Abstract
AIM: To investigate the epidemiology of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection in the two major ethnic groups in Kazakhstan.
METHODS: A cross-sectional prospective study of HBV and HCV seroprevalence was performed among individuals born in Kazakhstan with no history of chronic hepatitis or liver disease.
RESULTS: There were 290 volunteers (140 Russians and 150 Kazakhs) aged 10 to 64 years, males accounted for 46%. Active HBV infection (HBsAg positive) was present in 3.8%, anti-HBc in 30%. The prevalence was similar in females and males (33% vs 25%) (P = 0.18). The prevalence of anti-HBc increased from 19% in 10-29 years old volunteers to 53% in 50-years and older volunteers. The prevalence of HBV infection was higher in married than in single adults (38% vs 26%, respectively) (P = 0.2) and more common in Kazakhs (35%) than in Russians (24%) (P = 0.07). HCV infection was present in 9 subjects (3.2%), 5 of them also were positive for anti-HBc in the absence of HBsAg.
CONCLUSION: The frequency of active HBV infection (3.8%) coupled with a high prevalence of HBV exposure in those > 50 years of age increases with age, which suggests that horizontal transmission likely relates to the use of contaminated needles. The low prevalence of HCV infection suggests that HBV and HCV are acquired differently in this group of subjects.
Keywords: Viral hepatitis B, Viral hepatitis C, Hepatitis B virus, Transmission, Epidemiology, Sero-epidemiology, Kazakhstan
INTRODUCTION
Kazakhstan has two major ethnic groups: Kazakhs who trace their origins back to Genghis Khan, and ethnic Russians. Each constitutes approximately 40% of the population. The different ethnic groups share similar living and socioeconomic conditions.
Kazakhstan is part of a nine-country subregion in which the mortality due to chronic liver disease and cirrhosis has been estimated to be 21.8 per 100 000 population[1]. The mortality rate of hepatocellular carcinoma is also high, with liver cancer representing approximately 4% of all malignant neoplasms[2]. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are both common causes of hepatocellular carcinoma. We conducted a cross-sectional study in Kazakhstan to examine the epidemiology and risk factors associated with HBV and HCV infections. We gave particular emphasis on possible relationships between chronic hepatitis B or C virus infections and a history of intravenous injections, blood transfusions, vaccinations, needle sticks, surgeries, having tattoos, and shared use of tooth brushes, body brushes and towels.
MATERIALS AND METHODS
We performed a cross-sectional seroepidemiologic study in Almaty, Kazakhstan. Both Russians and Kazakhs were entered if they were unrelated and born in Kazakhstan with no prior history of clinical hepatitis or chronic liver disease. An attempt was made to obtain an equal number of volunteers per decade from each of these two major ethnic groups.
A trained physician interviewed each volunteer and completed a detailed questionnaire. Demographic data focusing on the environment during the subject's childhood included social and economic data (parent's education, occupation and family income) as well as histories of blood transfusions, intravenous injections, vaccinations, needle sticks, surgeries, having tattoos, and the shared use of tooth brushes, body brushes or towels. In women, we obtained data about miscarriages and abortions.
Serologic methods
Each volunteer provided a blood sample. Sera were stored at -20°C until analyzed. Serum samples were tested for hepatitis A and B serology using kits (Abbott Diagnostic Laboratories, Abbott Park, IL), including enzyme immunoassays (EIA) for the qualitative detection of total antibody to hepatitis A virus (anti-HAV; HAVAB EIA), hepatitis B surface antigen (HBsAg; AUSZYME Monoclonal), and total antibody to hepatitis B core antigen (anti-HBc; Corzyme). Antibody to hepatitis B surface antigen (anti-HBs; AUSAB, human subtypes ad and ay) was evaluated by solid phase radioimmunoassay. Antibody to hepatitis C (anti-HCV; HCV Version 3.0, Ortho-Clinical Diagnostics, Inc, Raritan, NJ) was detected by EIA. The interpretation of the results was done according to the manufacturer’s instructions.
RESULTS
Two hundred and ninety volunteers were enrolled in the study including 150 Kazakhs and 140 ethnic Russians ranging in age from 10 to 64 years. One hundred and thirty-four (46%) of the subjects were men.
Risk factors and prevalence of HBV
Total anti-HBc was found in 30% of the population, being similar in women (33.3%) and men (25.4%) (P = 0.18). There was an age-specific significant increase in the prevalence of anti-HBc antibody: from 19.2% in 10-29 years old individuals to 53.4% in those who were 50- years old and older (Figure 1A) (P < 0.01). The prevalence of anti-HBc was more common in Kazakhs (34.7%) than in Russians (24.3%) (P = 0.07). Among those who were 21-years old and older, the prevalence of hepatitis B infection was similar in married and single adults (38% vs 26%, P = 0.2) (data not shown).
Figure 1.
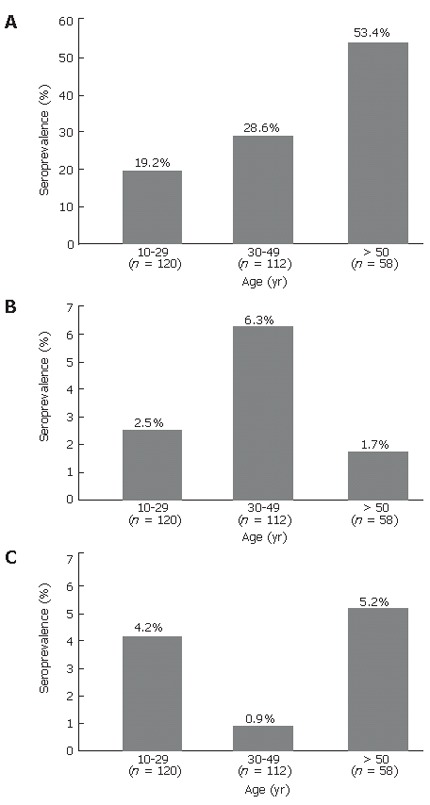
Prevalence of anti-HBc antibodies (A), HBsAg (B), and anti-HCV antibodies (C) in relation to age in Kazakhstan.
The overall seroprevalence rate of active HBV infection (HBsAg positive) was 3.8% (11 of 290 subjects), none of these individuals tested was positive for anti-HCV. There was no significant difference in the prevalence of detectable HBsAg in men and women (3.7% of men vs 3.8% of women) (P = 0.7) nor between ethnic groups (4.3% of Russians vs 3.3% of Kazakhs) (P = 0.9). The prevalence of HBsAg peaked in those whose age was 30-49 years (6.3%) and was lower in those whose age was 10-29 years (2.5%) (P = 0.3) and lowest in those whose age was 50 years and more (1.7%) (P = 0.4, Figure 1B). Four of the 11 HBsAg positive individuals (36%) were anti-HBs positive. However, the concentrations of antibodies were low in this group of individuals (Table 1). Three of four individuals with concurrent HBsAg and anti-HBs positivity were Kazakhs, but gender distribution was equal.
Table 1.
Concentration of anti-HBs in four HBsAg and anti-HBc positive subjects
| I.D. | mIU/mL |
| 11 | 16.1 |
| 180 | 3.4 |
| 249 | 4.0 |
| 143 | 5.7 |
Table 2 shows unadjusted and adjusted (for socio-economic status, gender, and ethnicity) incidence rates and risk factors possibly associated with acquisition of hepatitis B. The highest risks associated with hepatitis B infection were having been a blood donor or having a history of dental surgery. The risk factors for positive anti-HBc and HBsAg included dental surgery (73%), frequent injections (64%), and ear piercing (54%).
Table 2.
Effect of selected risk factors on the incidence rate of anti-HBc in a study population from Kazakhstan (n = 86)
| Risk factor | Unadjusted OR | P | 95% CI | Adjusted1 OR | P | 95% CI |
| Blood transfusion | 2.11 | 0.19 | 0.67-6.48 | 1.26 | 0.72 | 0.36-4.40 |
| Blood donor | 1.77 | 0.03 | 1.05-2.98 | 1.38 | 0.48 | 0.56-3.42 |
| Tattoo | 1.67 | 0.19 | 0.77-3.65 | 1.26 | 0.62 | 0.51-3.12 |
| Ear piercing | 1.43 | 0.16 | 0.87-2.39 | 1.20 | 0.57 | 0.64-2.24 |
| Dental surgery | 1.93 | 0.02 | 1.10-3.31 | 1.29 | 0.29 | 0.80-2.08 |
Adjusted OR for socioeconomic status, ethnicity and gender.
Risk factors and prevalence of viral hepatitis C
Anti-HCV was detected in only 9 subjects (3.2%) including 3 Kazakh women and 6 Russians (three women and three men). Five of the 9 subjects also had detectable levels of anti-HBc, but were negative for anti-HBs. Figure 1C and Table 3 display age-specific prevalence rates of anti-HCV in this study population and selected risk factors associated with anti-HCV seropositivity. A history of tattoos was a common risk factor for hepatitis C with a trend in risk toward towel sharing.
Table 3.
Effect of selected risk factors on the incidence rate of anti-HCV in a study population from Kazakhstan (n = 9)
| Risk factor | Unadjusted OR | P | 95% CI | Adjusted1 OR | P | 95% CI |
| Blood transfusion | 2.48 | 0.41 | 0.29-21.20 | 1.42 | 0.76 | 0.15-13.38 |
| Tattoo | 4.02 | 0.05 | 0.90-16.44 | 14.40 | 0.01 | 1.76-118.32 |
| Ear piercing | 2.58 | 0.18 | 0.65-10.18 | 3.05 | 0.29 | 0.38-24.4 |
| Dental surgery | 1.65 | 0.48 | 0.42-6.52 | 1.46 | 0.64 | 0.29-7.26 |
| Towel sharing | 3.25 | 0.09 | 0.82-12.83 | 3.87 | 0.07 | 0.91-16.4 |
Adjusted OR for socioeconomic status, ethnicity and gender.
DISCUSSION
Almost 30% of the study population examined had evidence of current or past HBV infection which is similar to the results of a prior study in northwestern Kazakhstan, showing that 22% (n = 579) of the healthy population had detectable anti-HBc antibodies[3]. The prevalence of anti-HBc seropositivity in Kazakhstan is substantially lower than that reported in 110 Bukharian Jews who immigrated from Uzbekistan and Tajikistan (former Soviet Union) to Israel, 66% of them had evidence of exposure to HBV[4]. However, the prevalence of past or current hepatitis B infection is much higher in Central Asia than in Pakistan, Ethiopia, Sweden, England and Wales[5-8].
The primary mode of transmission of HBV in Kazakhstan is unsettled. The prevalence of infection increasing with age could result from either birth cohort effects and continuing gradual horizontal acquisition, or both. Demographic factors associated with risk of acquisition (i.e. history of blood donation and dental surgery) found in a recent study in Pakistan are blood transfusions and use of contaminated syringes, and contaminated dental and surgery equipment are the main risk factors for acquiring HBV infections[9]. However, in our study, adjustments for socioeconomic status, ethnicity and gender eliminated these significantly associated risk factors (Table 2).
In countries of European Region C, including Kazakhstan, the annual number of injections was reported to be 11.3 per person in 2000 and this estimate is the highest among countries recognized as having a global burden of disease. The same is true for the Russian Federation where the annual number of injections per person is also 11.3 and different from neighboring countries such as Kyrgyzstan and Uzbekistan where this estimate is 5.2 or China where the annual number of injections per person is only 2.4[10]. Several studies conducted in Russia showed that 85%-99% of injections are unnecessary with a ratio of 20:1 for injections being given with therapeutic intent compared to those for disease prevention[11,12]. In addition, the proportion of equipments being reused was reported to be 11%. Of interest in this study, the common factor for having both anti-HBc and HBsAg was a history of frequent injections.
In the current study, 4 of the 11 HBsAg positive subjects also had anti-HBs antibodies (36%). It was reported that the rate of concurrent HBsAg and anti-HBs positivity is between 21% and 32%[13-16] and is associated with evidence of viral replication and features of active inflammation[13] or with progressive liver disease[14]. Most probably, these discordant serologic results reflect a low concentration of non-complexed anti-HBs that fails to recognize insertions or deletions that occur in the pre-S/S region of the HBV genome[16,17]. Heterotypic antibody is often observed in these situations[13,15].
The prevalence of anti-HCV in this study population was 3.2% which is somewhat higher than the prevalence of anti-HCV antibodies observed in northwestern Kazakhstan (1.7%; P = 0.3)[3], or in Siberian natives from the Kamchatka Peninsula of Russia (1.4%, n = 348)[18]. In contrast, in the Republic of Azerbaijan[19], 8.7% of screened serum samples are anti-HCV reactive[20]. We feel certain that virtually all of our reactive samples represented a past or present infection with HCV based on strong EIA ratios of 3.0 or greater. However, we did not do HCV RNA testing to confirm its active infection. It also is possible that we underestimated the prevalence of hepatitis C in that a proportion of the population may have resolved their infection and subsequently lost their anti-HCV[21].
The risk factors related to HCV infection (Table 3) have very wide confidence intervals indicative of the small sample size which precludes making firm conclusions regarding transmission. However, the low prevalence of hepatitis C infection compared with the relatively high prevalence of HBV infection suggests that different modes of transmission are active in this population due to the sexual or vertical transmission of HBV and the parenteral transmission of HCV. Overall, the use of unsafe (contaminated) needles remains of great concern in this population as a vehicle for transmission of these viruses.
Footnotes
Supported by the Office of Research and Development Medical Research Service Department of Veterans Affairs; Public Health Service grant DK56338 which funds the Texas Gulf Coast Digestive Diseases Center; the Eugene B. Casey Foundation and the William and Sonya Carpenter Fund, Baylor College of Medicine
S- Editor Liu Y L- Editor Wang XL E- Editor Lu W
References
- 1.The European health report. World Health Organization 2005: Public health action for healthier children and populations. Available from: http: //www.euro.who.int/ehr2005/20050809_1.
- 2.WHO Global Infobase Online: National/Subnational country profiles. Kazakhstan: WHO Estimates of country level mortality. Available from: http: //www.who.int/ncd_surveillance/infobase/web.
- 3.Kruglov IV, Iashina TL, Tsvetova GV, Seliutina IA, Klimkin AIu, Aksenova NF, Sobina GV, Gerasimenko NA, Bakulin VS, Ivanov SV. The spread of hepatitis B and C markers and the etiological structure of the morbidity with acute viral hepatitis of the population in the Kuznetsk Basin and northwestern Kazakhstan. Zh Mikrobiol Epidemiol Immunobiol. 1995;6:36–37. [PubMed] [Google Scholar]
- 4.Glikberg F, Brawer-Ostrovsky J, Ackerman Z. Very high prevalence of hepatitis B and C in Bukharian Jewish immigrants to Israel. J Clin Gastroenterol. 1997;24:30–33. doi: 10.1097/00004836-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann G, Berglund G, Elmståhl S, Eriksson S, Verbaan H, Widell A, Lindgren S. Prevalence and clinical spectrum of chronic viral hepatitis in a middle-aged Swedish general urban population. Scand J Gastroenterol. 2000;35:861–865. doi: 10.1080/003655200750023246. [DOI] [PubMed] [Google Scholar]
- 6.Gay NJ, Hesketh LM, Osborne KP, Farrington CP, Morgan-Capner P, Miller E. The prevalence of hepatitis B infection in adults in England and Wales. Epidemiol Infect. 1999;122:133–138. doi: 10.1017/s0950268898001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsega E, Mengesha B, Hansson BG, Lindberg J, Nordenfelt E. Hepatitis A, B, and delta infection in Ethiopia: a serologic survey with demographic data. Am J Epidemiol. 1986;123:344–351. doi: 10.1093/oxfordjournals.aje.a114243. [DOI] [PubMed] [Google Scholar]
- 8.Khan AJ, Luby SP, Fikree F, Karim A, Obaid S, Dellawala S, Mirza S, Malik T, Fisher-Hoch S, McCormick JB. Unsafe injections and the transmission of hepatitis B and C in a periurban community in Pakistan. Bull World Health Organ. 2000;78:956–963. [PMC free article] [PubMed] [Google Scholar]
- 9.Tassaduqe K, Muhammad , Abdus S, Humera K, Asma S, Soban U. Studies on the prevalence of hepatitis B virus in relation to sex, age, promotive factors, associated symptoms and season among human urban population of Multan, Pakistan. J Biol Sci. 2004;4:183–187. [Google Scholar]
- 10.Hutin YJ, Hauri AM, Armstrong GL. Use of injections in healthcare settings worldwide, 2000: literature review and regional estimates. BMJ. 2003;327:1075. doi: 10.1136/bmj.327.7423.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loukina TN. Current treatment methods for the respiratory infections in children. Thesis for the degree of candidate of medical sciences. Moscow: Institute of Pediatrics; 1993. [Google Scholar]
- 12.Stekolschikova IA. Diagnostic and treatment tactics in the management of ARI in children. Thesis for the degree of candidate of medical sciences. Moscow: Institute of Pediatrics, RAMS; 1993. [Google Scholar]
- 13.Shiels MT, Taswell HF, Czaja AJ, Nelson C, Swenke P. Frequency and significance of concurrent hepatitis B surface antigen and antibody in acute and chronic hepatitis B. Gastroenterology. 1987;93:675–680. doi: 10.1016/0016-5085(87)90427-6. [DOI] [PubMed] [Google Scholar]
- 14.Heijtink RA, van Hattum J, Schalm SW, Masurel N. Co-occurrence of HBsAg and anti-HBs: two consecutive infections or a sign of advanced chronic liver disease? J Med Virol. 1982;10:83–90. doi: 10.1002/jmv.1890100202. [DOI] [PubMed] [Google Scholar]
- 15.Tsang TK, Blei AT, O'Reilly DJ, Decker R. Clinical significance of concurrent hepatitis B surface antigen and antibody positivity. Dig Dis Sci. 1986;31:620–624. doi: 10.1007/BF01318693. [DOI] [PubMed] [Google Scholar]
- 16.Wang YM, Ng WC, Kang JY, Yap I, Seet BL, Teo J, Smith R, Guan R. Serological profiles of hepatitis B carrier patients in Singapore with special reference to the frequency and significance of concurrent presence of HBsAg and anti-HBs. Singapore Med J. 1996;37:150–152. [PubMed] [Google Scholar]
- 17.Ohba K, Mizokami M, Kato T, Ueda R, Gurtsenvitch V, Senyuta N, Syrtsev A, Zoya K, Yamashita M, Hayami M. Seroprevalence of hepatitis B virus, hepatitis C virus and GB virus-C infections in Siberia. Epidemiol Infect. 1999;122:139–143. doi: 10.1017/s0950268898001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman PF. Surveillance for hepatitis B surface antigen mutants. J Med Virol. 2006;78 Suppl 1:S56–S58. doi: 10.1002/jmv.20609. [DOI] [PubMed] [Google Scholar]
- 19.Galetskiĭ SA, Seniuta NB, Syrtsev AV, Abdullaev OM, Aliev DA, Kerimov AA, Yamashita M, Hayami M, Kato T, Mizokami M. Analysis of some viral infections, transmitted by parenteral and sexual routes, in the Republic of Azerbaijan. Vopr Virusol. 1999;44:232–236. [PubMed] [Google Scholar]
- 20.Pasha O, Luby SP, Khan AJ, Shah SA, McCormick JB, Fisher-Hoch SP. Household members of hepatitis C virus-infected people in Hafizabad, Pakistan: infection by injections from health care providers. Epidemiol Infect. 1999;123:515–518. doi: 10.1017/s0950268899002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A, et al. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]


