Abstract
AIM: To study the expression of ether à go-go (Eag1) potassium channel in colorectal cancer and the relation-ship between their expression and clinico-pathological features.
METHODS: The expression levels of Eag1 protein were determined in 76 cancer tissues with paired non-cancerous matched tissues as well as 9 colorectal adenoma tissues by immunohistochemistry. Eag1 mRNA expression was detected in 13 colorectal cancer tissues with paired non-cancerous matched tissues and 4 colorectal adenoma tissues as well as two colorectal cancer cell lines (LoVo and HT-29) by reverse transcription PCR.
RESULTS: The frequency of positive expression of Eag1 protein was 76.3% (58/76) and Eag1 mRNA was 76.9% (10/13) in colorectal cancer tissue. Expression level of Eag1 protein was dependent on the tumor size, lymphatic node metastasis, other organ metastases and Dukes’ stage (P < 0.05), while not dependent on age, sex, site and degree of differentiation. Eag1 protein and mRNA were negative in normal colorectal tissue, and absolutely negative in colorectal adenomas except that one case was positively stained for Eag1 protein.
CONCLUSION: Eag1 protein and mRNA are aberrantly expressed in colorectal cancer and occasionally expressed in colorectal adenoma. The high frequency of expression of Eag1 in tumors and the restriction of normal expression to the brain suggest the potential of this protein for diagnostic, prognostic and therapeutic purposes.
Keywords: Colorectal cancer, Adenoma, Ether à go-go gene, Potassium channels
INTRODUCTION
Ion channels play a vital role in the function of diverse cell types. In recent years, the concept of ion channels as therapeutic targets and diagnostic and prognostic biomarkers has attracted increasing interest, and trigged a wave of experimental results, identifying individual ion channels relevant with specific cancer types.
In 1969, several mutants from the fruit fly Drosophila melanogaster were produced following the treatment of adult males with ethyl methane sulfonate[1]. The mutants presented shaking of the legs under ether anesthesia and independent gene loci were found to be involved. One of these mutants exhibited a slow and rhythmic leg-shaking behavior; the locus involved was then named ether à go-go. Homology screening helped to identify other two sequences closely related to eag: the eag-related gene (erg) and the eag-like gene (elk); thus the EAG channels family comprises three subfamilies: EAG, ERG and ELK[2]. Two members for the eag subfamily, three for erg and two for elk are differentially expressed in different species including rat, bovine and humans. Two members of the eag subfamily are Eag1 (KCNH1, Kv10.1) and Eag2 (KCNH5, Kv10.2)[3].
Eag1 is detected only in the brain and placenta in the process of myoblast fusion, indicating that the channel is not expressed in differentiated peripheral tissues[4]. On the other hand, eag1 is expressed in several cell lines derived from human malignant tumors, such as neuroblastoma[5,6], melanoma[7], breast[5], and cervical carcinoma[5]. In these cell lines, Eag1 enhances the proliferation of the cells, and is required for the maintenance of growth. One of the most intriguing aspects of human eag1 channels is its relationship to cellular transformation. Cells transfected with Eag1 are able to grow in the absence of serum, lose contact inhibition, and induce aggressive tumors when implanted into immune-depressed mice[5]. Recently, functional expression of Eag1 has been described in clinical samples of cervical carcinoma[8]. Similarly, aberrant expression of the channel has also been detected in sarcomas[9] and other tumors from diverse origins[12], while the surrounding tissues are devoid of Eag1 expression. Moreover, specific inhibition of Eag1 expression by antisense technique[5], siRNA[9,10] or by non-specific blockers[7,11] leads to a reduction in tumor cell proliferation in vitro.
The membrane protein Eag1 is accessible from the extracellular side and is predominantly present in tumor cells, represents a potential tumor marker and an interesting therapeutic target for cancer. For any potential clinical application it is an essential prerequisite that samples from human tumors overexpress the target Eag1. Colorectal cancer is a malignancy with a worldwide distribution, and the actual frequency of expression of Eag1 in colorectal tissue remained unknown. In this study, we explored the expression of Eag1 potassium channel in colorectal cancer and the relationship between their expression and clinicopathological features.
MATERIALS AND METHODS
Patients
Samples were obtained from Renmin Hospital of Wuhan University, according to a protocol approved by the Committee for the Conduct of Human Research. These patients had surgery between 2004 and 2005. Resected tissues were formalin-fixed and paraffin-embedded in the Department of Pathology. For immunohistochemical analysis, colorectal cancer tissue with paired non-cancerous matched tissue (NCMT) (n = 76) as well as colorectal adenoma tissue (from endoscopic biopsy) (n = 9) were obtained. For reverse transcription polymerase chain reaction, 13 colorectal cancer tissues with NCMT as well as 4 colorectal adenoma tissues (obtained from endoscopic biopsy) were examined during March to June 2006. These fresh specimens were kept in liquid nitrogen immediately after excision until use. Two pathologists screened histological sections and selected areas of the representative tumor cells. Tumor stage was classified according to Dukes’ criteria.
Immunohistochemistry
For immunohistochemical analysis, 5 μm sections were sliced and mounted on poly-L-lysine-coated slides the day before use. Immunohistochemistry was conducted according to instructions of HistostainTM-Plus kits (Beijing Zhongshan Golden Bridge Biotechnology Co., LTD). The primary antibody Eag1 (Sigma-Aldrich, USA) was diluted 1:200 with 0.1% bovine serum albumin. As negative controls, the slides were treated by replacement of primary antibody with non-immune serum.
TTo achieve a semi-quantitative estimation of Eag1 expression levels, we used an immunohistochemical score method: Scores were 0, less than 10% of the tumor cells stained; 1+, faint staining in more than 10% of the cells; 2+, moderate staining in more than 10% of tumor cells; and 3+, strong staining in more than 10% of the cells. The immunohistochemical score was evaluated as negative (0), weakly positive (1+), and strongly positive (2+, 3+). Each stained slide was scored by two independent observers. There were no major disagreements regarding scoring and the average scoring was reported.
Cell culture
HT29 and LoVo cells (obtained from Cell Bank, Chinese Academy of Sciences) were maintained in T75 flasks in a humidified atmosphere at 37°C with 50 ml/L carbon dioxide and passaged every 4-5 d. The HT-29 line was isolated from primary tumor, and LoVo line was isolated from metastatic tumor nodules in the left supraclavicular region. HT29 cells were cultured in McCoy's 5a medium (modified) with 1.5 mmol/L L-glutamine adjusted to contain 2.2 g/L 90% sodium bicarbonate, 10% fetal bovine serum. LoVo cells were grown in Ham's F12K medium with 2 mmol/L L-glutamine adjusted to contain 1.5 g/L 90% sodium bicarbonate, 10% fetal bovine serum. All media were also supplemented with 100 units/mL penicillin plus 100 μg/mL streptomycin.
RNA preparation and reverse transcription PCR
Total RNA was isolated from colorectal tissue and HT29 and LoVo cells using TRIZOL® reagent (Invitrogen Corporation, USA) following instructions of the TRIZOL kit.
We designed specific primers for Eag1 ( Genbank accession: AF078741) and β-actin. The primers were as follows: For Eag1 (bp966-bp1441, 475 bp), sense primer 5’-GCTTTTGAGAACGTGGATGAG-3’; antisense primer 5’-CGAAGATGGTGGCATAGAGAA-3’. For β-actin (479 bp): sense primer 5’-TGACGGGGTCACCCACACTGTGCC-3’; antisense primer: 5’-CTGCAFCCTGTCGGCAATGCCAG-3’ (479 bp). The primers were synthesized by Shanghai Sangon (China).
One step reverse transcription PCR (RT-PCR) was performed using One Step mRNA Selective PCR Kit 1.1 (TaKaRa Dalian, China) according to the manufacturer’s specifications. The RT-PCR reaction mixture contained 25 μL of 2 × mRNA selective PCR buffer reaction buffer I, 10 μL of MgCl2, 5 μL of dNTP/analog mixture, 1 μL of RNase Inhibitor, 1 μL of AMV Rtase XL, 1 μL of AMV-Optimized taq, 1 μL sense primer (20 μmol/L), 1 μL of antisense primer (20 μmol/L), 1 μL of total RNA, 4 μL of RNase free dH2O to a final volume of 50 μL. Reactions without template RNA were used as a negative control. The RT-PCR for β-actin was used to check the quality of the RNA extraction and RT-PCR. The following RT-PCR conditions were used for Eag1: 1 cycle of 45°C for 25 min; 30 cycles of 88°C for 30 s, 50°C for 45 s, and 72°C for 1 min; and a final cycle of 72°C for 7 min. The conditions for β-actin: 1 cycle of 50°C for 15 min; 30 cycles of 85°Cfor 30 s, 45°C for 45 s, and 72°C for 1 min; and a final cycle of 72°C for 7 min.
Fifteen microliters of PCR products were analyzed by electrophoresis on a 1.5% agarose gel. DNA fragments were visualized and photographed under UV light after ethidium bromide staining. The expected band for each marker was identified by comigration of a DNA marker ladder electrophoresed in an adjacent lane.
Statistical analysis
The χ2-test was used for analysis. Differences between the values were considered significant when P < 0.05.
RESULTS
Eag1 channel is aberrantly expressed in colorectal cancer
Immunopositivity for Eag1 protein was clearly marked on the cytoplasm and/or membrane of cells. Using immunohistochemistry, aberrant expression of Eag1 was detected in tissue of colorectal cancer and negative expression was detected in NCMT. The frequency of expression of Eag1 in colorectal cancer averaged 76.3% (58/76 ) of the cases studied. Figure 1 shows representative positive expression of neoplastic tissue of colorectal cancer and metastatic cancer. Again, using reverse transcription PCR, positive expression of Eag1 was found in 10/13 (76.9%) of colorectal cancer samples and negative expression was found in 13 cases of NCMT (Figure 2, negative expression of NCMT was not shown here). Positive expression of Eag1 was also detected in HT29 and LoVo colorectal cancer cell lines (Figure 2).
Figure 1.
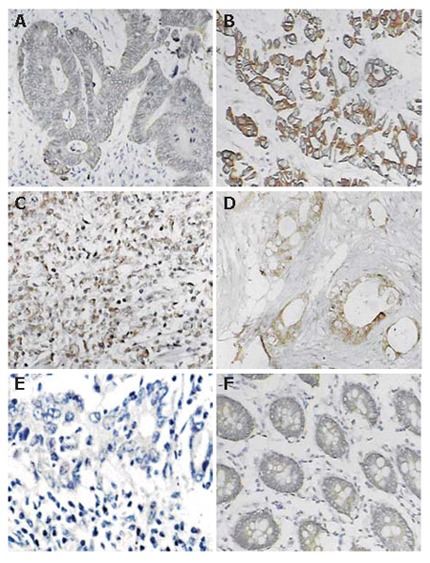
Typical immunohistochemical staining for Eag1 in colorectal cancer and adenoma. A, B, C: Positive staining of colorectal cancers; D: Positive staining of metastasis tissue from greater omentum; E: Negative control of colorectal cancer; F: Positive staining from one case of colonic adenoma.
Figure 2.
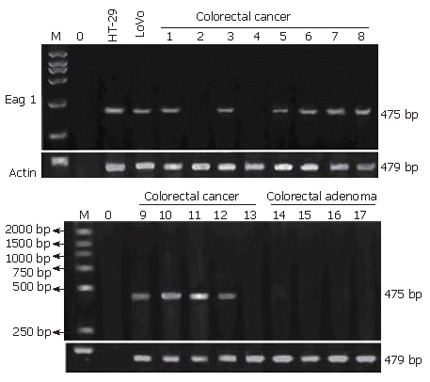
Eag1 mRNA expression in colorectal cancer and adenoma. Eag1 mRNA positively expressed in LoVo and HT29 cell lines and in 10/13 of colorectal cancer tissues, but negatively expressed in NCMT( not shown) and colorectal adenoma. M, molecular weight marker; 0, water.
Eag1 channel is occasionally expressed in colorectal adenoma
Nine cases of colorectal adenoma were also studied by immunohistochemistry, which were negative for Eag1 protein in most of the cases except that in 1 case weak positive expression was detected (Figure 1). In addition, Eag1 mRNA expression was not detected in 4 samples of colorectal adenomas by RT- PCR (Figure 2).
Relationship of Eag1 expression and clinic-pathological features
The immunohistochemistry results showed that no association was detected between Eag1 expression and sex, age, site, differentiation, while expression levels of Eag1 protein were found to be dependent on the tumor size, lymphatic node metastasis, other organ metastases and Dukes’ stage (P < 0.05). Especially, all the tissues from metastatic cancer of other organ or Dukes’ D stage cancer showed an intensively positive immunostaining (Figure 1 and Table 2). Table 1 summarizes the relationship of Eag1 protein expression with clinico-pathological features.
Table 2.
Clinico-pathological features and Eag1 protein expression
| Clinico-pathological features | Number of tumor n (%) | Positive Eag1 expression n (%) | χ2 value |
| Sex | 0.70 | ||
| Male | 44 (57.9) | 32(72.2) | |
| Female | 32 (42.1) | 26(81.3) | |
| Age (yr) | 0.002 | ||
| < 60 | 51 (67.1) | 39 (76.5) | |
| ≥ 60 | 25 (32.9) | 19 (76.0) | |
| Location | 4.52 | ||
| Recta | 28 (36.8) | 22 (78.6) | |
| Left colon | 21 (27.6) | 16 (76.2) | |
| Transverse colon | 9 (11.8) | 6 (66.7) | |
| Right colon | 18 (23.7) | 14 (77.8) | |
| Differentiation | 2.09 | ||
| High | 18 (23.7) | 12 (66.7) | |
| Moderate | 22 (28.9) | 16 (72.7) | |
| Low | 29 (38.2) | 24 (82.8) | |
| Undifferentiated | 7 (9.2) | 6 (85.7) | |
| Size | 6.41a | ||
| < 5 cm | 48 (63.2) | 32 (66.7) | |
| ≥ 5 cm | 28 (36.8) | 26 (92.9) | |
| Lymphatic node metastasis | 5.14a | ||
| N0 | 46 (60.5) | 31 (67.4) | |
| N1 | 30 (39.5) | 27 (90.0) | |
| Other organs metastasis | 4.59a | ||
| M0 | 62 (81.6) | 44 (71.0) | |
| M1 | 14 (18.4) | 14 (100) | |
| Dukes’ stage | 8.89a | ||
| A | 7 (9.2) | 4 (57.1) | |
| B | 39 (51.3) | 26 (66.7) | |
| C | 16 (21.1) | 14 (87.5) | |
| D | 14 (18.4) | 14 (100) |
P < 0.05.
Table 1.
Eag1 immunoreactivity in the colorectal cancers
| Stage |
Eag1 expression1 |
Positive cases | ||
| Negative | Weak | Strong | n (%) | |
| A | 3 | 2 | 2 | 4/7 (57.1) |
| B | 13 | 14 | 12 | 26/39 (66.7) |
| C | 2 | 5 | 9 | 14/16 (87.5) |
| D | 0 | 5 | 9 | 14/14 (100) |
| Total | 18 | 26 | 32 | 58/76 (76.3) |
Intensity of expression was interpreted as negative, weakly positive, and strongly positive, P < 0.05.
DISCUSSION
Because of its restricted distribution in normal tissue and its more ubiquitous distribution in cancer cells and its oncogenic properties, Eag1 channels have gained interest as promising tools for the development of novel diagnostic and therapeutic methods of cancers.
Recently, a few published papers have documented the contribution of potassium channels in the pathological status of cells other than neural or lymphoid origin. A paper of Pardo’s group represents a milestone in the link between Eag1 and cancer[5]. Furthermore, they found that Eag1 expression was limited outside the CNS, but was frequently expressed in clinic tumors from diverse origins, including colon carcinoma[12]. They reported that 6/8 of colon carcinomas were positively expressed. However, the actual frequency of expression of hEAG1 in colorectal cancer remained unknown. Here, we have provided that EAG1 protein and Eag1 mRNA were negatively expressed in normal colorectal tissue, but aberrantly expressed in a large fraction of colorectal cancer tissue and colorectal cell lines. These results strongly suggest the possibility of the use of Eag1 as a tool for the differential diagnosis of malignant transformation together with morphological criteria.
We also found that EAG1 protein was occasionally expressed in colorectal adenoma. Similar, Farias’s group found one of negative pap smears of control cervical samples that presented human papilloma virus infection (the most important etiological factor for cervical cancer) was positive for Eag1 expression[8]. A plausible scenario is that the increase in Eag1 expression in precancer pathological changes could be an early sign of tumor development, namely, Eag1 may be involved in the early development of cancer.
We analyzed relationship of Eag1 expression detected by immunohistochemistry with clinico-pathological features, and found that there was no association between Eag1 expression and sex, age, site, differentiation, while expression of Eag1 was dependent on the tumor size, lymphatic node metastasis, metastases to other organs and Dukes’ stage. Especially, all the tissues from metastatic cancers of other organ showed intensively positive immunostaining. Such a correlation would obviously be of great interest, since the channel could thus serve as a prognostic marker. These conclusions are somewhat different from opinions held by the Pardo’s group. They found there were no correlations between Eag1 expression and demographic factors (sex, age, grade and site of tumor) of soft tissue sarcoma[9]. However, they also found that the tumors from patients who died over the two-year follow up period were much more likely to express significant levels of Eag1. To address this question precisely, quantitative studies of Eag1 expression and prospective studies with larger samples and longer time period will be needed.
Studies of Eag1 in cancer treatment have also provided some promising conclusions. Recently, the Pardo’s group reported siRNA sequences acting specifically on Eag1, reproducibly induced a significant decrease in the proliferation of tumor cell lines while did not trigger any observable non-specific responses[10]. So, siRNA would serve as tools in the future to facilitate the elucidation of both the physiological and pathophysiological functions of this intriguing protein. We are also investigating the roles of Eag1-siRNAs served as tools in colorectal cancer therapy, and trying to clarify the effect of silencing the channel activity on tumor cells.
In conclusion, Eag1 potassium channels are aberrantly expressed in colorectal cancer patients and cell lines. These findings strongly suggest that Eag1 might be used as a potential diagnostic and prognostic marker as well as a potential membrane therapeutic target for colorectal cancer.
Footnotes
S- Editor Wang J L- Editor Zhu LH E- Editor Lu W
References
- 1.Kaplan WD, Trout WE. The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer CK, Schwarz JR. Physiology of EAG K+ channels. J Membr Biol. 2001;182:1–15. doi: 10.1007/s00232-001-0031-3. [DOI] [PubMed] [Google Scholar]
- 4.Occhiodoro T, Bernheim L, Liu JH, Bijlenga P, Sinnreich M, Bader CR, Fischer-Lougheed J. Cloning of a human ether-a-go-go potassium channel expressed in myoblasts at the onset of fusion. FEBS Lett. 1998;434:177–182. doi: 10.1016/s0014-5793(98)00973-9. [DOI] [PubMed] [Google Scholar]
- 5.Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer R, Heinemann SH. Characterization of an eag-like potassium channel in human neuroblastoma cells. J Physiol. 1998;508(Pt 1):49–56. doi: 10.1111/j.1469-7793.1998.049br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavrilova-Ruch O, Schönherr K, Gessner G, Schönherr R, Klapperstück T, Wohlrab W, Heinemann SH. Effects of imipramine on ion channels and proliferation of IGR1 melanoma cells. J Membr Biol. 2002;188:137–149. doi: 10.1007/s00232-001-0181-3. [DOI] [PubMed] [Google Scholar]
- 8.Farias LM, Ocaña DB, Díaz L, Larrea F, Avila-Chávez E, Cadena A, Hinojosa LM, Lara G, Villanueva LA, Vargas C, et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 9.Mello de Queiroz F, Suarez-Kurtz G, Stühmer W, Pardo LA. Ether à go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer. 2006;5:42. doi: 10.1186/1476-4598-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber C, Mello de Queiroz F, Downie BR, Suckow A, Stühmer W, Pardo LA. Silencing the activity and proliferative properties of the human EagI Potassium Channel by RNA Interference. J Biol Chem. 2006;281:13030–13037. doi: 10.1074/jbc.M600883200. [DOI] [PubMed] [Google Scholar]
- 11.Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001;7:345–356. [PubMed] [Google Scholar]
- 12.Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun , et al. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. doi: 10.1186/1476-4598-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


