Abstract
Background
Nonalcoholic steatohepatitis (NASH) is common and severe in patients with diabetes mellitus. Although, there are no effective treatments for NASH in diabetic patients, preliminary reports suggest that polyunsaturated fatty acids (PUFA) may be beneficial in these patients.
Aim
A prospective, randomized, double blind placebo controlled study (NCT 00323414) was performed in NASH patients with diabetes.
Methods
37 patients (50.6±9.8y) with well controlled diabetes (HbA1C<8.5%) were randomized to receive either PUFA containing eicosapentaenoic acid 2160 mg and docosahexaenoic acid 1440 mg daily or an isocaloric, identical placebo containing corn oil for 48 weeks under CONSORT guidelines. Clinical, demographics, biochemical laboratory tests, body composition using DEXA® and liver biopsy were done at randomization and at the end of treatment. Liver biopsy was scored by the NASH CRN criteria. An intention to treat analysis was performed.
Results
At inclusion, gender, age, body weight, biochemical tests, glucose control and liver histology were similar in the 2 treatment groups. There was no change in liver enzymes, body weight or body composition during the study in either group. At the end of treatment, hepatic steatosis and the activity score improved (p<0.05) and lobular inflammation worsened (p<0.001) with placebo but was unchanged with PUFA. At the end of treatment, insulin resistance (serum glucose and HOMA) worsened with PUFA but not placebo.
Conclusions
PUFA provided no benefit over placebo in NASH patients with diabetes. The effects of PUFA on histology and insulin resistance were inferior to placebo. These data provide no support for PUFA supplements in NASH.
Keywords: Diabetes mellitus, nonalcoholic steatohepatitis, polyunsaturated fatty acids
Introduction
Both nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes (DM), which affect 30% and 10% of the US adult population respectively(3;13), are common complex metabolic diseases associated with insulin resistance (30). NAFLD is the most common cause of chronic liver disease (56). Nonalcoholic steatohepatitis (NASH) is the most severe form of NAFLD(37). One third of NASH patients have advanced fibrosis and 20% develop cirrhosis (37). Thus, it is estimated that NAFLD has or will cause 6-8 million Americans to develop cirrhosis. Supporting these estimates is the fact that NAFLD is now the third most common indication for liver transplantation with a trajectory to become the most common in 10 years (5). DM, which is present in 30% of NAFLD patients (35), is now recognized as a major risk factor for liver injury in these patients (55;57).
The recognition of the clinical consequences and underlying molecular mechanisms of NASH (51) has led to a number of treatment strategies that have been studied, predominantly in non-diabetic patients (33). To date, only vitamin E (44) and weight loss (40) have been shown to be safe and effective therapies for reversing NASH. There are no established therapies for NASH patients with DM.
N-3 polyunsaturated fatty acids (PUFA) have been shown in nascent human and animal studies to have a beneficial impact in improving hypertension, hyperlipidemia, endothelial dysfunction, cardiovascular disease (25) and improving hepatic steatosis in NAFLD (38). The n-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to regulate a number of transcription factors that control critical components of hepatic fatty acid metabolism (11;22). N-3 PUFAs are potent activators of PPARα that in turn stimulates fatty acid oxidation (39;60) and PPARγ that increases insulin sensitivity (29), inhibits hepatic lipogenesis via sterol regulatory binding protein-1 expression (54), down regulates pro-inflammatory genes (1;21;27) and reduces hepatic reactive oxygen species (ROS) (20). Human studies with n-3 EPA supplements resulted in improved lipid profile (15;41). Long term treatment with EPA in humans has reported them to be well tolerated and safe (46). These data provide compelling evidence for a therapeutic role of n-3 PUFA in fatty liver; specifically in patients with DM who have multiple metabolic risk factors that can potentially be reversed by the administration of n-3 fatty acids, EPA and DHA. Therefore, we performed a randomized double blind controlled trial in NASH patients with DM.
Subjects and Methods
Selection of patients
Patients were recruited from two medical centers, Cleveland Clinic and MetroHealth Medical Center, in Cleveland, Ohio. Patients were considered for the study if they had an established diagnosis of NASH and a NAFLD activity score (NAS) ≥ 4 on liver biopsy performed within 6 months of entry into the study. Other inclusion criteria were (1) adult diabetic patients (age >18) with at least moderate control of blood sugar (HbA1c <8.5%), (2) a stable regimen of anti-diabetic agents (> 4 months) prior to the biopsy and during the time between biopsy and randomization, (3) appropriate exclusion of other liver disease, (4) ability to give informed consent.
The exclusion criteria were (1) cirrhosis defined on liver biopsy or unequivocal clinical evidence of cirrhosis, (2) daily alcohol intake > 30 g for male and > 20 g for females for at least three consecutive months during the previous 5 years assessed by the Skinner lifetime history questionnaire and the self-administered Audit , (3) end stage organ disease associated with diabetes (renal failure defined as a serum creatinine >2, severe neuropathy, advanced peripheral vascular disease), (4) heart failure (NYHA class 2-4), (5) the use of any amount of fish oil supplements during the 6 months prior to biopsy, (6) the use of medications known to cause steatosis, (7) the use of medications that have shown benefits in previous studies (vitamin E, thiazolidinedione, S-adenosylmethione) (8) other types of liver disease suspected by history, clinical finding or serum biochemistries.
Study Design
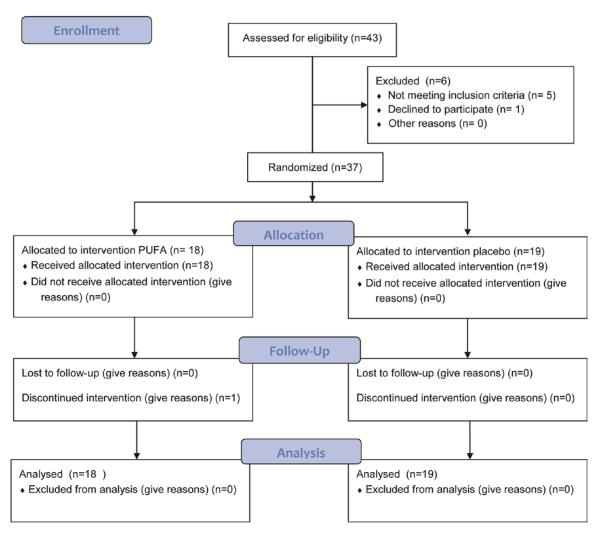
This was a pilot and feasibility study of the NASH Clinical Research Network (CRN) designed as a prospective double blinded, randomized, placebo controlled trial. The study was approved by the Institutional Review Boards at both participating centers and conformed to the Helsinki accord on human subjects in research. It was undertaken in accordance with the CONSORT guidelines (Figure 1). An independent Data and Safety Monitoring Board and a regulatory and compliance monitor provided oversight and supervised the conduction of the study. The study was registered in clinicaltrials.gov NCT00323414.
Figure 1.
CONSORT statement of study design.
Patients, who met the study criteria, were randomized to one of two study groups and received either an oral dose of purified EPA/DHA or placebo (corn oil).The duration of the study was 48 weeks. At entry and at each study visit, study patients were instructed to perform 30 minutes of aerobic exercises 5 days a week and follow a healthy heart diet.
The EPA/DHA supplementation included 2160 mg of EPA and 1440 mg of DHA in 2 divided doses and contained 72 calories. An identical placebo containing corn oil (72 calories) was administered in the same manner. Patients were randomized by the sealed envelope technique using a random numbers table. The codes were broken only after primary analysis was completed.
Both formulations were yellow, oblong capsules without markings and with no discernible differences in odor or taste. The PUFA formulation was a marine fish (anchovy and sardines) concentrate (0pti-EPA™ ) and provided by Douglas Laboratories (Pittsburg PA), which tested the quality and purity of the PUFA. The oil is molecularly distilled fish oil and meets all the specific health limits for dioxins, PCBs and heavy metals as established by the Council for Responsible Nutrition(www.crnusa.org).
Evaluation and Monitoring
Clinical history and physical examination were performed at entry, at 12, 24, 36 and 48 weeks. A follow up liver biopsy was performed at 48 weeks at discontinuation of the study medication. Laboratory studies were performed at entry and at 48 weeks and included blood counts, hepatic function, prothrombin time, renal function, fasting glucose and insulin, HbA1c, and a fasting lipid panel. Patients were followed for 24 weeks after completing the study and clinical and biochemical assessments were repeated. Precise weight and height measurements were obtained. The metabolic syndrome was diagnosed using previously defined criteria (16). Body composition measured using dual energy X-ray absorptiometry (DEXA®) was performed at entry and at the completion of the study. All subjects were evaluated by a dietician for education regarding an American Diabetic Association recommended diet as part of clinical care. However, no specific dietary intervention was planned to avoid additional confounders.
Assessment of Liver Histology
Liver pathologists at each center (A.K at MetroHealth and L.Y. at the Cleveland Clinic) established the histological diagnosis of NASH that was required for entry into the study. Adequacy of the liver biopsy samples was assessed by the study pathologists, who reviewed all entry and end of study biopsies. The histological review used the NASH CRN criteria (23). The grade was based on the individual scores for steatosis, lobular inflammation, ballooning and the composite NAFLD activity score (NAS).
Fibrosis was staged from 0-4 (0 absent, 1a: mild perisinusoidal (seen on Masson Trichrome stain), 1b: moderate perisinusoidal (seen on hematoxylin and eosin stain), 1c: portal/periportal, 2 perisinusoidal and periportal/portal, 3: bridging fibrosis, 4: cirrhosis.
Outcome measures
The primary outcome was defined as an improvement of ≥ 2 points in the NAS. Secondary outcome measures included change in serum transaminases, insulin resistance (HOMA score) and measures of diabetes control (fasting blood glucose and HbA1C) before and after therapy.
Statistical Analysis
Qualitative variables were compared using the chi square test. Quantitative and rating variables were compared using the student’s ‘t’ test for independent variables and the paired ‘t’ test for serial measurements. For multiple group comparisons, analysis of variance with Bonferroni post hoc analysis was used. The primary outcome measure was the change in the composite histological score between the two groups. Since this outcome measure is ordinal, a conservative approach to estimating sample size was to convert it to a dichotomous variable (improved histology versus no change or worsening histology). For the placebo group, the percentage with improved histology was estimated to be 15%. This value represents the percentage of patients with NASH secondary to diabetes who have improved histology one year following their initial biopsy (37). For the PUFA group, the percentage of patients with improved histology is estimated to be 60% (improvement of 45%). This difference (about 50% improvement) has been documented in patients treated with PUFA for other components of the metabolic syndrome (i.e. hypertriglyceridemia)(32). Hence, 18 patients per group will allow us to show a rate of improvement of 15% versus 60% with a power of 80% and a type I error of 0.05 (two-tail). This sample size would also allow us to detect an effect size of 0.83 (Difference / Standard Deviation) with a power of 80% and a Type I Error of 0.05(two-tail) for our secondary outcome measures that are interval in nature (i.e., insulin sensitivity, aminotransferases). The difference in the secondary outcome variable: insulin sensitivity and aminotransferases were anticipated to be 50-80% between the treatment and control groups. This number of patients was more than adequate for the secondary outcome measures that are expected to change between the 2 groups by at least 50-100 %.
An intention to treat analysis was performed. The effect of PUFA and placebo were compared for the primary and secondary outcomes on both an absolute difference and delta change during treatment.
Analysis for Efficacy
Even though the primary intent of this study was to obtain pilot data for a larger, multi-centered study, the data was analyzed (as stated above) for efficacy. Patient compliance was monitored and reinforced with protocol telephone calls and study visits with our research personnel. In addition, compliance with the medication was monitored by research personnel using pill counts and patient self-reporting.
Results
Study Patients
Thirty seven subjects met the above criteria and completed the study. The medication was well tolerated by all subjects. Their baseline clinical and demographic features are shown in table 1. Both groups were comparable at entry regarding demographics, laboratory tests, and metabolic parameters. Females were the majority in both study groups. All patients had the metabolic syndrome with at least 3 components and these were similar in the two treatment groups. The mean serum transaminases did not differ between groups. Serum ALT and AST were greater than 40 IU/dl in 8 patients in the PUFA and 12 patients in the placebo group. Measures of diabetes control including fasting blood glucose and HbA1C were similar (p>0.1) in the 2 treatment groups. Histological evaluation showed that the NAS and its individual components that included steatosis, inflammation, hepatocyte ballooning, and fibrosis were also similar in the 2 groups.
Table 1.
Clinical and laboratory characteristics
| Characteristic | PUFA | Placebo |
|---|---|---|
|
| ||
| Number | 18 | 19 |
|
| ||
| Gender (M:F) | 6:12 | 2:17 |
|
| ||
| Age (y) | 51.5±6.9 | 49.8±12.1 |
|
| ||
| Ethnicity | ||
| Caucasian | 17 (94.4%) | 17 (89.5%) |
| Hispanic | 0 | 2 (10.5%) |
| Black | 1 (5.6%) | 0 |
|
| ||
| Hypertension | 17 (94.4%) | 13 (68.4%) |
|
| ||
| Hyperlipidemia | 9 (50%) | 11 (47.9%) |
|
| ||
| Laboratory results | ||
|
| ||
| AST (units/l) | 47.7±22.8 | 49.1±22.1 |
|
| ||
| ALT (units/l) | 60.1±27.6 | 66.0±43.7 |
|
| ||
| Total bilirubin (mg/dl) | 1.1±0.3 | 1.0±0.1 |
|
| ||
| Fasting glucose (mg/dl) | 129.9±36.5 | 120.5±37.6 |
|
| ||
| HOMA IR | 12.01±6.8 | 15.5±4.9 |
|
| ||
| HbA1C (g/dl) | 6.7±0.9 | 6.7±0.7 |
|
| ||
| Total cholesterol (mg/dl) | 177.9±40.2 | 189.6±47.4 |
|
| ||
| HDL (mg/dl) | 40.3±7.0 | 41.1±9.7 |
|
| ||
| Triglycerides (mg/dl) | 190.2±108.9 | 233.0±169.6 |
|
| ||
| Liver biopsy findings | ||
|
| ||
| Steatosis grade | ||
| 1 | 3 (16.7%) | 4 (21.1%) |
| 2 | 7 (38.9%) | 9 (47.4%) |
| 3 | 8 (44.4%) | 6 (31.6%) |
|
| ||
| Lobular inflammation grade | ||
| 1 | 2 (11.1%) | 4 (21.0%) |
| 2 | 12 (66.6%) | 9 (47.4%) |
| 3 | 4 (22.2%) | 6 (31.6%) |
|
| ||
| Ballooning grade | ||
| 1 | 6 (33.3%) | 7 (36.8%) |
| 2 | 12 (66.7%) | 12 (63.2%) |
|
| ||
| Fibrosis grade | ||
| 1 | 6 (33.3%) | 7 (36.8%) |
| 2 | 6 (33.3%) | 7 (36.8%) |
| 3 | 6 (33.3%) | 5 (26.3%) |
All values mean±SD
Body composition
As displayed in table 2, anthropomorphic and body composition measurements including body weight, body mass index (BMI), whole body fat mass, trunk fat, and total lean mass and bone mass were also similar in the 2 study groups. All patients in both groups were overweight with 17 patients in each group being obese (BMI> 30 kg/m2). There was no significant change in body weight or in any of the measures of body composition in either of the groups during the course of the study.
Table 2.
Body composition in diabetic patients with NASH.
| PUFA | Placebo | |||
|---|---|---|---|---|
| Weight | 98.2±14.8 | 96.4±14.3 | 97.9±27.4 | 98.6±25.1 |
| BMI (kg/m2) | 34.8±4.6 | 34.1±4.6 | 35.7±7.0 | 35.9±6.2 |
| Waist circumference (cm) |
113.1±10.2 | 113.0±11.7 | 110.8±15.7 | 112.1±14.6 |
| Trunk fat | 21.6±5.6 | 20.8±5.5 | 20.9±6.7 | 18.7±5.0 |
| Total body fat | 35.2±8.8 | 33.1±8.9 | 39.5±15.3 | 35.1±8.3 |
| Total body lean mass | 57.6±6.2 | 58.6±6.3 | 52.3±7.3 | 52.7±6.1 |
| Total body bone mass | 24.6±3.6 | 24.8±3.8 | 23.9±4.3 | 24.6±5.4 |
All values mean±SD
All measurements in kg.
Outcome measures
The primary outcomes are displayed in table 3 and figure 2. End of treatment histological outcome measures showed that steatosis and the NAS significantly improved while lobular inflammation worsened in the placebo group (Table 3). In contrast, there was no significant change in any of the histological measures in the PUFA group (Table 3). However, the mean change from initiation to end of treatment in individual components or the composite NAFLD activity score on liver biopsy in the PUFA and placebo groups were not significantly different (Figure 2). Changes in histological components in individual subjects are shown in table 3. Eight patients in the PUFA group and 9 subjects in the placebo group had at least a 2 point reduction in the NAS. Steatosis was unchanged in 7 patients in the PUFA group and in 8 in the placebo group, worsened in 3 patients on PUFA and 1 in the placebo group. In the remainder, steatosis decreased by at least 1 point on the NAS system. Lobular inflammation was unchanged in 8 and worsened by 1 point in 3 patients in the PUFA group while it was unchanged in 7 and did not worsen in any of those treated with placebo. There was improvement in hepatocyte ballooning by at least 1 point in 6 patients in the PUFA group and in 10 patients in the placebo group. Ballooning score was unchanged in 11 patients in PUFA and 7 in the placebo group while it worsened in 1 in the PUFA and 2 in the placebo group, respectively. In the majority of patients, the fibrosis score did not change (12 in PUFA and 9 in placebo group). These were not significantly different between PUFA and placebo treated subjects.
Table 3.
Primary histological outcomes
| Characteristic | PUFA | Placebo | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number | 18 | 19 | ||||
| Entry | End of trt | Entry | End of trt. | |||
|
| ||||||
| NAFLD activity score | 6.12±0.99 | 5.41±1.20 | 5.83±1.25 | 4.11±1.45a | ||
| Decrease by ≥ 2 points | 8 (44.4%) | 9 (47.4%) | ||||
| Unchanged | 7 (38.9%) | 8 (42.1%) | ||||
| Worsened by ≥ 2 points | 3 (16.7%) | 2 (10.5%) | ||||
|
| ||||||
| Steatosis | 2.29±0.77 | 2.06±0.75 | 2.06±0.75 | 1.47±0.72b | ||
| Improved | 8 (44.4%) | 10 (52.6%) | ||||
| Unchanged | 7 (38.9%) | 8 (42.1%) | ||||
| Worsened | 3 (16.7%) | 1 (5.3%) | ||||
|
| ||||||
| Lobular inflammation | 1.88±0.60 | 2.12±0.60 | 1.50±0.51 | 2.17±0.51a | ||
| Improved | 7 (38.9%) | 10 (52.6%) | ||||
| Unchanged | 8 (44.4%) | 8 (42.1%) | ||||
| Worsened | 3 (16.7%) | 1 (5.3%) | ||||
|
| ||||||
| Ballooning | 1 (5.3%) | 1.47±0.51 | 1.69±0.48 | 1.38±0.50 | ||
| Improved | 6 (33.3%) | 10 (52.6%) | ||||
| Unchanged | 11 (61.1%) | 7 (36.8%) | ||||
| Worsened | 1 (5.6%) | 1 (5.6%) | ||||
|
| ||||||
| Fibrosis | 2.13±1.02 | 2.06±0.85 | 1.94±0.77 | 2.00±0.82 | ||
| Improved | 3 (16.7%) | 3 (16.7%) | ||||
| Unchanged | 12 (66.7%) | 9 (47.4%) | ||||
| Worsened | 3 (16.6%) | 4 (21.1%) | ||||
p<0.001 compared to Entry;
p<0.05 compared to entry.
Figure 2.
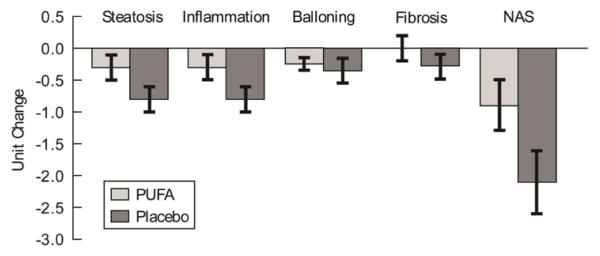
Histograms showing mean (±SEM) of difference in histological scores on liver biopsy in the PUFA and placebo groups.
Displayed in table 4 are the predefined secondary outcome measures. There were no significant changes in hepatic transaminases or total bilirubin in either group. Improvement in serum ALT compared to baseline was observed in 11 subjects in the PUFA and 13 in the placebo. Blood glucose, insulin, HOMA IR, HbA1C, serum triglycerides, HDL and total cholesterol all increased in the PUFA group but only the increases in glucose and HbA1C were significant.
Table 4.
Secondary outcome measures
| Group | PUFA | Placebo | ||
|---|---|---|---|---|
| Characteristic | Baseline | 48 w | Baseline | 48 w |
| Aspartate amino transferase (IU/dl) | 47.7±22.8 | 41.7±17.4 | 47.8±22.1 | 47.9±38.1 |
| Alanine amino transferase (IU/dl) |
60.1±27.6 | 56.9±30.9 | 66.7±44.9 | 59.6±43.8 |
| Serum bilirubin (mg/dl.) | 1.1±0.3 | 1.1±0.3 | 1.0±0.1 | 1.0±0.0 |
| Blood glucose (mg/dl) | 129.9±36.5 | 150.4±43.7a | 121.5±38.4 | 123.5±22.9 |
| Insulin (mIU/L) | 37.7±17.7 | 42.3±20.7 | 55.4±50.6 | 43.6±21.6 |
| HOMA | 12.0±6.8 | 16.1±10.3a | 15.8±15.2 | 13.1±7.3 |
| HbA1C (g/dl) | 6.7±0.9 | 7.5±2.2b | 6.7±0.7 | 6.9±1.1 |
| Serum triglycerides (mg/dl) | 175.2±91.6 | 211.5±160.8 | 232.1±174.5 | 177.4±66.0 |
| Serum HDL (mg/dl.) | 40.6±7.1 | 43.2±8.9 | 40.8±9.4 | 41.6±9.3 |
| Total cholesterol | 177.9±40.2 | 182.5±55.5 | 189.6±47.4 | 187.2±32.1 |
All values mean±SD
p<0.05 compared to baseline;
p= 0.059 compared to baseline
Discussion
This is the first randomized, placebo controlled double blind study comparing omega 3 fatty acid supplementation and isocaloric placebo that used predefined histology as the primary endpoint in diabetic patients with NASH. In these patients with well controlled diabetes, in whom the compliance was excellent and the diagnosis of NASH used well-established histological criteria, PUFA supplementation was not beneficial for either histological or biochemical improvement. In contrast, there was evidence that insulin resistance worsened with PUFA supplementation. These results differ from previous studies that used different endpoints to evaluate PUFA in NASH.
There have been 10 studies (4;6;9;18;36;47;48;50;52;59) [see table, supplementary digital content] and one meta-analysis (38) that have evaluated the efficacy of PUFA in NAFLD. Five were randomized placebo controlled trials with a total of 286 patients (6;32;36;47;59), one was diet controlled with 40 patients (48) and four were open label with a total of 124 patients (4;18;50;52). Table 5 displays the characteristics of our study and the 5 previously published placebo controlled trials. All of the published trials except one performed in children (36) included adults with nonalcoholic fatty liver seen on ultrasound. The duration of the placebo controlled trials ranged from 8 weeks to 12 months and the dose varied between 830 mg to 6 grams. One study used pure DHA (36), one used pure EPA (50), while all the other trials used a mixture of both EPA and DHA. Only 2 of the published trials (36;50) performed entry liver biopsies and only one (50) obtained post treatment biopsies but only in seven of the 23 (30.4%) enrolled patients. Improvement in the amount of fat on ultrasound was the primary outcome for both the placebo (6;9;36;47;59) and non-placebo (4;18;48;50;52) controlled studies. There was significant heterogeneity in the design, type of PUFA used and the duration of follow-up (supplementary table). Furthermore, most studies did not include diabetic patients.
Table 5.
Placebo Controlled Trials in NAFLD
| Author (Year) | n | Method of Diagnosis |
Dose | Duration | Outcome1 |
|---|---|---|---|---|---|
| Zhu [34] | 144 | Ultrasound | 6,000 | 24 weeks | FOU, ALT, Lipids |
| Chen [35] | 46 | Ultrasound | 5,000 | 24 weeks | FOU |
| Cussons [36] 2 | 25 | Ultrasound | 4,000 | 8 weeks | FOU |
| Sofi [33] | 11 | Ultrasound | 830 | 12 months | FOU |
| Nohili [32] 3 | 60 | Ultrasound/ Liver Biopsy |
250/500 | 6 months | FOU |
| Dasarathy (present study) |
37 | NASH on Biopsy | 3,600 | 48 weeks | Liver Histology |
FOU = Fat on ultrasound
This was a crossover study
Only DHA used at two different doses: 250 and 500 mg
PUFA supplements significantly decreased the amount of hepatic fat observed on ultrasound in most of these studies (4;6;9;36;47;48;50;59), as was the case in a meta-analysis that included most but not all of the PUFA trials (38).These results support the strategy of PUFA supplements in NAFLD patients, who have been reported to consume less polyunsaturated fat intake (34) and fish (58) and a higher ratio of n-6/n-3 (8) in their diet, as compared to controls.
Although these studies suggest that PUFA are effective in improving liver fat content, the effect of PUFA on other components of NASH, specifically those histological features believed to predict progression, including hepatocyte ballooning and fibrosis, were not assessed. An additional limitation to these trials is the lack of standardized, protocol liver biopsies with predetermined histological end points. This is especially relevant given the limitation of ultrasound to precisely quantify hepatic fat content, and inability to identify histological measures of hepatocellular injury (10). To our knowledge, the present study is the first of its kind to examine the role of both EPA and DHA in a double blind, randomized study in well characterized NASH patients with diabetes. In the current study, both DHA and EPA were used rather than purified DHA or EPA because each has a different effect on hepatic fatty acid metabolism (14;53). Although the optimal dose of PUFA supplements remains unclear (28), we chose the dose of 3.6 g because similar doses have shown to be beneficial for cardiovascular disease (45) hypertriglyceridemia (32) and NAFLD.
Our data showed that over 48 weeks of treatment, there were no significant beneficial effects of PUFA in this population. The two treatment groups were similar in terms of demographic characteristics and diabetic control as determined by HbA1C. Importantly, there was no change in body weight or body composition over the course of study in either study group. Of note, patients treated with PUFA did not show a statistically significant improvement in histology while patients on placebo had significant improvement in steatosis and in the NAFLD activity score. In addition, markers of glycemic control worsened in the PUFA treated patients while lipid control did not change. These data are in contrast to previous data on the analysis of 23 trials using omega 3 PUFA in type 2 diabetes mellitus (17). Plasma triglyceride levels decreased while glycemic control and HDL did not change. The dose of PUFA in the present study was similar to those previously reported with beneficial effects.
Our observations in the present study differ from the randomized and open labeled studies previously reported. Even though the number of patients who showed improvement in overall histology was similar between the PUFA and placebo groups, it is interesting that the mean NAS improved in the placebo group but not in the PUFA. Improvement of patients in the placebo arm in this study is consistent with previous reports in randomized controlled studies on non diabetic patients with NASH that spontaneous improvement in liver histology occurs in 15-26% (7;19). These observations also suggest that PUFA might adversely affect NASH patients with DM. This could be due to worsening of glucose control as seen in the current study. Another possible explanation could be the use of other supplements including vitamin E but none of these patients had reported ingestion of vitamin E or other food supplements for the 6 months prior to or during the study period. Use of n-3 PUFA could have increased hepatic mitochondrial fatty acid oxidation by activation of PPARα with increased oxidative stress (26;49) that could also have resulted in failure of beneficial effects. In addition, recent studies have shown a lack of clinical efficacy of PUFA in cardiovascular trials (24;25;42;43) and even potential harm in a cancer trial (2).
One of the limitations of the present study was that neither tissue nor plasma EPA and DHA were quantified and this could have contributed to the negative results. However, compliance was carefully assessed by clinical criteria (pill count technique and patient recall). Variable absorption and metabolism may also contribute to differential effects of the medications. Another limitation is the relatively small sample size that might have allowed for a type 2 error and potentially missing a positive effect of PUFA. However, this is unlikely given the relative positive effect in the placebo group compared to PUFA. Finally, patients were not rigorously monitored for dietary compliance and this may have contributed to worsening glycemic control and failure to respond to PUFA.
We therefore believe that it is principally our study design of using change in liver histology as an outcome measure and the study population of diabetic patients that explains our essentially negative observations. In fact, as mentioned earlier, PUFA may be worse than placebo in this patient group. Our data suggests that patients with type 2 diabetes may demonstrate metabolic heterogeneity determined by the presence or absence of NASH. The potential mechanisms by which omega 3 PUFAs lower plasma triglycerides include suppression of SREBP-1 with resultant reduced lipogenesis, decreased VLDL secretion and enhanced hepatic clearance of lipoproteins requiring a hepatic response (12). It is possible that patients with NASH do not respond appropriately to PUFA. This is the first study of its kind to specifically evaluate the response of an intervention that depends on its hepatic effects in diabetic patients with NASH. These data therefore provide a paradigm shift in developing therapeutic interventions for diabetic patients and suggest that response to therapy may be determined by the severity of underlying hepatic dysfunction.
We conclude that despite strong rationale for the use of PUFA in NAFLD (31) the present study shows that in a well characterized population of NASH patients with diabetes, supplementation of n-PUFA provided no beneficial effects, and may potentially be inferior to placebo in terms of histological progression of the disease. Results from long term studies that are ongoing on the use of n-3 PUFA in NASH will need to be evaluated before PUFA can be recommended as a therapeutic intervention in this population at high risk of progression to cirrhosis. (Clinicaltrials.gov NCT 00323414).
Supplementary Material
Acknowledgements
We acknowledge the assistance provided by the staff, nurses and laboratory staff of the Clinical research units at MetroHealth Medical Center and the Cleveland Clinic. Research Funding for the study was provided by the National Institutes of Health:
U01061732
DK83414
CTSC grant UL1TR000439
Douglas Laboratories and their Vice President Andrew Hoelpner assisted with providing the PUFA and placebo in a masked manner.
Statement of Interests
SD, JD, LY, CH, RS and AJM were partly funded by NIH U01061732
SD was also partly funded by DK83414
The CRU was funded by the CTSC grant UL1TR000439
List of abbreviations
- NASH
Non alcoholic steatohepatitis
- PUFA
polyunsaturated fatty acids
- HOMA
homeostatic model of assessment
- DEXA
dual energy Xray absorptiometry
- NAFLD
non alcoholic fatty liver disease
- DM
diabetes mellitus
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- ROS
reactive oxygen species
- NAS
NAFLD activity score
- CRN
clinical research network
- BMI
body mass index
- SD
standard deviation
- SEM
standard error of mean
Footnotes
Trial Registration. Clinicaltrials.gov(NCT 00323414)
No conflict of interest for any of the authors
Reference List
- 1.Al-Gayyar MM, Shams ME, Barakat EA. Fish oil improves lipid metabolism and ameliorates inflammation in patients with metabolic syndrome: impact of nonalcoholic fatty liver disease. Pharm Biol. 2012;50:297–303. doi: 10.3109/13880209.2011.604088. [DOI] [PubMed] [Google Scholar]
- 2.Brasky TM, Darke AK, Song X, et al. Plasma Phospholipid Fatty Acids and Prostate Cancer Risk in the SELECT Trial. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Capanni M, Calella F, Biagini MR, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 5.Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Chen R, Guo Q, Zhu W, et al. Therapeutic efficacy of (omega)-3 polyunsaturated fatty acid capsule in the treatment of patients with non-alcoholic fatty liver disease. 2008. pp. 2002–2008.
- 7.Chitturi S. Treatment options for nonalcoholic Fatty liver disease. Therap Adv Gastroenterol. 2008;1:173–189. doi: 10.1177/1756283X08096951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez-Pinto H, Jesus L, Barros H, et al. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr. 2006;25:816–823. doi: 10.1016/j.clnu.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 9.Cussons AJ, Watts GF, Mori TA, et al. Omega-3 fatty acid supplementation decreases liver fat content in polycystic ovary syndrome: a randomized controlled trial employing proton magnetic resonance spectroscopy. J Clin Endocrinol Metab. 2009;94:3842–3848. doi: 10.1210/jc.2009-0870. [DOI] [PubMed] [Google Scholar]
- 10.Dasarathy S, Dasarathy J, Khiyami A, et al. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducheix S, Montagner A, Polizzi A, et al. Essential fatty acids deficiency promotes lipogenic gene expression and hepatic steatosis through the liver X receptor. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr. 2005;135:2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 14.Froyland L, Vaagenes H, Asiedu DK, et al. Chronic administration of eicosapentaenoic acid and docosahexaenoic acid as ethyl esters reduced plasma cholesterol and changed the fatty acid composition in rat blood and organs. Lipids. 1996;31:169–178. doi: 10.1007/BF02522617. [DOI] [PubMed] [Google Scholar]
- 15.Grimsgaard S, Bonaa KH, Hansen JB, et al. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am J Clin Nutr. 1997;66:649–659. doi: 10.1093/ajcn/66.3.649. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit Pathw Cardiol. 2005;4:198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Hartweg J, Perera R, Montori V, et al. Omega-3 polyunsaturated fatty acids (PUFA) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008:CD003205. doi: 10.1002/14651858.CD003205.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzitolios A, Savopoulos C, Lazaraki G, et al. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23:131–134. [PubMed] [Google Scholar]
- 19.Hoofnagle JH, Van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. doi: 10.1111/apt.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishii H, Horie Y, Ohshima S, et al. Eicosapentaenoic acid ameliorates steatohepatitis and hepatocellular carcinoma in hepatocyte-specific Pten-deficient mice. J Hepatol. 2009;50:562–571. doi: 10.1016/j.jhep.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 21.Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–247. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, Lee KT, Park YB, et al. Dietary docosahexaenoic acid-rich diacylglycerols ameliorate hepatic steatosis and alter hepatic gene expressions in C57BL/6J-Lep(ob/ob) mice. Mol Nutr Food Res. 2008;52:965–973. doi: 10.1002/mnfr.200700315. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van NM, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Kotwal S, Jun M, Sullivan D, et al. Omega 3 Fatty acids and cardiovascular outcomes: systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:808–818. doi: 10.1161/CIRCOUTCOMES.112.966168. [DOI] [PubMed] [Google Scholar]
- 25.Kromhout D, Yasuda S, Geleijnse JM, et al. Fish oil and omega-3 fatty acids in cardiovascular disease: do they really work? Eur Heart J. 2012;33:436–443. doi: 10.1093/eurheartj/ehr362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larter CZ, Yeh MM, Cheng J, et al. Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. J Gastroenterol Hepatol. 2008;23:267–275. doi: 10.1111/j.1440-1746.2007.05157.x. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Ruan XZ, Powis SH, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Chen D. The optimal dose of omega-3 supplementation for non-alcoholic fatty liver disease. J Hepatol. 2012;57:468–469. doi: 10.1016/j.jhep.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem. 2006;17:1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010;31:679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 32.McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715–728. doi: 10.1592/phco.27.5.715. [DOI] [PubMed] [Google Scholar]
- 33.Musso G, Gambino R, Cassader M, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 34.Musso G, Gambino R, De MF, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 35.Neuschwander-Tetri BA, Clark JM, Bass NM, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 37.Pagadala MR, McCullough AJ. The relevance of liver histology to predicting clinically meaningful outcomes in nonalcoholic steatohepatitis. Clin Liver Dis. 2012;16:487–504. doi: 10.1016/j.cld.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56:944–951. doi: 10.1016/j.jhep.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem. 2003;278:35931–35939. doi: 10.1074/jbc.M306238200. [DOI] [PubMed] [Google Scholar]
- 40.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rambjor GS, Walen AI, Windsor SL, et al. Eicosapentaenoic acid is primarily responsible for hypotriglyceridemic effect of fish oil in humans. Lipids. 1996;31(Suppl):S45–S49. doi: 10.1007/BF02637050. [DOI] [PubMed] [Google Scholar]
- 42.Rizos EC, Ntzani EE, Bika E, et al. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 43.Roncaglioni MC, Tombesi M, Avanzini F, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 44.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saravanan P, Davidson NC, Schmidt EB, et al. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376:540–550. doi: 10.1016/S0140-6736(10)60445-X. [DOI] [PubMed] [Google Scholar]
- 46.Saynor R, Gillott T. Changes in blood lipids and fibrinogen with a note on safety in a long term study on the effects of n-3 fatty acids in subjects receiving fish oil supplements and followed for seven years. Lipids. 1992;27:533–538. doi: 10.1007/BF02536136. [DOI] [PubMed] [Google Scholar]
- 47.Sofi F, Giangrandi I, Cesari F, et al. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: a preliminary study. Int J Food Sci Nutr. 2010;61:792–802. doi: 10.3109/09637486.2010.487480. [DOI] [PubMed] [Google Scholar]
- 48.Spadaro L, Magliocco O, Spampinato D, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis. 2008;40:194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Sugiyama E, Ishikawa Y, Li Y, et al. Eicosapentaenoic acid lowers plasma and liver cholesterol levels in the presence of peroxisome proliferators-activated receptor alpha. Life Sci. 2008;83:19–28. doi: 10.1016/j.lfs.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka N, Sano K, Horiuchi A, et al. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42:413–418. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 51.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 52.Vega GL, Chandalia M, Szczepaniak LS, et al. Effects of N-3 fatty acids on hepatic triglyceride content in humans. J Investig Med. 2008;56:780–785. doi: 10.2310/JIM.0b013e318177024d. [DOI] [PubMed] [Google Scholar]
- 53.Willumsen N, Hexeberg S, Skorve J, et al. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J Lipid Res. 1993;34:13–22. [PubMed] [Google Scholar]
- 54.Xu J, Nakamura MT, Cho HP, et al. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- 55.Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- 56.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Zein CO, Unalp A, Colvin R, et al. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:753–759. doi: 10.1016/j.jhep.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zelber-Sagi S, Lotan R, Shlomai A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–1151. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Zhu FS, Liu S, Chen XM, et al. Effects of n-3 polyunsaturated fatty acids from seal oils on nonalcoholic fatty liver disease associated with hyperlipidemia. World J Gastroenterol. 2008;14:6395–6400. doi: 10.3748/wjg.14.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuniga J, Cancino M, Medina F, et al. N-3 PUFA supplementation triggers PPAR-alpha activation and PPAR-alpha/NF-kappaB interaction: anti-inflammatory implications in liver ischemiareperfusion injury. PLoS One. 2011;6:e28502. doi: 10.1371/journal.pone.0028502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.