Abstract
Malignant fibrous histiocytoma (MFH) occurs most commonly in the extremities and trunk, but rarely in the intestine. Here we report two cases of primary intestinal MFH. The first case was a 70-year old man admitted for recurrent right lower quadrant abdominal pain. At laparotomy, a tumor was found originating from the cecum, with a suspicious metastatic nodule on the surface of the right lobe of the liver. A right hemicolectomy was performed followed by an ileotransverse end-to-end anastomotic reconstruction. The second case was a 43-year old man with intussusceptions of the small intestine. An emergent laparotomy revealed 4 pedunculated masses in the small bowel and a partial resection of the small intestine was performed. Though the symptoms were not typical, based on histological and immunohistochemical studies, the patients were diagnosed as MFH of the intestine. They were not treated with chemotherapy or radiotherapy and both died within 3 mo. MFH of the intestine is an extremely rare neoplasm with an aggressive biological behavior. The pathogenesis of this disease has not been clarified to date. Complete surgical excision is preferred, adjuvant chemotherapy or radiotherapy may be advisable.
Keywords: Malignant fibrous histiocytoma, Intestinal neoplasms, Abdominal pain
INTRODUCTION
Malignant fibrous histiocytoma (MFH) is a common soft tissue sarcoma, usually occurring in the extremities. Primary intestinal MFH is a very rare neoplasm, with not more than 40 cases reported in international literatures[1-5]. Here we report two cases of primary intestinal MFH and review other reported cases.
CASE REPORTS
Case 1
A 70-year old man was admitted for recurrent right lower quadrant abdominal pain for about 50 d. He had no recent history of fever, vomiting, diarrhea, or constipation. Physical examination revealed a slightly tender fist-size mass in the right lower quadrant of the abdomen. Laboratory examination showed a white blood cell count of 5.02 × 109/L, and a neutrophil count of 68.3%. Abdominal ultrasonography revealed a large hypo-echoic mass in the right lower quadrant and an abdominal computed tomography demonstrated a large soft-tissue mass in the right lumbar region and iliac fossa (Figure 1A). Chest X-ray and computed tomography showed a mass on the hilum of the right lung (Figure 1B). Barium enema and colonofiberscopy were not performed owing to the patient’s abdominal pain. At laparotomy, a tumor was found originating from the cecum and infiltrating into the right lateral peritoneum. There was a suspicious metastatic nodule located on the surface of the right lobe of the liver. A right hemicolectomy was carried out, and reconstruction was performed by an ileotransverse end-to-end anastomosis. Grossly, an ulcerative annular tumor undergone necrosis was seen in the cecum, measuring 12 cm × 10 cm. There was a tumor nodule on the mesentery, measuring 3 cm × 2 cm. On cut section, the tumor was soft in consistency, solid and grayish-white in color. Microscopic examination revealed that the tumor was pleomorphic and consisted of spindle-shaped cells, oval cells, pleomorphic giant cells, and inflammatory cells. There were involvements of two lymph nodes. Immunohistochemical stains were positive for vimentin (Figure 2), KP-1, α 1-antitrypsin, LYS, P53, Ki67, and negative for S-100, CD1a, CD34, CD117, HMB45, CK, and SMA. The final histopathological diagnosis was MFH of the cecum. Postoperatively, the patient developed pyrexia for about one week which was subsequently followed by breathlessness as a result of lung metastasis. He was not treated with chemotherapy or radiotherapy, and died a month later.
Figure 1.
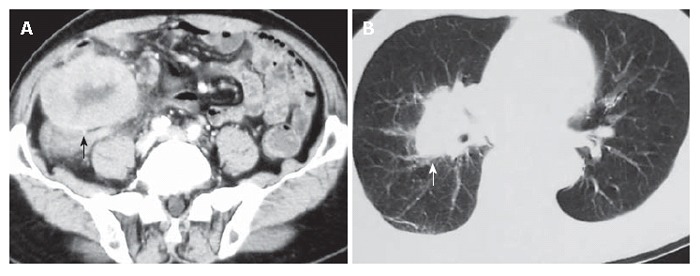
Computed tomography showing a large soft-tissue mass (black arrow) in the right lumbar region and iliac fossa (A) and a mass (white arrow) on the hilum of the right lung (B).
Figure 2.
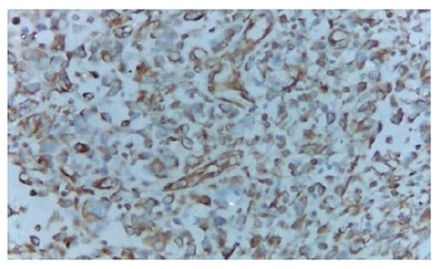
Tumor cells showing positive staining for vimentin (EnVision × 200).
Case 2
A 43-year old man with a history of melena for 10 d had intermittent abdominal pain, fever, vomiting and diarrhea. Physical examination revealed a slightly distended tender abdomen. Laboratory findings showed a white blood cell count of 23.39 × 109/L, and an elevated C-reactive protein level of 193.0 mg/L. Plain abdominal X-ray films showed small bowel obstruction. Computed tomography demonstrated features of multiple intussusceptions, i.e. some portions of the small intestine were seen invaginating into contiguous segments (Figure 3). Chest X-ray and computed tomography showed a mass on the apex of the right lung. The diagnosis of intussusceptions of the small intestine was made, and an emergency operation was performed. At laparotomy, 4 masses measuring 8, 5, 5, 3 cm in diameter were seen in the small bowel and local partial resections of the small intestine were performed. The resected specimens contained pedunculated tumors. Some metastatic nodules were also noted on the mesentery. Microscopic examination demonstrated fibroblast-like cells, histiocyte-like cells and pleomorphic giant cells within the tumor substance (Figure 4). Immunohistochemical stains were positive for vimentin, Ki67 (Figure 5A), P53 (Figure 5B), CK, KP-1 (Figure 5C), LYS, and negative for ER, PR, c-erbB2, MMP2, MMP9, S-100 and HMB45. The pathological findings were compatible with MFH of the small intestine. Postoperatively, the patient had high fever, vomiting and abdominal pain. White blood cell count varied from 26.64 × 109/L to 55.5 × 109/L, and plain abdominal X-ray films demonstrated partial bowel obstruction. The patient was not treated with chemotherapy or radiotherapy, and died 2 mo later.
Figure 3.
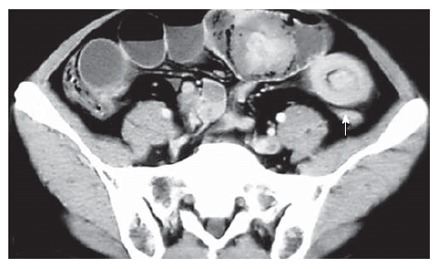
Computed tomography showing features of multiple intussusceptions in the small intestine (white arrow).
Figure 4.
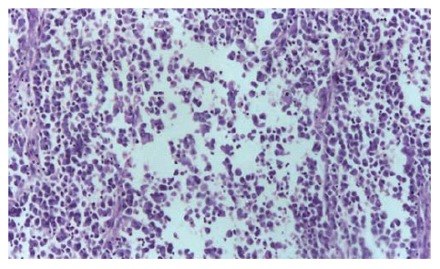
Histology revealing tumor cells consisting of fibroblast-like cells, histiocyte-like cells and pleomorphic giant cells (HE, × 40).
Figure 5.
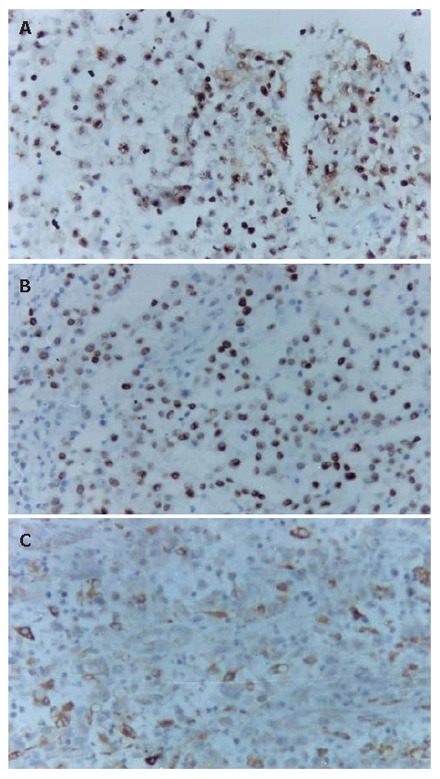
Tumor cells showing positive staining for Ki67 (A), P53 (B), and KP-1 (C) (EnVision × 200).
DISCUSSION
Malignant fibrous histiocytoma (MFH) was first described as malignant histiocytoma and fibrous xanthoma by Ozello et al in 1963, and was established by O’Brien and Stout in 1964 to describe soft tissue sarcomas arising from fibroblasts and histiocytes[3,5]. The peak incidence of extraintestinal MFH occurs in the sixth and seventh decades of life. It usually occurs in the extremities, presenting as a painless mass, and less commonly in the retroperitoneal space, associated with weight loss and increased intra-abdominal pressure[6]. Five histological subtypes of MFH have been described: pleomorphic storiform, myxoid, giant cell, inflammatory, and angiomatoid; the most common being pleomorphic storiform and myxoid types. The first two subtypes tend to be high-grade neoplasms, while the others are usually low-grade sarcomas[7]. The karyotypic abnormalities in MFH are usually complex, with multiple numerical and structural rearrangements[8]. Schmidt[9] reported that chromosomes 1, 3, 6, 9, 12, 16, 18, and 20 are involved in structural aberrations, and that the breakpoint regions are most frequently observed in 1p32, 3p25, and in the centromeric region of chromosomes 1 and 16. The pathogenesis of MFH has not been clarified to date. However, it has been recognized as a complication of radiation, resulting from chronic postoperative repair, trauma, surgical incisions or burn scars[10-13].
Primary MFH of the intestine is very rare. Not more than 40 cases have been reported in international literatures[1-4]. We reviewed the total 37 cases of intestinal MFH, including ours, and summarized the findings as follow. The ratio of men to women was 2.4:1, with an average age of 54.2 years and a range of 12-84 years. The tumor originated from the duodenum in 2 patients, the jejunum in 5, the ileum in 6, the jejunum and the ileum in 1, the cecum in 4, the ascending colon in 6, the transverse colon in 5, the descending colon in 2, the sigmoid colon in 2, the rectum in 3, and from the anal canal in 1. In most patients, the tumor was solitary, while in 4 cases multiple tumors were observed. The distribution of the colorectal MFH was different from that of carcinoma. Despite the fact that most of the tumors were large, ranging from 2 to 19 cm in diameter, their early detection was difficult. The symptoms were not typical: most patients complained of abdominal pain, fever, anorexia, bloody stools and diarrhea, but few had constipation. Thirty-five of the patients were treated surgically, 3 received adjuvant chemotherapy, one was given radiotherapy, and one underwent chemoradiation. In some patients, initial laparatomy revealed metastatic masses, especially in the regional lymph nodes, liver and lungs. Local recurrence was the most common pattern of recurrence in MFH of the intestine. Several patients died of local recurrence or metastasis including our reported two cases.
The diagnosis of MFH depends on an accurate differential diagnosis from other sarcomas, observation of karyomorphism and differential figures, and positive results on immunohistological staining. It was reported that MFH frequently expresses vimentin, actin, CD-68, and α 1-antitrypsin and α 1-antichymotrypsin[14]. Thus, the final diagnosis of our patients was MFH. The differential diagnosis of MFH should include pleomorphic liposarcoma and rhabdomyosarcoma. The former lacks the storiform pattern and shows evidence of cellular differentiation, while the latter shows cross striations on histological examination.
Murata[15] reported that colorectal MFH of the inflammatory type produces granulocyte colony-stimulating factor (G-CSF). Liesveld[16] reported that patients with MFH have leukocytosis, leukemoid reaction, and paraneoplastic syndrome because of various cytokines produced by tumor cells. Thus, postoperative recurrent leukocytosis and elevated CRP level might be predictors for recurrence of MFH. The second case presented to our center had leukocytosis and leukemoid reaction postoperatively.
The treatment for MFH is early and complete surgical excision with en-bloc regional lymph node dissection. Postoperatively, our patients were not given chemotherapy due to their poor general condition. Chemotherapy and radiotherapy were used, but without success. Zagars[17] reported that adjuvant chemotherapy cannot minimize the rate of metastasis. Patients with myxoid tumors do not require systemic therapy. However, patients with nonmyxoid disease exceeding 5 cm are at a significant risk of developing metastases and the development of effective adjuvant treatment is an important research goal. Another report stated that inadequate peritumoral excision is a cause for recurrence[18].
Most of the reports suggest that the prognosis associated with colonic MFH is poor. Weiss and Einzinger’s analysis of MFH[6] showed that the 2-year survival rate of patients with pleomorphic/storiform type of MFH is 60% and the rate of metastases is 42%. Tumor site (extremity vs nonextremity), location (proximal vs distal), size (≤ 5 cm vs > 5 cm), and histology (myxoid vs nonmyxoid) are not significant determinants for final outcome [17]. For metastatic relapse, the major determinants were histology (myxoid vs nonmyxoid) and tumor size. Myxoid tumors have a low metastatic propensity (13% 10-year metastatic rate) compared to nonmyxoid tumors (40% 10-year metastatic rate).
In conclusion, primary intestinal MFH is an extremely rare neoplasm with an aggressive biological behavior. Clinicians and pathologists must consider the possibility of this tumor and include it in the differential diagnoses of intestinal lesions. Complete surgical excision is preferred and adjuvant chemotherapy or radiotherapy may be advisable. Due to the high recurrence, life-long surveillance should be carried out.
Footnotes
S- Editor Wang J L- Editor Wang XL E- Editor Ma WH
References
- 1.Udaka T, Suzuki Y, Kimura H, Miyashita K, Suwaki T, Yoshino T. Primary malignant fibrous histiocytoma of the ascending colon: report of a case. Surg Today. 1999;29:160–164. doi: 10.1007/BF02482242. [DOI] [PubMed] [Google Scholar]
- 2.Singh DR, Aryya NC, Sahi UP, Shukla VK. Malignant fibrous histiocytoma of the rectum. Eur J Surg Oncol. 1999;25:447–448. doi: 10.1053/ejso.1999.0677. [DOI] [PubMed] [Google Scholar]
- 3.Okubo H, Ozeki K, Tanaka T, Matsuo T, Mochinaga N. Primary malignant fibrous histiocytoma of the ascending colon: report of a case. Surg Today. 2005;35:323–327. doi: 10.1007/s00595-004-2915-1. [DOI] [PubMed] [Google Scholar]
- 4.Hiraoka N, Mukai M, Suzuki M, Maeda K, Nakajima K, Hashimoto M, Hosoda Y, Hata J. Malignant fibrous histiocytoma of the cecum: report of a case and review of the literature. Pathol Int. 1997;47:718–724. doi: 10.1111/j.1440-1827.1997.tb04448.x. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa S, Kawachi H, Kurosawa H, Obi Y, Yamanaka K, Nakamura K, Abe T. Malignant fibrous histiocytoma in the ileum associated with intussusception. Dig Dis Sci. 2004;49:1156–1160. doi: 10.1023/b:ddas.0000037804.91628.8d. [DOI] [PubMed] [Google Scholar]
- 6.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250–2266. doi: 10.1002/1097-0142(197806)41:6<2250::aid-cncr2820410626>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Anagnostopoulos G, Sakorafas GH, Grigoriadis K, Kostopoulos P. Malignant fibrous histiocytoma of the liver: a case report and review of the literature. Mt Sinai J Med. 2005;72:50–52. [PubMed] [Google Scholar]
- 8.Szymanska J, Tarkkanen M, Wiklund T, Virolainen M, Blomqvist C, Asko-Seljavaara S, Tukiainen E, Elomaa I, Knuutila S. A cytogenetic study of malignant fibrous histiocytoma. Cancer Genet Cytogenet. 1995;85:91–96. doi: 10.1016/0165-4608(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt H, Körber S, Hinze R, Taubert H, Meye A, Würl P, Holzhausen HJ, Dralle H, Rath FW. Cytogenetic characterization of ten malignant fibrous histiocytomas. Cancer Genet Cytogenet. 1998;100:134–142. doi: 10.1016/s0165-4608(97)00019-8. [DOI] [PubMed] [Google Scholar]
- 10.Fukunaga M, Endo Y, Ushigome S. Radiation-induced inflammatory malignant fibrous histiocytoma of the ileum. APMIS. 1999;107:837–842. doi: 10.1111/j.1699-0463.1999.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 11.Froehner M, Gaertner HJ, Hakenberg OW, Wirth MP. Malignant fibrous histiocytoma of the ileum at a site of previous surgery: report of a case. Surg Today. 2001;31:242–245. doi: 10.1007/s005950170177. [DOI] [PubMed] [Google Scholar]
- 12.Kim GI, Lee JH, Kim HK, Park SH, Kim CH. Malignant fibrous histiocytoma in a chronic burn scar: a rare case report and review of the literature. Burns. 2004;30:742–745. doi: 10.1016/j.burns.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Yücel A, Yazar S, Demirkesen C, Durak H, Dervişoğlu S, Altintaş M. An unusual long-term complication of burn injury: malignant fibrous histiocytoma developed in chronic burn scar. Burns. 2000;26:305–310. doi: 10.1016/s0305-4179(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg AE. Malignant fibrous histiocytoma: past, present, and future. Skeletal Radiol. 2003;32:613–618. doi: 10.1007/s00256-003-0686-1. [DOI] [PubMed] [Google Scholar]
- 15.Murata I, Makiyama K, Miyazaki K, Kawamoto AS, Yoshida N, Muta K, Itsuno M, Hara K, Nakagoe T, Tomita M. A case of inflammatory malignant fibrous histiocytoma of the colon. Gastroenterol Jpn. 1993;28:554–563. doi: 10.1007/BF02776955. [DOI] [PubMed] [Google Scholar]
- 16.Liesveld JL, Rush S, Kempski MC, Turner AR, Brennan JK, Gasson JC, Abboud CN. Phenotypic characterization of the human fibrous histiocytoma giant cell tumor (GCT) cell line and its cytokine repertoire. Exp Hematol. 1993;21:1342–1352. [PubMed] [Google Scholar]
- 17.Zagars GK, Mullen JR, Pollack A. Malignant fibrous histiocytoma: outcome and prognostic factors following conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:983–994. doi: 10.1016/0360-3016(95)02262-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto S, Ahmed AR, Kawaguchi N, Manabe J, Matsushita Y. Results of surgery for malignant fibrous histiocytomas of soft tissue. Int J Clin Oncol. 2003;8:104–109. doi: 10.1007/s101470300018. [DOI] [PubMed] [Google Scholar]


