Abstract
AIM: To evaluate the effect of antisense oligonucleotide targeting midkine (MK-AS) on angiogenesis in chick chorioallantoic membrane (CAM) and in situ human hepatocellular carcinoma (HCC).
METHODS: An in situ human hepatocellular carcinoma (HCC) model and CAM assay were used in this experiment. The effect of MK-AS on angiogenesis was evaluated by cell proliferation assay and hematoxylin-eosin (HE) staining.
RESULTS: MK-AS significantly inhibited human umbilical vein endothelial cells (HUVEC) and in situ human HCC growth. At the same time, MK-AS suppressed the angiogenesis both in human hepatocellular carcinoma cell line (HEPG2)-induced CAM and in situ human HCC tissues.
CONCLUSION: MK-AS is an effective antiangiogenesis agent in vivo.
Keywords: Midkine, Angiogenesis, Antisense oligonucleo-tide, Tumor, Chick chorioallantoic membrane assay, Hepatocellular carcinoma
INTRODUCTION
Angiogenesis is a process leading to formation of new blood vessels, which plays a central role in survival of cancer cells, growth of local tumors, and development of distant metastasis[1]. It is believed that tumor cells require the ability to stimulate angiogenesis, and angiogenesis induced by tumor cells, leads to hypervascularization of tumor tissue[2]. More recently, it has been found that tumor cells also produce angiogenesis inhibitors[3]. The angiogenic phenotype of a solid tumor is accepted as a result of the net balance between the activities of angiogenesis promoters and inhibitors[4]. Preclinical testing of these antiangiogenic factors in animal models suggested that they can effectively suppress the growth and/or metastasis of experimental tumors[5-7]. Inevitably, identification of angiogenic inhibitors and demonstration of their angiostatic functions would provide rational foundations for the development of tumor-specific antiangiogenic therapy.
Up to now, different growth factors, such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), transforming growth factor-alpha (TGF-α) and midkine (MK) have been identified as positive regulators of angiogenesis and are secreted by cancer cells to stimulate normal endothelial cell growth through paracrine mechanisms[8-11]. MK was first identified in early stage embryonal carcinoma cells during retinoic acid-induced differentiation[12]. MK and pleiotrophin comprise a family of heparin-binding growth/differentiation factors, which are different from other heparin-binding growth factors such as fibroblast growth factor and hepatocyte growth factor[13-15]. MK is over-expressed in various malignant tumors[16-24], and low or undetectable in normal adult tissues[13,24]. It is accepted that MK promotes the survival[25,26], growth[27,28] and migration[29-31] of many cells, involved in activating mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinases 1 and 2 or protein kinase B (PKB/AKT) pathways[25,32]. Recently, it was reported that midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types[33].
There is evidence that MK plays an important role in angiogenesis, which is an important event in tumor development and progression. It was reported that enhanced tumor growth in MCF-7 breast carcinoma cells due to high MK expression is correlated with increased vascular density and endothelial proliferation, implicating an angiogenic role of MK in tumor growth[11]. Additionally, MK, an angiogenic factor, is expressed in bladder cancer, and its over-expression correlates with a poor outcome in patients with invasive cancers[20]. In fact, we have confirmed that MK-AS transfer can significantly inhibit the growth of hepatocellular carcinoma cells, which is associated with increased Caspase-3 activity[34]. The present study was to determine whether MK-AS can suppress angiogenesis, which is an important mechanism underlying inhibition of tumor cell proliferation.
MATERIALS AND METHODS
Antisense oligodeoxynucleotides
MK-AS (5’-CCCCGGGCCGCCCTTCTTCA-3’) and MK-SEN phosphorothioate oligonucleotide (5’-TGAAGAAGGGCGGCCCGGGG-3’) were synthesized with an applied biosystem model 391 DNA synthesizer using Oligo Pilot II DNA (Amersham-Pharmacia, Piscataway, NJ, USA) and purified by high-performance liquid chromatography (HPLC) (Waters Delta Prep 4000, Milfordd, MA, USA) with SOURCE 15Q (Amersham Pharmacia, Piscataway, NJ, USA) as previously described[34].
Cultured cell lines
Human umbilical vein endothelial cells (HUVECs), a gift of the Chinese Academy of Medical Sciences, Beijing, China, were pooled after collagenase type I treatment and seeded on cell culture plates. The cells were grown in RPMI 1640 medium (Invitrogen Corporation, CA, USA) supplemented with 20% fetal calf serum (FBS; GIBCO BRL, Grand Island, NY, USA), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in an atmosphere containing 5% CO2.
Cell proliferation assay
A total of 3 × 103 cells were seeded in each well of a 96-well microtiter plate and allowed them to attach overnight. Oligonucleotides at the concentrations of 0.2, 0.4, 0.8 μmol/L were transfected into the cells with Lipofectin (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Transfection medium was replaced by normal culture medium after 6 h. The effects of antisense oligodeoxynucleotide (ASODN) on cellular viability were measured by 3-[4, 5-dimethythiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt (MTS) assay. After 48 h of incubation following transfection, 20 μL MTS (Sigma, St Louis, MO, USA) was added to each well and incubated at 37°C for 2 h. The absorbance value was determined at 490 nm by a MR600 microplate reader (Wallac 1420 Multilable counter, Wallac, Turku, Finland).
In vivo tumor studies
In situ HCC models were established as previously described[35]. Two days after in situ HCC models were established, mice were injected intravenously with saline (vehicle control) and MK-AS (25, 50 and 100 mg/kg per day) for 20 d. Body weight and general physical status of the animals were recorded daily. At the endpoint of the study, mice were killed by cervical dislocation and tumors were removed and weighed. Tumor sizes were monitored with calipers, the tumor volume (V, mm3) was calculated as (L × W2)/2, where L = length (mm) and W = width (mm). The percentage of tumor growth inhibition was calculated as: inhibitory rate (%) = (Wcontrol-Wtreat)/Wcontrol × 100.
Immunohistochemistry analysis for microvessel formation
Tumor specimens were fixed and frozen in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC). Five-μm thick cryosections were cut and stained with HE for histopathological analysis. To analyze the microvessel formation in tumors, sections were stained with anti-CD34 monoclonal antibody (DAKO Corp., Carpinteria, CA) and subsequently with the avidin-biotin-peroxidase (ABC) method. Positively stained vascular endothelial cells (brown) were visualized and imaged using a digital camera attached to an Olympus microscope. Micro-vessel density was determined as previously described[36]. Briefly, regions of the highest vessel density (“hot spot” regions) were scanned at low magnification (× 40-100) and counted at higher magnification (× 200). Three such “hotspot” fields were counted in each tumor section, and the mean microvessel density value was recorded. Any endothelial cell or endothelial cell cluster that was clearly separated from adjacent microvessels was considered a single, countable microvessel. Positively stained vascular endothelial cells were visualized and imaged using a Magnifire camera (Olympus, Melville, NY) attached to an Olympus Provis microscope.
Western blot analysis
After transfection with MK-AS for 24 h, cells in the microplates were collected into 1.5 mL Eppendorf tubes on ice and centrifuged at 2000 r/min for 5 min at 4°C. Pellets were then lysed with lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 0.5 mmol/L EDTA, 0.5% NP40, and 150 mmol/L NaCl] in the presence of protease inhibitors. Lysates were centrifuged at 12 000 × g for 15 min to remove debris. Proteins (30 μg) were separated from samples by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto hybond-polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences). Midkine protein was identified using the primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The reactive band was visualized with an ECL-plus detection kit (Amersham Biosciences, Piscataway, NJ) and scanned by Gel Doc 1000 (Bio-Rad CA, USA). β-actin was used as a control.
Chick chorioallantoic membrane assay
Angiogenesis assay in chick chorioallantoic membranes (CAM) was performed as previously described[37]. Fertilized eggs were incubated at 38°C. On d 7, a window was opened on the eggshell to expose the CAM, and the window was covered with a tape for further incubation. Filter paper discs (0.5 cm in diameter) containing HepG2 cells (18 000/disc) or PBS was placed on the surface of each CAM on d 8. After 24 h, 100 μL of 0.4 μmol/L MK-AS was given using a 30-gauge needle. Two days later, the CAM were fixed in 3.7% formaldehyde. Pictures were taken with a stereoscope, and the number of vessel branches was counted. Ten eggs were used for each experimental condition.
Statistical analysis
Data were expressed as mean ± SD, statistical analysis was carried out using Student’s t-test (two tailed), P < 0.05 was considered statistically significant.
RESULTS
Effect of MK-AS on MK expression in HUVEC
Our previous studies showed that antisense oligonucleotide targeting MK down regulates MK expression in hepatocellular carcinoma cells[34]. In this study, we measured the effect of MK-AS on MK expression in HUVEC. After MK compounds were transfected for 24 h, total RNA and protein of HUVEC were extracted for analysis of the effect of MK-AS transfer on MK expression. Our results indicated that MK-AS could efficiently decrease the mRNA and protein content of MK in HUVEC (Figure 1A and B).
Figure 1.
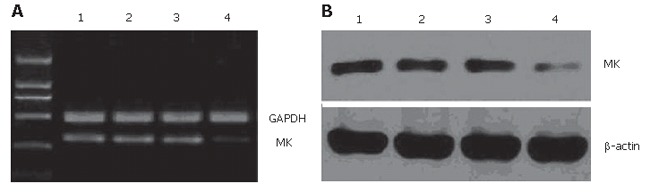
RT-PCR (A) and Western-blotting analysis (B) of MK expression in human umbilical vein endothelial cells (HUVEC) after transfection with 0.4 μmol/L MK-AS (lane 4), MK-SEN (lane 3), Lipofectin alone (lane 2) for 24 h. Lane 1 represents cells without transfection. GAPDH and β-actin were used as control respectively.
Effects of MK-AS treatment on HUVEC growth
To investigate the effect of MK-AS on endothelial cell proliferation, we analyzed the growth of HUVEC transfected with MK-SEN or MK-AS. MK-AS transfer significantly inhibited HUVEC proliferation (Figure 2), suggesting that MK signaling might directly contribute to endothelial cell growth. In contrast, no significant inhibition of HUVEC transfected with MK-SEN was observed.
Figure 2.
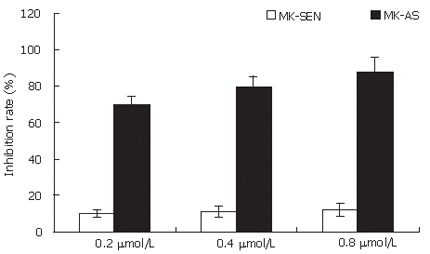
Effect of MK on growth of HUVEC. Cells after transfection with 0.4 μmol/L MK-AS or MK-SEN for 24 h were analyzed by MTS assay. Data were expressed as mean ± SD from four independent experiments.
MK-AS inhibited angiogenesis in chick CAM
It is widely accepted that tumors can stimulate angiogenesis by releasing angiogenesis stimulators such as bFGF and VEGF[8,9]. HepG2 induced noticeable angiogenesis (Figure 3B). When paper discs absorbing PBS alone were placed onto the CAM, a few vessels occupied the CAM area covered by the paper discs (Figure 3A). Interestingly, when CAM implanted with HepG2 were treated with MK-AS (0.4 μmol/L), the number of vessels under the paper discs was significantly reduced (Figure 3C). Similar results were obtained when the mean vessel length was measured (data not shown). Chick embryos treated with MK-AS were alive and showed the same extent of motility as embryos from PBS-treated eggs at the end of the experiment.
Figure 3.
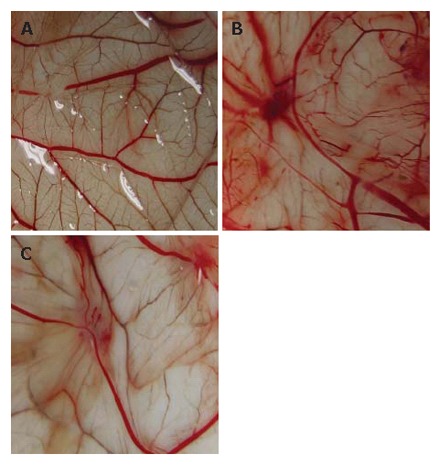
Angiogenesis in normal chick CAM (A), HepG2-induced CAM (B), and HepG2-induced CAM treated with 0.4 μmol/L MK-AS (C). Chick CAM assays were used to assess the impact of MK-AS on angiogenesis in vivo.
Effects of MK-AS treatment on in situ HCC xenograft growth
We have reported that MK-AS inhibits tumor proliferation[38]. In the present study, an in situ HCC model of mice was used to evaluate the effect of MK-AS on in vivo tumor proliferation. MK-AS at 25 mg/kg, 50 mg/kg, 100 mg/kg per day and saline were intravenously administered for 20 d. Tumors were removed, measured and weighed. MK-AS treatment significantly inhibited tumor growth compared to saline treatment (Table 1). The highest inhibitory efficacy was 65.89% in MK-AS treatment group compared to saline treatment group. The effects of MK-AS on tumor growth are shown in Table 1. The final tumor volumes after 20-d treatment are shown in Table 2 (P < 0.01). No difference was found in the mean body weight of mice between the groups during the study (data not shown).
Table 1.
Effect of MK-AS on weight and inhibition of in situ HCC
| Treatment group | Tumor weight (g) | Inhibition rate (%) |
| Saline | 1.29 ± 0.13 | - |
| MK-AS 100 mg/kg per day | 0.44 ± 0.18b | 65.89 |
| MK-AS 50 mg/kg per day | 0.64 ± 0.18b | 50.39 |
| MK-AS 25 mg/kg per day | 0.70 ± 0.14b | 45.74 |
| MK-Sen 50 mg/kg per day | 1.04 ± 0.14a | 19.38 |
P < 0.05,
P < 0.01 vs control group.
Table 2.
Effect of MK-AS on growth of in situ human HCC
| Treatment group | Tumor volume (mm3) |
| Saline | 807.75 ± 195.19 |
| MK-AS 100 mg/kg per day | 294.50 ± 70.66b |
| MK-AS 50 mg/kg per day | 532.00 ± 121.57b |
| MK-AS 25 mg/kg per day | 552.75 ± 84.38b |
| MK-Sen 50 mg/kg per day | 630.88 ± 188.76a |
P < 0.05,
P < 0.01 vs control group.
Effects of MK-AS treatment on in situ HCC xenograft angiogenesis
To determine whether the antitumor effect of MK-AS therapy is associated with angiogenesis suppression, the status of vessel formation in in situ HCC xenograft was assessed by immunohistochemistry with endothelial cell-specific CD34 staining. A representative result of immunostaining is shown in Figure 4. High density of microvessels was present in untreated tumor samples, whereas MK-AS treatment significantly reduced vascularization (Figures 4 and 5).
Figure 4.
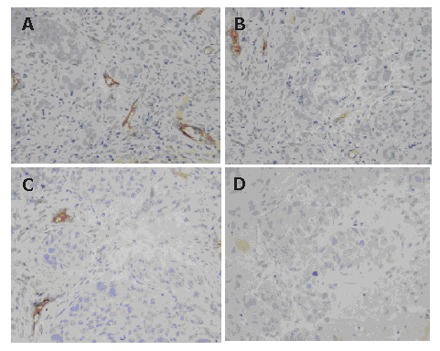
Immunohistochemical analysis of HCC xenograft angiogenesis after treatment with MK-SEN (A), 25 mg/kg of MK-AS per day (B), 50 mg/kg of MK-AS per day (C), and 100 mg/kg of MK-AS per day (D). Representative views of anti-CD34 antibody-stained vascular endothelium (brown) (х 200).
Figure 5.
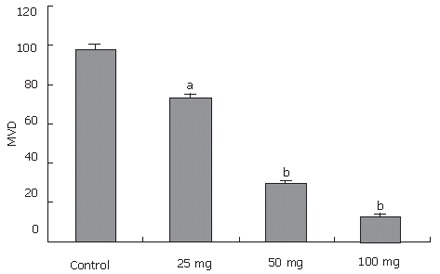
Tumors resected from mice at the experimental end point. Resected tumors were sectioned and stained as described in the text. Microvessel densities (MVD) were determined by counting CD34-positive endothelial cells in the sections and presented as mean ± SE positive cells/field from the three “hot-spot” fields. aP < 0.05, bP < 0.01 vs control group.
DISCUSSION
Tumor-induced neovascularization plays a key role in the development, progression and metastasis of neoplasm[39]. Angiogenesis is regulated by several endogenous stimulators and inhibitors of endothelial cell migration, proliferation and tube formation[4]. Tumor growth progression is correlated to the serum concentration of angiogenic mediators[40]. Furthermore, vascular density of tumor tissues is correlated to the clinical course of tumors[2]. MK is heparin binding to cytokines involved in neurogenesis, cell migration and mesoderm-epithelial interactions[41]. Choudhuri et al[11] reported that in vitro tumor cell growth is not affected by these growth factors, while in vivo tumor cell growth is correlated to increased vascular density of tumors. Since MK plays an important role in angiogenesis, it may be a possible therapy target to suppress angiogenesis of tumor proliferation.
Under physiological conditions the vasculature is quiescent in normal adults. Only 0.01% of endothelial cells in normal adult vessels are in the cell division cycle at any given time[42]. However, in response to an appropriate angiogenic stimulus, endothelial cells can be activated to grow new vessels. In the present study, down regulation of MK expression could restrain HUVEC proliferation, suggesting that endothelial cells can also inhibit angiogenesis in vivo. Chick CAM assay showed that MK-AS could inhibit local CAM vasculogenesis.
Furthermore, we developed an in situ HCC model of mice to determine the effect of MK-AS on tumor proliferation and angiogenesis in vivo. Our results indicate that MK-AS administration significantly inhibited tumor growth in the in situ HCC model of mice (Tables 1 and 2). Furthermore, Mk-AS administration also suppressed tumor angiogenesis in vivo (Figures 4 and 5). It should be noted that the tumor cell line originated from human HCC maintained the complete characteristics of human HCC tissue, such as AFP secretion, drug sensitivity. In addition, the pathological evidence also suggests that this model exhibits various features seen in clinical HCC patients, indicating that the information provided by this model can reflect the true clinic results of patients. In fact, angiogenesis suppressed by transfection with SiRNA targeting MK in human prostate cancer cell line (PC-3) has also been reported[43].
It has been well established that tumor growth and metastasis depend on the induction of new blood supply[44,45]. Angiogenic activity as determined by MVD, has been shown to correlate with worse prognosis in a number of solid tumors[46-48]. Therefore, antiangiogenic agents targeting either these factors or vascular endothelial cells can be used as promising therapeutic modalities in treatment of tumors[49]. In the present study, significant inhibition of angiogenesis was achieved using MK-AS, indicating that MK-AS is an effective antiangiogenesis agent.
COMMENTS
Background
Midkine (MK) is a 13kDa protein, a heparin-binding growth factor. Subsequent studies suggest that MK plays an important role in carcinogenesis.
Research frontiers
MK plays an important role in angiogenesis, which is an important event in tumor development and progression. Antisense oligonucleotide targeting MK is employed in cancer therapy as it can inhibit hypervascularization of tumors and is of great clinical interest.
Innovations and breakthroughs
In this study, MK inhibited angiogenesis in chicken chorioallantoic membrane and human hepatocellular carcinoma (HCC) xenograft. Meanwhile MK inhibited proliferation of human umbilical vein endothelial cells (HUVEC) and growth of HCC xenograft.
Applications
In this study, we addressed the potential therapeutic effect of MK on angiogenesis. Significant inhibition of angiogenesis was achieved using MK, indicating that MK is an effective anti-angiogenesis agent.
Peer review
The manuscript by Li-Cheng Dai et al describes the successful use of midkine to inhibit angiogenesis in chicken chorioallantoic membrane and human HCC xenograft. The results were found to be important for HCC therapy. The findings are of clinical interest.
Footnotes
Supported by grants from Medical and Sanitary Research Foundation of Zhejiang Province, (No. 2003A077), Huzhou Natural Science Foundation, (No. 2004SZX07-11)
S-Editor Liu Y L-Editor Wang XL E-Editor Zhou T
References
- 1.Folkman J, Tumor angiogenesis. In: Mendelsohn J, Howley P, Liotta LA, and Israel M, editors. The Molecular Basis of Cancer. Philadelphia: Saunders WB; 1995. p. 206–232. [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Campbell SC, Volpert OV, Ivanovich M, Bouck NP. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998;58:1298–1304. [PubMed] [Google Scholar]
- 4.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47:149–161. [PubMed] [Google Scholar]
- 5.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 6.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 7.O'Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Antiangiogenic activity of the cleaved conformation of the serpin antithrombin. Science. 1999;285:1926–1928. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- 8.Goldfarb M. The fibroblast growth factor family. Cell Growth Differ. 1990;1:439–445. [PubMed] [Google Scholar]
- 9.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992;13:18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 11.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–1819. [PubMed] [Google Scholar]
- 12.Kadomatsu K, Tomomura M, Muramatsu T. cDNA cloning and sequencing of a new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem Biophys Res Commun. 1988;151:1312–1318. doi: 10.1016/s0006-291x(88)80505-9. [DOI] [PubMed] [Google Scholar]
- 13.Li YS, Milner PG, Chauhan AK, Watson MA, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science. 1990;250:1690–1694. doi: 10.1126/science.2270483. [DOI] [PubMed] [Google Scholar]
- 14.Merenmies J, Rauvala H. Molecular cloning of the 18-kDa growth-associated protein of developing brain. J Biol Chem. 1990;265:16721–16724. [PubMed] [Google Scholar]
- 15.Muramatsu T. Midkine (MK), the product of a retinoic acid responsive gene, and pleiotrophin constitute a new protein family regulating growth and differentiation. Int J Dev Biol. 1993;37:183–188. [PubMed] [Google Scholar]
- 16.Garver RI, Chan CS, Milner PG. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am J Respir Cell Mol Biol. 1993;9:463–466. doi: 10.1165/ajrcmb/9.5.463. [DOI] [PubMed] [Google Scholar]
- 17.Garver RI, Radford DM, Donis-Keller H, Wick MR, Milner PG. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584–1590. doi: 10.1002/1097-0142(19940901)74:5<1584::aid-cncr2820740514>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Aridome K, Tsutsui J, Takao S, Kadomatsu K, Ozawa M, Aikou T, Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995;86:655–661. doi: 10.1111/j.1349-7006.1995.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi T, Kadomatsu K, Okamoto T, Tomoda Y, Muramatsu T. Expression of midkine and pleiotropin in ovarian tumors. Obstet Gynecol. 1997;90:285–290. doi: 10.1016/S0029-7844(97)00237-8. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 1996;56:2515–2518. [PubMed] [Google Scholar]
- 21.Konishi N, Nakamura M, Nakaoka S, Hiasa Y, Cho M, Uemura H, Hirao Y, Muramatsu T, Kadomatsu K. Immunohistochemical analysis of midkine expression in human prostate carcinoma. Oncology. 1999;57:253–257. doi: 10.1159/000012039. [DOI] [PubMed] [Google Scholar]
- 22.Mishima K, Asai A, Kadomatsu K, Ino Y, Nomura K, Narita Y, Muramatsu T, Kirino T. Increased expression of midkine during the progression of human astrocytomas. Neurosci Lett. 1997;233:29–32. doi: 10.1016/s0304-3940(97)00619-8. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawara A, Milbrandt J, Muramatsu T, Deuel TF, Zhao H, Cnaan A, Brodeur GM. Differential expression of pleiotrophin and midkine in advanced neuroblastomas. Cancer Res. 1995;55:1792–1797. [PubMed] [Google Scholar]
- 24.Hidaka H, Yagasaki H, Takahashi Y, Hama A, Nishio N, Tanaka M, Yoshida N, Villalobos IB, Wang Y, Xu Y, et al. Increased midkine gene expression in childhood B-precursor acute lymphoblastic leukemia. Leuk Res. 2007;31:1045–1051. doi: 10.1016/j.leukres.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Owada K, Sanjo N, Kobayashi T, Mizusawa H, Muramatsu H, Muramatsu T, Michikawa M. Midkine inhibits caspase-dependent apoptosis via the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase in cultured neurons. J Neurochem. 1999;73:2084–2092. [PubMed] [Google Scholar]
- 26.Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T, Kadomatsu K. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem. 2000;127:269–277. doi: 10.1093/oxfordjournals.jbchem.a022604. [DOI] [PubMed] [Google Scholar]
- 27.Muramatsu H, Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991;177:652–658. doi: 10.1016/0006-291x(91)91838-4. [DOI] [PubMed] [Google Scholar]
- 28.Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993;159:392–402. doi: 10.1006/dbio.1993.1250. [DOI] [PubMed] [Google Scholar]
- 29.Takada T, Toriyama K, Muramatsu H, Song XJ, Torii S, Muramatsu T. Midkine, a retinoic acid-inducible heparin-binding cytokine in inflammatory responses: chemotactic activity to neutrophils and association with inflammatory synovitis. J Biochem. 1997;122:453–458. doi: 10.1093/oxfordjournals.jbchem.a021773. [DOI] [PubMed] [Google Scholar]
- 30.Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 31.Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, et al. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J Clin Invest. 2000;105:489–495. doi: 10.1172/JCI7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandra F, Harada H, Nakamura N, Ohishi M. Midkine induced growth of ameloblastoma through MAPK and Akt pathways. Oral Oncol. 2004;40:274–280. doi: 10.1016/j.oraloncology.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277:35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 34.Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Antisense oligonucleotides targeting midkine induced apoptosis and increased chemosensitivity in hepatocellular carcinoma cells. Acta Pharmacol Sin. 2006;27:1630–1636. doi: 10.1111/j.1745-7254.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin RX, Tuo CW, Lü QJ, Zhang W, Wang SQ. Inhibition of tumor growth and metastasis with antisense oligonucleotides (Cantide) targeting hTERT in an in situ human hepatocellular carcinoma model. Acta Pharmacol Sin. 2005;26:762–768. doi: 10.1111/j.1745-7254.2005.00762.x. [DOI] [PubMed] [Google Scholar]
- 36.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 37.Martins-Green M, Feugate JE. The 9E3/CEF4 gene product is a chemotactic and angiogenic factor that can initiate the wound-healing cascade in vivo. Cytokine. 1998;10:522–535. doi: 10.1006/cyto.1997.0311. [DOI] [PubMed] [Google Scholar]
- 38.Dai LC, Wang X, Yao X, Min LS, Ping JL, He JF. Antisense oligonucleotides targeting midkine inhibit tumor growth in an in situ human hepatocellular carcinoma model. Acta Pharmacol Sin. 2007;28:453–458. doi: 10.1111/j.1745-7254.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 39.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770–4775. [PubMed] [Google Scholar]
- 40.Beecken WD, Engl T, Hofmann J, Jonas D, Blaheta R. Clinical relevance of serum angiogenic activity in patients with transitional cell carcinoma of the bladder. J Cell Mol Med. 2005;9:655–661. doi: 10.1111/j.1582-4934.2005.tb00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang N, Deuel TF. Pleiotrophin and midkine, a family of mitogenic and angiogenic heparin-binding growth and differentiation factors. Curr Opin Hematol. 1999;6:44–50. doi: 10.1097/00062752-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 42.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takei Y, Kadomatsu K, Goto T, Muramatsu T. Combinational antitumor effect of siRNA against midkine and paclitaxel on growth of human prostate cancer xenografts. Cancer. 2006;107:864–873. doi: 10.1002/cncr.22068. [DOI] [PubMed] [Google Scholar]
- 44.Plate KH, Breier G, Risau W. Molecular mechanisms of developmental and tumor angiogenesis. Brain Pathol. 1994;4:207–218. doi: 10.1111/j.1750-3639.1994.tb00835.x. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 46.Brawer MK, Deering RE, Brown M, Preston SD, Bigler SA. Predictors of pathologic stage in prostatic carcinoma. The role of neovascularity. Cancer. 1994;73:678–687. doi: 10.1002/1097-0142(19940201)73:3<678::aid-cncr2820730329>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol. 1997;80:401–404. doi: 10.1046/j.1464-410x.1997.00374.x. [DOI] [PubMed] [Google Scholar]
- 48.Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, Nichols PW. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 49.Streeter EH, Harris AL. Angiogenesis in bladder cancer--prognostic marker and target for future therapy. Surg Oncol. 2002;11:85–100. doi: 10.1016/s0960-7404(02)00013-0. [DOI] [PubMed] [Google Scholar]


