis
Fifty-four male Sprague-Dawley (SD) rats (Animal Center, Medical College of Nanchang University), weighing 160-200 g, were used in this experiment. Animals were housed in cages under standard conditions at room temperature in a 12-h light-dark cycle. Prior to the experiments, rats were deprived of food with free access to water. All procedures including ketamine hydrochloride intra-peritoneal injection (10 mg/100 g) for anesthesia were performed under sterile conditions. SD rats were divided into severe acute pancreatitis (SAP) group, pretreatment group with pioglitazone (PP) and sham group. One mL/kg body weight of sodium taurocholate (STC, 50 g/L, Sigma) was retrograde injected into the biliopancreatic duct of the rats to induce SAP, 10% dimethyl sulphoxide (DMSO, 1 mL/100 g) was injected intraperitoneally two hours prior to STC injection[26]. PP group received pioglitazone replacing 10% DMSO administered intraperitoneally two hours prior to STC injection (2 mg/100 g). Rats in the sham group underwent operation with nothing infused. The pancreas was flipped and striked gently three times. After operation, rats were fasted with free access to water and killed by abdominal aorta exsanguination 3, 6 and 12 h after the induction of pancreatitis.
Serum and ascites were obtained to measure amylase levels and ascitic capacity. Pancreata were quickly removed and fixed in 10% formalin for morphologic studies. Portions of the pancreas were freshly processed for determining pancreatic water contents.
Macroscopic assessment of pancreas
Edema, hemorrhage and necrosis of the pancreas were each graded from 0 to 3 as follows as previously described[27]: pancreatic edema: 0 = absent, 1 = mild gland swelling with no cysts, 2 = moderate gland swelling with cysts in the duodenal region, 3 = severe gland swelling with multiple cysts extending to the splenic region; pancreatic fat necrosis: 0 = absent, 1 = confined to the pancreas, 2 = extending to the omentum, 3 = widespread in the retroperitoneum, pancreatic hemorrhage: 0 = absent, 1 = periductal punctate, 2 = diffuse in pancreas, 3 = peripancreatic or pancreatic blood with clots.
Histological scoring of pancreas
Pancreas was removed from each rat and fixed in 10% buffered formalin at 4°C overnight. The pancreas was then embedded in paraffin. Full-length (4-μm) sections were taken and stained with hematoxylin and eosin for histologic evaluation. Edema, inflammation, hemorrhage and necrosis of the pancreas were each graded from 0 to 3 as follows as previously described[28]: edema: 0 = absent, 1 = focally increased between lobules, 2 = diffusely increased between lobules, 3 = tense acini and widely separated lobules, 4 = gross lobular separation; inflammation: 0 = absent, 1 = around ductal margins, 2 = in parenchyma (< 50% of lobules), 3 = in parenchyma (51%-75% of lobules), 4 = massive collections and abscesses; hemorrhage: 0 = absent, 1 = blood in parenchyma(< 25%), 2 = blood in parenchyma (25%-50%), 3 = blood in parenchyma (50%-75%), 4 = blood in 100% of lobules; necrosis: 0 = absent, 1 = periductal parenchymal destruction, 2 = focal parenchymal necrosis (< 20%), 3 = diffuse loss of lobules (20%-50%), 4 = severe loss of lobules (> 50%).
Biochemical assay
Serum amylase levels were measured with the CNPG3 amylase test and spectrophotometrically according to the manufacturer’s instructions.
Pancreatic water content
The wet/dry weight ratio of the pancreas was obtained when the freshly prepared pancreatic tissue was dried for 24 h at 90°C.
NF-κB p65 in pancreatic tissue
NF-κB p65 in pancreatic tissues was detected by immunohistochemistry. In brief, endogenous peroxidase was blocked for 30 min with 0.3% methanol-H2O2. After several washes, samples were incubated overnight in a humidified chamber at 4°C with the rabbit and mouse antibody against NF-κB p65 diluted 1:100, followed by non-biotinylated second antibody diluted 1:200 for 30 min. Negative controls were carried out by omission of the primary antibody. Several sections were counterstained with hematoxylin.
ICAM-1 in pancreatic tissue
ICAM-1 in pancreatic tissue was determined by immunohistochemistry. In brief, endogenous peroxidase was blocked for 30 min with 0.3% H2O2. After several washes, samples were incubated overnight in a humidified chamber at 4°C with rabbit antibody against ICAM-1 diluted 1:50, followed by non-biotinylated second antibody diluted 1:200 for 30 min. Several sections were counterstained with hematoxylin.
Statistical analysis
The values were expressed as mean ± SE. As the values in some data sets were not normally distributed or exhibited unequal standard deviations, the nonparametric Wilcoxon rank sum test was used to test for statistical differences between groups. P < 0.05 was considered statistically significant.
RESULTS
Amylase assay and ascites
After the establishment of the model, in SAP group, the serum level of amylase was significantly higher in SAP group and PP group than that in sham group at different time points (P < 0.05, Table 1). The serum levels of amylase in PP group were significantly lower than those in SAP group at 12 h after the establishment of the studied model (P < 0.01, Table 1).
Table 1.
Levels of amylase in three subgroups (mean ± SE)
| Group |
Amylase (IU/L) |
||
| 3 | 6 | 12 | |
| Sham | 2376.00 ± 413.88 | 2104.67 ± 377.16 | 1879.83 ± 528.42 |
| PP | 5364.83 ± 14082bc | 5645.67 ± 1907.8b | 2979.50 ± 1080.12 |
| SAP | 5053.33 ± 2632.1c | 6969.50 ± 1368.9b | 7598.00 ± 1071.92d |
aP < 0.05,
P < 0.01 vs sham,
P < 0.05 vs 12 h,
P < 0.01 vs PP, fP < 0.01 vs 12 h.
No ascites was found in sham group. No difference in ascites was found between SAP group and PP group at all time points (P > 0.05, Table 2).
Table 2.
Ascites in three subgroups (mean ± SE)
| Group |
Ascites (mL) |
||
| 3 | 6 | 12 | |
| Sham | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| PP | 4.42 ± 1.85a | 4.87 ± 1.04a | 5.70 ± 1.87a |
| SAP | 6.70 ± 1.70b | 7.67 ± 2.47b | 8.37 ± 1.19b |
P < 0.05,
P < 0.01 vs sham.
Morphological examination
After infusion of STC, the pancreas was enlarged with a visible collection of edematous fluid and the pancreatic mass was almost doubled. Three and six hours after establishment of the studied model, typical pathological changes in SAP, such as a large number of inflammatory cells, edema, hemorrhage, necrosis, a large amount of ascites was found. The same pathological changes were observed in PP group (Table 3, Figures 1, 2 and 3).
Table 3.
Morphological score (mean ± SE)
| Group |
Time point |
||
| 3 h | 6 h | 12 h | |
| Sham | 0.50 ± 0.55 | 0.17 ± 0.41 | 0.17 ± 0.41 |
| PP | 5.50 ± 1.64b | 6.83 ± 0.75b | 4.50 ± 2.07ab |
| SAP | 7.17 ± 1.83b | 7.67 ± 0.82b | 7.17 ± 1.47bd |
P < 0.05 vs PP at 6 h;
P < 0.01 vs Sham,
P < 0.01 vs PP at 12 h.
Figure 1.
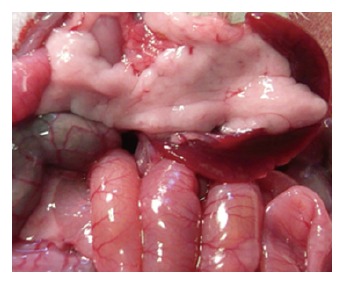
Normal pancreas in sham group after the establishment of the studied model.
Figure 2.
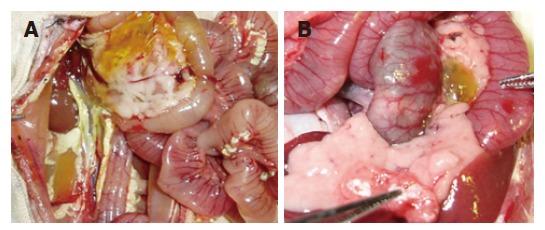
Edema, hemorrhage, necrosis, large amount of ascites in SAP group (A) and PP group (B) after the establishment of the studied model.
Figure 3.
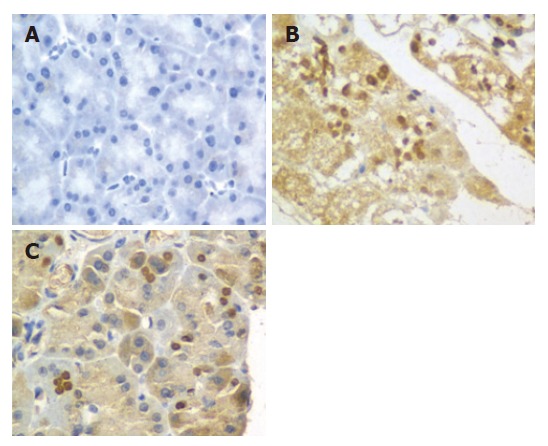
Expression of p65 in sham group (A), SAP group (B), and PP group (C) after the establishment of the studied model (10 × 400).
Histological examination
Under light microscope, interlobular and intralobular edema, vacuolization of acinar cells, and moderate perivascular and scare diffuse leukocyte infiltration were observed. Inflammatory changes in the pancreas were significantly less in PP group than in SAP group (Table 4).
Table 4.
Histological score (mean ± SE)
| Group |
Time point |
||
| 3 | 6 | 12 | |
| Sham | 1.67 ± 2.16 | 0.50 ± 0.55 | 0.50 ± 0.55 |
| PP | 8.83 ± 1.94b | 7.83 ± 0.75b | 7.50 ± 1.05b |
| SAP | 9.67 ± 1.37b | 10.33 ± 1.21bd | 11.33 ± 1.75bd |
P < 0.01 vs sham,
P < 0.01 vs PP.
Pancreatic water content
The extent of pancreatic edema and water content was estimated as the wet/dry weight ratio of the tissue (Table 5). In sham group and PP group, the pancreatic water content was reduced. However, it was increased in SAP group (▲P < 0.05 vs the PP group and sham group at 12 h, *P < 0.05 vs PP group at 3 h).
Table 5.
Pancreatic water content (mean ± SE)
| Group |
ww/dw |
||
| 3 h | 6 h | 12 h | |
| Sham | 3.10 ± 1.06 | 2.70 ± 0.78 | 2.44 ± 0.68 |
| PP | 4.01 ± 1.32 | 2.88 ± 0.96 | 2.48 ± 0.74a |
| SAP | 3.22 ± 0.81 | 3.65 ± 1.72 | 3.99 ± 1.22c |
P < 0.05 vs PP at 3 h,
P < 0.05 vs PP and sham at 12 h.
Expression of NF-κBp65
The expression of NF-κB p65 at 3, 6 and 12 h was determined by immunohistochemistry. In sham group, no expression of NF-κB p65 in pancreas was found in pancreas at 6 h (Figure 3A). However, the expression of NF-κB p65 in pancreas was significantly increased in SAP and PP groups compared to sham group at all time points (P < 0.01). In SAP group, the expression of NF-κB p65 in pancreas was up-regulated and differed significantly at 6, 12 and 3 h (P < 0.01, Figure 3B). In PP group, the expression of NF-κB p65 in pancreas was the highest at 6 h (Figure 3C), but was significantly lower than that in SAP group. The expression of NF-κB p65 in pancreas in PP group was significantly lower at 12 h than that in SAP group (P < 0.01).
Expression of ICAM-1
In sham group, mild expression of ICAM-1 in pancreas was found only at 3 h. The expression of ICAM-1 in pancreas was significantly higher in SAP group than in sham group at all time points (P < 0.01). In SAP group, the expression of ICAM-1 in pancreas was up-regulated and differed significantly at 12 and 3 h (P < 0.01). However, in PP group, the expression of ICAM-1 in pancreas was the highest at 6 h, but was significantly lower than that in SAP group. The expression of ICAM-1 in pancreas differed significantly between PP group and SAP group at 12 h (P < 0.05, Table 6).
Table 6.
ICAM-1 expression in acinar cells of the pancreas (mean ± SE)
| Group |
Time point |
||
| 3 h | 6 h | 12 h | |
| Sham | 0.19 ± 0.10 | 0.24 ± 0.21 | 0.11 ± 0.12 |
| PP | 0.57 ± 0.21b | 0.86 ± 0.40b | 0.80 ± 0.53a |
| SAP | 0.73 ± 0.27b | 0.93 ± 0.41b | 1.36 ± 0.54bce |
P < 0.05,
P < 0.01 vs sham,
P < 0.05 vs PP,
P < 0.05 vs 3 h.
DISCUSSION
Although previous reports indicated that PPARγ agonists exhibit anti-inflammatory effects on AP in vitro and in vivo[22,29], the effect of PPARγ on AP has not been fully elucidated. This anti-inflammatory effect seems to be related to multiple mechanisms. Ricote et al[24] demonstrated that PPARγ ligands inhibit macrophage activation and inflammatory cytokine production. In our study, pancreatic PPARγ was induced and activated in STC-induced pancreatitis, suggesting that PPARγ plays a potential role in regulating acute inflammation.
In the present study, the serum levels of amylase and ascites decreased significantly in STC- induced pancreatitis after petreatment with pioglitazone. The pathological changes were less in pacreas such as the number of inflammatory cells, edema, hemorrhage, necrosis after pretreatment with pioglitazone. The reason for this is not entirely known, but it may be associated with an altered population of activated macrophages in the pancreas. The initiation of acute pancreatitis involves intrapancreatic activation of digestive zymogens and subsequent autodigestion, which triggers the sequestial activation of monocytes/macrophages within the pancreas and the overproduction of macrophages-derived inflammatory mediators, which result in local pancreatic injury[30,31].
Furthermore, PPARγ activators are negative regulators of macrophage activation[24] and induce macrophage apoptosis by interfering with the antiapoptotic NF-κB pathway[32]. Therefore, we postulate that the anti-inflammatory effect of pioglitazone in STC-induced pancreatitis may be due, in part, to the inhibition of macrophage activation. Further studies are required to determine whether pioglitazone affects pancreatic macrophage activation and PPARγ expression.
Pro-inflammatory cytokines play a main role in acute pancreatitis and its complications[33,34]. Failure of different organ systems is a frequent problem in SAP. The majority of fatalities in patients with SAP are associated with the failure of at least one or more organ systems. Organ failure in SAP has to be regarded as part of the inflammatory response following the liberation of activated enzymes from the pancreas.
NF-κB plays a key role in the transcriptional regulation of adhesion molecules, enzymes and cytokines involved in acute inflammatory diseases. NF-κB is a key transcription factor for the expression of various proinflammatory molecules such as chemokines, cytokines and adhesion molecules. This notion has recently been verified by adenoviral-mediated gene transfer of an active subunit, RelA/p65, into acinar cells into the rat pancreatic duct[35]. Over-expressed RelA/p65 protein transactivates NF-κB and induces pancreatic inflammation, a pathological state very similar to acute pancreatitis. In addition to the importance of NF-κB in the pathogenesis of acute pancreatitis, it was also reported that NF-κB activation in macrophages is a key event in the development of generalized complications and a cause of the high mortality of SAP. Studies also showed that glucocorticoid is one of the most potent inhibitors of NF-κB, which might be attributable to the inhibition of this key transcription factor both in acinar cells and in inflammatory cells such as monocytes/macrophages. Because the activation of NF-κB is an early event in acute pancreatitis, corticosteroid has beneficial effects on acute pancreatitis[36].
The transcription factor NF-kB is activated in the course of acute pancreatitis[16,37] and regulates several inflammatory processes by mediating the induction of numerous inflammatory mediators, including proinflammatory cytokines and ICAM-1[15,38,39]. Our results suggest that pioglitazone could attenuate the severity of STC-induced pancreatitis by inhibiting NF-κB. Therefore, the observed beneficial effects of pioglitazone could be mediated, in part, by inhibiting NF-κB activity.
Similarly, other studies have demonstrated that nonspecific NF-κB inhibitors ameliorate the course of experimental pancreatitis[16-18]. Our findings suggest that pioglitazone can inhibit NF-κB activity. The deleterious role of ICAM-1 in inducing acute pancreatitis has been reported[40]. Cerulein induced pancreatitis in ICAM-1 knockout mice showed reduced severity of pancreatitis and of pancreatitis-associated lung injury[14]. ICAM-1, present not only in endothelial cells but also in acinar cells of the pancreas, is up-regulated by cerulein and mediates direct binding of neutrophils to acinar cells[15].
Our present study demonstrated that the expression of ICAM-1 was increased in acinar cells of pancreas with STC-induced pancreatitis, which is consistent with previous reports[14,15,40]. Decreased expression of ICAM-1 in pancreas after treatment with pioglitazone provides a possible explanation for the decreased pancreatic infiltration of inflammatory cells, especially neutrophils.
In summary, pioglitazone, a specific PPARγ ligand, ameliorates significantly the severity of STC-induced SAP. The expression of NF-κB and ICAM-1 in pancreas can be significantly inhibited by pioglitazone. Treatment of acute pancreatitis with pioglitazone may offer a new therapeutic option.
Footnotes
Supported by Health Bureau Foundation of Jiangxi Province, No.20045019 and Natural Science Foundation of Jiangxi Province, No.0640069
S- Editor Wang J L- Editor Wang XL E- Editor Chin GJ
References
- 1.Chiang DT, Anozie A, Fleming WR, Kiroff GK. Comparative study on acute pancreatitis management. ANZ J Surg. 2004;74:218–221. doi: 10.1111/j.1445-2197.2004.02958.x. [DOI] [PubMed] [Google Scholar]
- 2.Saluja AK. Pathophysiology of pancreatitis. Role of cytokines and other mediators of inflammation. Digestion. 1999;60 Suppl 1:27–33. doi: 10.1159/000051450. [DOI] [PubMed] [Google Scholar]
- 3.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 4.Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–967. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 5.Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 6.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors and inflammation: from basic science to clinical applications. Int J Obes Relat Metab Disord. 2003;27 Suppl 3:S41–S45. doi: 10.1038/sj.ijo.0802499. [DOI] [PubMed] [Google Scholar]
- 7.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 9.Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1157–G1162. doi: 10.1152/ajpgi.2001.280.6.G1157. [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Seo JY, Kim KH. NF-kappaB and cytokines in pancreatic acinar cells. J Korean Med Sci. 2000;15 Suppl:S53–S54. doi: 10.3346/jkms.2000.15.S.S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffray C, Yang J, Carter G, Mendez C, Norman J. Pancreatic elastase activates pulmonary nuclear factor kappa B and inhibitory kappa B, mimicking pancreatitis-associated adult respiratory distress syndrome. Surgery. 2000;128:225–231. doi: 10.1067/msy.2000.107419. [DOI] [PubMed] [Google Scholar]
- 12.Rakonczay Z, Jármay K, Kaszaki J, Mándi Y, Duda E, Hegyi P, Boros I, Lonovics J, Takács T. NF-kappaB activation is detrimental in arginine-induced acute pancreatitis. Free Radic Biol Med. 2003;34:696–709. doi: 10.1016/s0891-5849(02)01373-4. [DOI] [PubMed] [Google Scholar]
- 13.Altavilla D, Famulari C, Passaniti M, Campo GM, Macrì A, Seminara P, Marini H, Calò M, Santamaria LB, Bono D, et al. Lipid peroxidation inhibition reduces NF-kappaB activation and attenuates cerulein-induced pancreatitis. Free Radic Res. 2003;37:425–435. doi: 10.1080/1071576031000070093. [DOI] [PubMed] [Google Scholar]
- 14.Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- 15.Zaninovic V, Gukovskaya AS, Gukovsky I, Mouria M, Pandol SJ. Cerulein upregulates ICAM-1 in pancreatic acinar cells, which mediates neutrophil adhesion to these cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G666–G676. doi: 10.1152/ajpgi.2000.279.4.G666. [DOI] [PubMed] [Google Scholar]
- 16.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 17.Satoh A, Shimosegawa T, Fujita M, Kimura K, Masamune A, Koizumi M, Toyota T. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–258. doi: 10.1136/gut.44.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya AS, Pandol SJ. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1197–G1208. doi: 10.1152/ajpgi.2001.280.6.G1197. [DOI] [PubMed] [Google Scholar]
- 19.Ethridge RT, Hashimoto K, Chung DH, Ehlers RA, Rajaraman S, Evers BM. Selective inhibition of NF-kappaB attenuates the severity of cerulein-induced acute pancreatitis. J Am Coll Surg. 2002;195:497–505. doi: 10.1016/s1072-7515(02)01222-x. [DOI] [PubMed] [Google Scholar]
- 20.Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 21.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 23.Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 24.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 25.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldacre MJ, Roberts SE. Hospital admission for acute pancreatitis in an English population, 1963-98: database study of incidence and mortality. BMJ. 2004;328:1466–1469. doi: 10.1136/bmj.328.7454.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg. 1996;171:274–280. doi: 10.1016/s0002-9610(97)89568-2. [DOI] [PubMed] [Google Scholar]
- 28.Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–288; discussion 289. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K, Ethridge RT, Saito H, Rajaraman S, Evers BM. The PPARgamma ligand, 15d-PGJ2, attenuates the severity of cerulein-induced acute pancreatitis. Pancreas. 2003;27:58–66. doi: 10.1097/00006676-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788. doi: 10.1023/a:1018886120711. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, Fruchart JC, Chapman J, Najib J, Staels B. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273:25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 33.Osman MO, Gesser B, Mortensen JT, Matsushima K, Jensen SL, Larsen CG. Profiles of pro-inflammatory cytokines in the serum of rabbits after experimentally induced acute pancreatitis. Cytokine. 2002;17:53–59. doi: 10.1006/cyto.2001.0977. [DOI] [PubMed] [Google Scholar]
- 34.Bidarkundi GK, Wig JD, Bhatnagar A, Majumdar S. Clinical relevance of intracellular cytokines IL-6 and IL-12 in acute pancreatitis, and correlation with APACHE III score. Br J Biomed Sci. 2002;59:85–89. doi: 10.1080/09674845.2002.11783640. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Ji B, Han B, Ernst SA, Simeone D, Logsdon CD. NF-kappaB activation in pancreas induces pancreatic and systemic inflammatory response. Gastroenterology. 2002;122:448–457. doi: 10.1053/gast.2002.31060. [DOI] [PubMed] [Google Scholar]
- 36.Takaoka K, Kataoka K, Sakagami J. The effect of steroid pulse therapy on the development of acute pancreatitis induced by closed duodenal loop in rats. J Gastroenterol. 2002;37:537–542. doi: 10.1007/s005350200083. [DOI] [PubMed] [Google Scholar]
- 37.Steinle AU, Weidenbach H, Wagner M, Adler G, Schmid RM. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. doi: 10.1016/s0016-5085(99)70140-x. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 39.Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- 40.Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World J Gastroenterol. 2006;12:5005–5009. doi: 10.3748/wjg.v12.i31.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]


