Abstract
NF-κB is involved in a variety of biological processes, including cancer development. In this issue of Cancer Cell, Kuo et al. show the essential role for IKK/NF-κB signaling in epigenetic regulation by MLL oncoproteins to maintain leukemia stem cells.
MLL fusion leukemia is an aggressive blood cancer carrying chimeric fusion of the MLL gene. The MLL gene encodes a DNA-binding protein that contains a C-terminal SET domain with histone H3 lysine 4 (H3K4) methyltransferase activity. MLL is targeted by at least 70 different chromosomal translocations, resulting in the fusion of the 5′ portion of MLL to a number of different partner genes in leukemia patients. MLL fusion proteins lose the SET domain and H3K4 methyltransferase activity, but instead acquire aberrant functions for epigenetic regulation. The H3K79-specific methyltransferase DOT1L is associated with many of the fusion partner proteins and enhances H3K79 dimethylation at MLL fusion loci. Wild-type MLL also collaborates with MLL fusion proteins in regulating target gene expression partly by increasing H3K4 trimethylation. As a result, MLL fusion proteins induce constitutive expression of MLL target genes, including HOXA9 and MEIS1, thereby promoting self-renewal capabilities in hematopoietic stem and progenitor cells. Recent studies have identified several crucial epigenetic regulators and chromatin modifiers in MLL fusion leukemia. Those include epigenetic modifying enzymes DOT1L and PRMT1, histone demethylase KDM1A, polycomb group proteins EZH2 and CBX8, the PAFc complex, and bromodomain-containing protein BRD4. Inhibition of these factors has shown a dramatic effect to reverse the inappropriate expression of key MLL target genes that drive leukemogenesis. Therefore, therapeutic potential of targeting these epigenetic regulators in MLL fusion leukemia has received much attention in recent years (Neff and Armstrong, 2013).
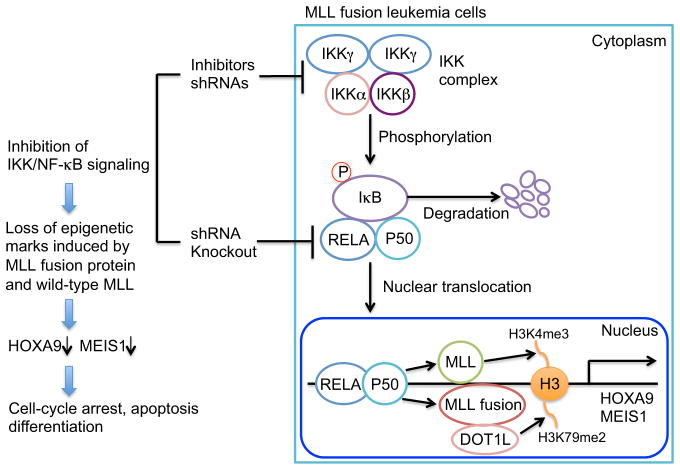
In this issue of Cancer Cell, Kuo et al. describe an unexpected role of IKK/NF-κB signaling as a coordinator for epigenetic regulation induced by MLL-fusion proteins (Kuo et al., 2013). NF-κB is a dimeric complex of transcription factors mainly consisting of RELA (p65)/p50 (NFKB1; canonical pathway) or RELB/p52 (NFKB2; noncanonical pathway). The canonical pathway broadly modulates cell proliferation, survival, and inflammation, whereas the noncanonical pathway mainly controls lymphomagenesis. In the canonical pathway, the RELA/p50 complex is held in the cytoplasm by the inhibitory IκB. During activation, IKKα/β/γ complex phosphorylates IκB and triggers its degradation, resulting in nuclear translocation of RELA/p50 and transactivation of target genes (Hayden and Ghosh, 2008). By employing a non-biased screening approach with a short hairpin RNA (shRNA) library, the authors found knockdown of multiple kinases/phosphatases involved in NF-κB signaling had substantial growth-inhibitory effects in MLL fusion leukemia. Network analyses using global DNA methylation and gene expression profiles in human acute myeloid leukemia (AML) also revealed the connection between NF-κB signaling and MLL fusion leukemia. Furthermore, NF-κB was strongly activated by lipopolysacccharide stimulation in MLL fusion leukemia cells compared with other types of leukemia cells. The authors then performed extensive functional studies using shRNAs, knockout mice, and pharmacological inhibitors for IKK/NF-κB signaling. IKK inhibitors and RELA knockdown inhibited the growth of human AML cell lines with MLL fusion. In murine transformation models, IKK inhibitors and shRNA-mediated knockdown of key NF-κB molecules (Ikkα, Ikkβ, Ikkγ, and RELA) impaired colony formation and in vivo leukemia development induced by MLL fusion proteins. Furthermore, cells derived from RELA-deficient mice were unable to sustain continuous replating of MLL-fusion-transduced cells, confirming a functional requirement for IKK/NF-κB signaling in MLL leukemogenesis. Conversely, forced RELA expression accelerated the development of MLL fusion leukemia. Mechanistically, IKK inhibitor treatment induced cell-cycle arrest, apoptosis, differentiation, and substantial reduction of leukemia stem cell frequency in both mouse and human MLL fusion leukemia cells. Gene expression analysis suggested that NF-κB is responsible for promoting the oncogenic program associated with MLL fusion leukemia. IKK inhibitors and RELA knockdown decreased expression of the primary MLL target genes Hoxa9 and Meis1. Promoter occupancy profile of RELA was similar to that of MLL-AF10, and occupancy of both was reduced by treatment with IKK inhibitors. Of note, IKK inhibition also led to a decrease in the epigenetic marks on the promoters of Hoxa9 and Meis1 genes. IKK inhibitors reduced promoter occupancy of MLL-AF10, resulting in a loss of DOT1L-mediated H3K79 dimethylation marks. Inhibition of IKK also reduced binding of wild-type MLL and the level of H3K4 trimethylation on Meis1 and Hoxa9 promoter regions. Together, these results suggest that IKK/NF-κB signaling is necessary for maintaining epigenetic marks induced by MLL fusion protein and wild-type MLL in leukemia cells (Figure 1).
Figure 1. Therapeutic Targeting of Canonical NF-κB Pathway in MLL-Fusion Leukemia.
Inhibition of IKK/NF-κB signaling results in loss of epigenetic marks on HOXA9 and MEIS1 promoters, which ultimately induces cell-cycle arrest, apoptosis, and differentiation in MLL fusion leukemia cells. me3, trimethylation; me2, dimethylation. The figure is modeled on the online graphical abstract in Kuo et al. (2013), with some modifications.
These exciting findings by Kuo et al. will be the basis for much future basic and preclinical research. Many studies have focused on downstream signals associated with oncogene expression in an attempt to reveal the addictive signals that can be therapeutically targeted in cells expressing the oncogene. One particularly novel outcome of this study is the identification of an upstream pre-existing signaling pathway that converges on MLL and MLL fusion protein recruitment to chromatin. Blockade of NF-κB signaling could be expected to interfere with all signals emanating from MLL fusion activation of HoxA9 and Meis1 expression. How broadly NF-κB regulation impacts on MLL and MLL fusion signaling and whether NF-κB plays a role in regulating other MLL target genes, such as MECOM/Evi1 (Arai et al., 2011), remain to be determined. The particular association between NF-κB and MLL fusion leukemia raises additional questions that warrant further study. Numerous other subclasses of AML show increased HoxA9 and Meis1 expression, including the NPM1c-associated subtype, but no overlapping gene sets were identified that would implicate NF-κB in HoxA9 regulation in this AML subset. How these genes are regulated independently of NF-κB signals could reveal novel signaling cascades that can be therapeutically targeted in these AMLs. Whether NF-κB is also required for MLL-partial tandem duplication mediated leukemia is of interest, as this genetic aberration is recognized as a frequent player in myelodysplastic syndrome and AML. In addition, the data from this study raise questions as to whether NF-κB signaling works collaboratively with other epigenetic regulators in various biological processes. Interestingly, recent studies have shown that wild-type MLL stabilizes RUNX1 (Huang et al., 2011), that RUNX1 attenuates NF-κB signaling through interaction with IKK complex (Nakagawa et al., 2011), and that deletion of Runx1 could accelerate MLL-ENL mediated AML in a mouse model (Nishimoto et al., 2011). Therefore, it is tempting to speculate that reduced RUNX1 expression may be required in MLL fusion leukemia for full NF-κB activation. We can also not ignore NF-κB functions independent of epigenetic regulation in MLL fusion leukemia. Crosstalk between NF-κB and other signaling pathways that are known to be active in MLL fusion leukemia, such as WNT (Wang et al., 2010) and Rac (Mizukawa et al., 2011; Wei et al., 2008), may play important roles in promoting leukemogenesis.
Although there are several unanswered mechanistic questions, the results presented in this article highlight the potential therapeutic implications of targeting IKK/NF-κB signaling in MLL fusion leukemia. Targeting this pathway may also be effective in other types of AML. For example, Nakagawa et al. recently showed that inhibition of NF-κB signaling efficiently blocked the growth of RUNX1-mutated leukemia cells (Nakagawa et al., 2011). Moreover, such therapies will perhaps have promise for treating other types of cancer that are dependent on similar aberrant transcriptional or epigenetic activity for survival. Given the diverse functions of NF-κB, however, side effects of NF-κB-directed therapy will be a concern. Indeed, Kuo et al. pretreated leukemia cells prior to transplantation for the in vivo study in this article, because IKK inhibitors used in their study were too toxic for chronic systemic administration to mice. Careful in vivo preclinical studies, ideally with primary AML cells and various inhibitors for the IKK/NF-κB signaling pathway, will be necessary before translating these findings into the clinic.
References
- Arai S, Yoshimi A, Shimabe M, Ichikawa M, Nakagawa M, Imai Y, Goyama S, Kurokawa M. Blood. 2011;117:6304–6314. doi: 10.1182/blood-2009-07-234310. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Huang G, Zhao X, Wang L, Elf S, Xu H, Zhao X, Sashida G, Zhang Y, Liu Y, Lee J, et al. Blood. 2011;118:6544–6552. doi: 10.1182/blood-2010-11-317909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H-P, Wang Z, Lee D-F, Iwasaki M, Duque-Afonso J, Wong SHK, Lin C-H, Figueroa ME, Su J, Lemischka IR, Cleary ML. Cancer Cell. 2013;24:423–437. doi: 10.1016/j.ccr.2013.08.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukawa B, Wei J, Shrestha M, Wunderlich M, Chou FS, Griesinger A, Harris CE, Kumar AR, Zheng Y, Williams DA, Mulloy JC. Blood. 2011;118:5235–5245. doi: 10.1182/blood-2011-04-351817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Shimabe M, Watanabe-Okochi N, Arai S, Yoshimi A, Shinohara A, Nishimoto N, Kataoka K, Sato T, Kumano K, et al. Blood. 2011;118:6626–6637. doi: 10.1182/blood-2010-12-326710. [DOI] [PubMed] [Google Scholar]
- Neff T, Armstrong SA. Blood. 2013;121:4847–4853. doi: 10.1182/blood-2013-02-474833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N, Arai S, Ichikawa M, Nakagawa M, Goyama S, Kumano K, Takahashi T, Kamikubo Y, Imai Y, Kurokawa M. Blood. 2011;118:2541–2550. doi: 10.1182/blood-2010-10-315440. [DOI] [PubMed] [Google Scholar]
- Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, Zheng Y, Cancelas JA, Gu Y, Jansen M, et al. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]