Abstract
Background
Peripheral neuropathy (PN) is a frequent complication of chronic HIV infection. We prospectively studied individuals with primary HIV infection (PHI, <1 year after transmission) to assess the presence of and laboratory associations with PN in this early stage.
Methods
Standardized examination and analysis of blood and cerebrospinal fluid (CSF) was performed in participants with laboratory-confirmed PHI. PN was defined as ≥1 of the following unilateral or bilateral signs: decreased distal limb position, vibration, or temperature sense, or hyporeflexia; symptomatic PN (SPN) as presence of these signs with symptoms. Analysis employed nonparametric statistics.
Results
20/58 (35%) antiretroviral-naïve male subjects without diabetes evaluated at a median 107 days post HIV transmission (dpt) met criteria for PN. 13/20 (65%) of PN subjects met criteria for SPN; 6/20 (30%) had bilateral findings. PN subjects and no PN subjects (NPN) did not differ in median age, dpt, blood CD4 or CD8 counts, CSF or plasma HIV RNA levels, CSF white blood cell counts, or CSF:blood albumin ratio. PN and SPN subjects had elevated CSF neopterin (p=0.003 and p=0.0005), CSF MCP-1 (p=0.006 and p=0.01) and blood neopterin (p=0.006 and p=0.009) compared to NPN. PN subjects had a higher percentage of activated phenotype CSF CD8+ T lymphocytes than NPN subjects (p=0.009).
Conclusions
Signs of PN were detected by detailed neurologic exam in 35% of men enrolled in a neurological study at a median 3.5 months after HIV transmission. PN during this early period may be mediated by systemic and nervous system immune responses to HIV.
Keywords: HIV, peripheral neuropathy, cerebrospinal fluid, immune activation
Introduction
HIV-1 (HIV) affects both the central nervous system (CNS) and peripheral nervous system (PNS)1,2. Nervous system infection with HIV produces a range of clinical disorders, with peripheral neuropathy as a frequent neurological complication3. Although many of the end-stage complications of advanced AIDS and immunosuppression are prevented or ameliorated by the use of potent combination antiretroviral therapy (ART), neurological abnormalities persist as detected by reduced performance on neuropsychological testing4,5, which may reflect damage to both the PNS and the CNS2,6. The extent of early PNS dysfunction during primary HIV infection (PHI) is unknown, though understanding the frequency and mechanism of PNS involvement may provide therapeutic approaches to neuroprotection in HIV-infected persons.
A distal sensory polyneuropathy (DSP) is the most common type of peripheral neuropathy seen in chronic HIV infection or with neurotoxic ART, presenting with symptoms of distal numbness and paresthesias, and signs of absent or decreased deep tendon reflexes7. The exact pathogenesis of HIV-DSP is unknown. Macrophage activation and pro-inflammatory cytokines associate with neurological disease development, and are implicated in the immunopathogenesis of HIV-DSP8. Pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-6 have been detected in the dorsal root ganglia (DRG) of HIV-infected patients, suggesting inflammation-mediated neuronal damage9,10. However, studies have been limited to patients with AIDS, and little is known about the inflammatory mediators of HIV-DSP in early infection. Although numerous case reports have described peripheral nerve abnormalities including DSP, demyelinating neuropathies, and focal neuritis following initial seroconversion11–13, systematic data assessing when peripheral nerve abnormalities first develop in recent HIV infection and what underlying pathophysiology causes such damage is lacking.
In the first weeks and months of HIV infection, cerebrospinal fluid (CSF) HIV RNA and intrathecal immune activation can be readily detected in untreated patients14–16. We hypothesized that peripheral neuropathy may be present during PHI, and that correlations may exist between levels of infectious and inflammatory biomarkers and signs of peripheral neuropathy in this setting. To assess whether specific markers of viral replication and immune activation, including monocyte chemoattractant protein-1 (MCP-1), neopterin, interferon gamma induced protein-10 (IP-10), and activated CD4+ and CD8+ T lymphocytes and monocytes associate with peripheral neuropathy in early HIV infection, we performed a cross-sectional neurological study of ART-naïve subjects during the first year of HIV infection.
Methods
Study participants
Baseline visits from a longitudinal neurological study of PHI, defined as within the first 12 months after HIV transmission, were analyzed. Timing of infection was confirmed by a combination of antibody seroconversion, nucleic acid testing, or less sensitive enzyme immunoassay result17, and days post HIV transmission (dpt) was defined by estimating infection as 14 days prior to the onset of seroconversion symptoms or, in those with asymptomatic seroconversion, as the date halfway between the last negative and first positive HIV test18,19. Subjects were excluded if they had diabetes, thyroid disease, or prior ART exposure. Written informed consent was obtained from all participants. The study was approved by the Institutional Review Boards at University of California San Francisco (USCF) and Yale.
Clinical evaluation
Presence of peripheral neuropathy (PN) was determined through a neurologist’s (RWP, MG, EH, or SS) examination and recording of signs and symptoms according to a standardized UCSF Macro Neurologic examination (MacroNeuro) created for the AIDS Clinical Trial Group. PN was defined as presence of unilateral or bilateral signs of decreased position, vibration, or temperature sense at great toes, or presence of distal pain or tingling on examination with or without hyporeflexia or absent or decreased ankle jerks. Diminished vibration was documented as absent sensation of vibration of a 128 Hz tuning fork over a distal bony joint, or reduction of vibration duration compared to testing on the sternum of the subject and to that detected by the examiner. A reduction in ‘cold’ sensation from the flat portion of the tuning fork in the distal limbs compared to face and/or proximal limbs was considered a temperature deficit. Symptomatic peripheral neuropathy (SPN) met more stringent criteria of having aforementioned signs as well as symptoms including numbness and paresthesias. Information about alcohol use, abuse (determined according to modified ‘CAGE’ questions) and intravenous (IV) drug use was ascertained by standardized written inventories. Subjects underwent concurrent general medical, neuropsychological, and laboratory assessments. Neuropsychological testing included dominant hand grooved pegboard, non-dominant hand finger tapping, digit symbol test, and timed gait. Z scores were calculated for each test using age-adjusted norms, and were averaged to a summary NPZ-4 score.
Specimen sampling and processing
Laboratory assessments included blood testing and lumbar puncture. CSF total white blood cell (WBC), protein, albumin, blood albumin, CD4+ and CD8+ counts by flow cytometry were measured on fresh samples. Cell-free CSF and blood plasma were also aliquoted and stored within 6 hours of collection in −70°C freezers monitored daily for temperature using NIST-certified thermometers.
Measurement of soluble immune activation
Concentrations of CSF and blood neopterin, IP-10 and MCP-1 were measured in previously frozen samples at UCSF or in the laboratory of Dr. Fuchs by commercial immunoassays (BRAHMS Aktiengesellschaft).
Measurement of cellular immune activation
Multiparameter flow cytometry was used to measure the percentage of CD38 and HLA-DR double positive CD8+ and CD4+ T lymphocytes in a subset of CSF and whole blood samples using previously described methods20,21. Lymphocyte activation antibody-dye panels were switched halfway through the study, though the two panels were compared in analysis and confirmed to have consistent T lymphocyte activation measures. A further subset of samples was analyzed by flow cytometry for monocyte activation, identifying CD3 negative cells with a CD14+CD16+ phenotype. Blood samples were stained with fluorescence minus one (FMO) controls in which one antibody was omitted. An unstained control and single stained samples were also prepared as compensation controls. Samples were run on a FACS DIVA (BD Biosciences) and analyzed with FlowJo (TreeStar, Ashland, OR).
Virological Methods
HIV RNA levels were measured in previously frozen cell-free CSF and plasma using the ultrasensitive (50 copies/mL lower limit of detection) Amplicor HIV Monitor (version 1.5; Roche Molecular Diagnostic Systems, Branchburg, NJ), or the Abbott RealTime HIV-1 (Abbot Laboratories, Abbot Park, IL, USA) assays. Paired blood and CSF measurements were made using the same assay, in the same PCR run.
Statistical Analysis
Descriptive statistics were performed using Stata/SE 11.0 (StataCorp LP, College Station, TX). Nonparametric Mann-Whitney rank sum test compared group differences between PN and NPN, as well as subgroup differences between SPN and NPN. Fisher’s exact test compared differences between categorical variables. A regression model compared group differences after adjusting for age, alcohol abuse, and race.
Results
Study Participant Characteristics
Clinical and laboratory characteristics of study subjects are presented in Table 1. Subjects were previously healthy men with a median age of 36 years and CD4+ T cell count of 575 cells/μL evaluated at a median estimated 107 dpt. Subjects had normal basal metabolic indices (BMI) and showed no evidence of nutritional deficiency. Additional demographic data including ethnicity, history of IV drug use, alcohol use, and hepatitis C infection status are presented in Table 1.
Table 1.
Clinical and demographic characteristics of study participants by group.
| Overall Median | NPN | PN | SPN | p value | |
|---|---|---|---|---|---|
|
| |||||
| Number | 38 | 20 | 13 | ||
|
| |||||
| Age (years) | 36 | 34 (27–42) | 40 (31–47) | 37 | 0.05* 0.33ψ |
|
| |||||
| Years of education | 16 | 16 | 15 | 14 | 0.13* 0.06ψ |
|
| |||||
| Days post HIV transmission | 107 | 104 (65–188) | 125 (74–164) | 137 (72–172) | 0.87* 0.91ψ |
|
| |||||
| CD4+ T cellcount (cells/ul) | 575 | 558 (408–729) | 582(453–715) | 601 (483–755) | 0.83* 0.41ψ |
|
| |||||
| CD8+ T cell count (cells/ul) | 985 | 701 (513–1202) | 1019 (674–1543) | 1053 (736–1635) | 0.59* 0.33ψ |
|
| |||||
| Plasma HIV RNA (log10 copies/mL) | 4.45 | 4.37 (3.94–4.77) | 4.79 (3.17–5.18) | 4.82 (3.00–5.28) | 0.29* 0.25ψ |
|
| |||||
| Race/Ethnicity | |||||
| White, n (%) | 29 (49.2) | 10 (17.2) | 8 (13.8) | 0.08* 0.35ψ |
|
|
| |||||
| History of IV drug use | |||||
| Yes, n (%) | 27 (46.6) | 16 (27.6) | 10 (17.2) | 0.72* 0.52ψ |
|
|
| |||||
| History of alcohol use | |||||
| Yes, n (%) | 12 (20.7) | 13 (22.4) | 8 (13.8) | 0.05* | |
| Unknown, n (%) | 3 (7.9) | 0 | 0 | 0.65ψ | |
|
| |||||
| Hepatitis C infection status | |||||
| Yes, n (%) | 1 (2.6) | 0 | 0 | ||
| Unknown, n (%) | 5 (13.2) | 0 | 0 | ||
Abbreviations:
NPN = no peripheral neuropathy. PN = peripheral neuropathy. SPN = symptomatic peripheral neuropathy.
Values are presented as medians (IQR) unless otherwise noted.
NPN vs. PN
SPN vs. PN
Clinical and Demographic Associations with Peripheral Neuropathy in Primary Infection
20 of 58 (35%) PHI subjects had signs of peripheral neuropathy (designated ‘PN subjects’) upon neurologic examination. There was a trend towards PN subjects being older with a median age of 40 years compared to 34 years in subjects with no peripheral neuropathy (NPN, p=0.05). There was no significant relationship between history of alcohol abuse and signs of peripheral neuropathy (p=0.05). Of the 20 PN subjects, 6 subjects had bilateral findings, 7 subjects had unilateral findings, and in the remaining 7 subjects, laterality was not specified. Neuropsychological performance (NPZ4) was not different between the PN and NPN groups (−0.24, IQR−0.70–0.22 vs. 0.02, IQR −0.50–0.65, p=0.18). There was no difference in dpt between the two groups. The PN group showed no significant difference compared to the NPN group in absolute blood CD4+ (582 cells/μL, IQR 408–729 vs. 558 cells/μL IQR 453–715, p=0.82) and CD8+ T cell counts (1019 cells/μL, IQR 674–1543, I vs. 901 cells/μL, IQR 701–1202, p=0.59). 13 of the 20 PN subjects (65% of PN, 22% of total) also had symptoms of peripheral neuropathy (SPN). Typical symptoms included “foot tingling and numbness.” There were no demographic differences identified between NPN and SPN subjects.
Laboratory Associations with Peripheral Neuropathy in Primary Infection
Median plasma HIV RNA levels and CSF HIV RNA levels were similar between PN and NPN groups, as well as between SPN and NPN groups. CSF WBC count (8.00 cells/μL, IQR 2.00–10.00 vs. 6.00 cells/μL, IQR 2.00–12.00, p=0.48) and albumin ratios (4.96, IQR 4.46–6.53 vs. 5.47, IQR 4.23–8.25, p=0.76) were similar between PN and NPN subjects. Median levels of blood neopterin (17.9 nmol/L, IQR 15.4–25.3, vs. 12.1 nmol/L, IQR 8.8–20.0, p=0.006), CSF neopterin (13.7 nmol/L, IQR 8.0–21.9, vs. 8.1 nmol/L, IQR 5.2–9.5, p=0.003), CSF MCP-1 (620 pg/mL, IQR 534–780, vs. 477 pg/mL, IQR 373–643, p=0.006), and IP-10 (910 pg/mL, IQR 401–1749, vs. 530 pg/mL, IQR 323–907, p=0.09) in the PN subjects were elevated as compared to the NPN subjects (Figure 1A–E). Additional analysis adjusted for age, alcohol use, and race showed continued significant elevation of CSF neopterin (p=0.02) and blood neopterin (p=0.008) in PN compared to NPN subjects. There remained a trend for MCP-1 to be elevated in PN subjects, but the association was not statistically significant (p=0.05).
Figure 1A–E. Markers of immune activation in blood and CSF of PHI subjects with and without signs of peripheral neuropathy.
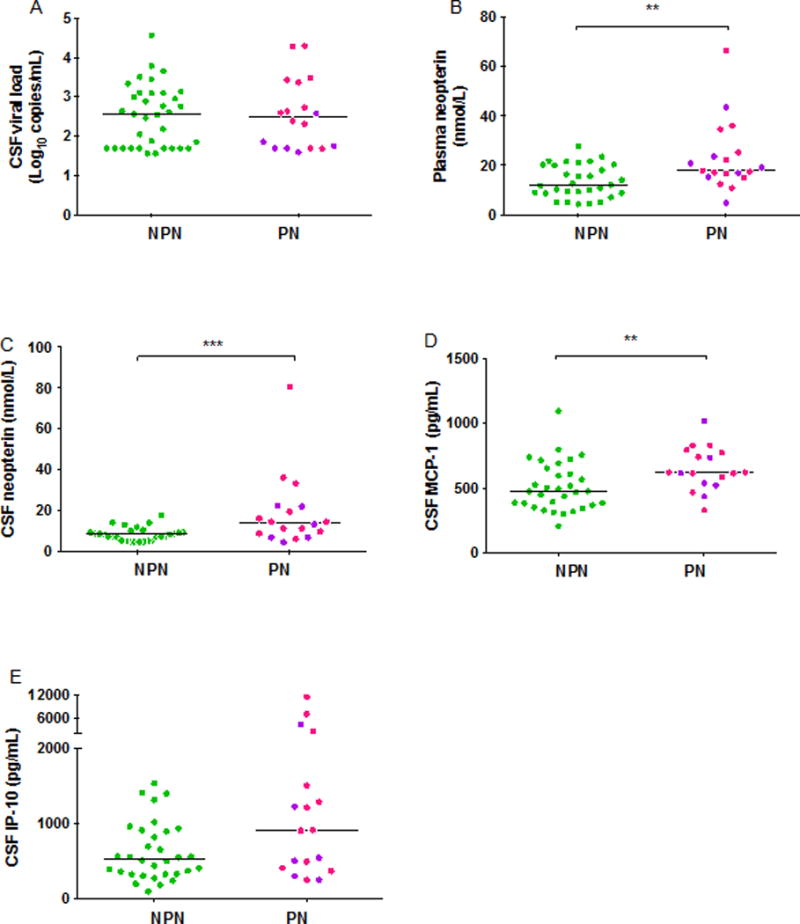
A) CSF viral load B) blood neopterin C) CSF neopterin D) CSF MCP-1 E) CSF IP-10. A single asterix represents P-value <0.05, double asterixes represent P-value <0.01, and triple asterixes represent P-value <0.005. Significant differences were detected between NPN and PN in blood neopterin (B, p=0.006), CSF neopterin (C, p=0.003), and CSF MCP-1 (D, p=0.006). Differences noted in (B) and (C) remained significant when single outlier was excluded. Solid bars indicate medians. Pink symbols indicate SPN subjects, purple symbols indicate PN subjects. Approximate reference values for blood neopterin <8.8 nmol; CSF neopterin <5.8 nmol; MCP-1: <500 pg/mL; IP-10 <250 pg/mL.
Flow cytometric analysis of CD8+ T lymphocyte activation revealed similar median percentage of activated CD38+/HLADR+ CD8+ T lymphocytes in PN subjects in blood (p=0.23) but elevated percentage in the CSF compartment (77.6%, IQR 69.4–86.8, vs. 60.9%, IQR 49.7–68.0, p=0.004, Figure 2A–D) compared to NPN subjects. There were no differences in percentages of activated CD4+ T lymphocytes or activated CD14+CD16+ monocytes in blood or CSF between PN and NPN.
Figure 2A–D. Cellular immune activation in PHI subjects with and without signs of peripheral neuropathy.
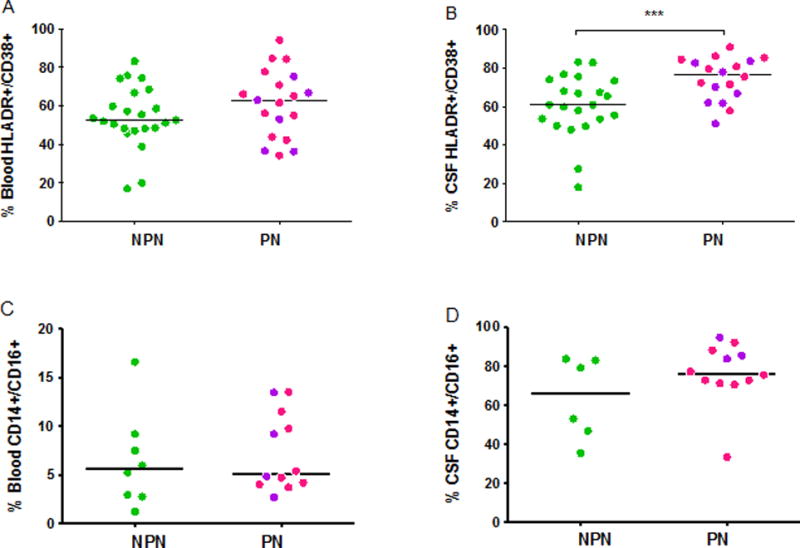
A) Blood CD38+/HLADR+ CD8+ T lymphocytes B) CSF CD38+/HLA-DR+ CD8+ T lymphocytes C) Blood CD14+/CD16+ monocytes D) CSF CD14+/CD16+ monocytes. A single asterix represents P-value <0.05, double asterixes represent P-value <0.01, and triple asterixes represent P-value <0.005. PHI subjects with signs of peripheral neuropathy (PN) had a significantly elevated proportion of activated (CD38+/HLADR+) CD8 T lymphocytes in CSF (B, p=0.004) compared to those without peripheral neuropathy (NPN).
When PN only subjects were excluded from the analysis, and CSF and blood samples for inflammatory biomarkers were compared between SPN and NPN subjects, elevated levels of CSF neopterin (14.3 nmol/L, IQR 5.9–29.7 vs. 8.1 nmol/L, IQR 4.4–9.5, p=0.0005), CSF MCP-1 (623 pg/mL, IQR 330–791 vs. 477 pg/mL, IQR 208–643, p=0.01), CSF IP-10 (1063 pg/mL, IQR 432–2242, vs. 530 pg/mL, IQR 327–907, p=0.03), and blood neopterin (17.7 nmol/L, IQR 10.8–32.4, vs. 12.1 nmol/L, IQR 4.3–12.1, p=0.009) were found in the SPN group. Percentage of activated CD4+ T lymphocytes and monocytes did not differ between the SPN and PN subjects in either compartment.
Discussion
Peripheral neuropathy is a frequent neurological disorder reported in HIV, classically in the setting of chronic untreated HIV infection or following exposure to certain antiretroviral medications. However, we found that signs (35%), or signs and symptoms (22%), of peripheral neuropathy were evident in a cohort of ART-naive subjects recruited to a neurological study at a median of 3.5 months after initial HIV transmission. We further examined mechanisms for peripheral nerve dysfunction identified during this stage, revealing that markers of systemic and CNS immune activation are elevated in subjects with signs of neuropathy (PN) compared to those with no signs of neuropathy (NPN).
Disorders of the PNS manifesting during PHI, typically around the period of HIV seroconversion, are well-described in case reports and series, and include acute inflammatory demyelinating peripheral neuropathies, meningoradiculitis and ataxic neuropathy12,22,23. However, subtle signs and symptoms of peripheral neuropathy during PHI have not been systematically studied. The pathogenesis of classic HIV-DSP is not clearly elucidated, but is thought to be due to a combination of direct viral toxicity and immune activation. Increased monocyte-macrophage markers, lymphocyte and macrophage infiltration, and cytokine expression are detected in the peripheral nerves and DRGs of patients with DSP in AIDS24, with resulting distal demyelination and axonal degeneration in a “dying back” pattern. The immunologic response to HIV begins in early disease with chemokine elevation, including MCP-1 and IP-10, as well as the macrophage activation marker neopterin, in the blood and CSF of HIV patients with normal CD4+ T cell counts, and even during PHI25,26.
We found high rates of peripheral neuropathy in our cohort of PHI subjects. As subjects with symptomatic seroconversion and neurologic symptoms may have been more likely to enroll in the study, it is possible that the prevalence of peripheral neuropathy in our cohort is higher than in all individuals with PHI. This may be one contributing factor to differences between our findings and that of a previous study, which observed a much lower rate of neuropathy (1.5%) in military recruits with HIV infection, mostly prior to advanced AIDS27. This discrepancy may also be due to different thresholds in assessment and reporting. It is possible that Barohn et al.’s examinations were performed to detect peripheral neuropathy affecting military performance, and that mild or subclinical signs were not recorded. In contrast, our diagnostic thresholds were used to detect and document evidence of neuropathy for research purposes. Furthermore, our subjects presented at an earlier stage of infection, with an overall median CD4+ T cell count of 575 cells/uL and none having a CD4 count <200, while in Barohn et al.’s study, 7.8% (62/798) had a CD4 count <200. The period of early infection characterizing our cohort is associated with rapid HIV virema, acute systemic and CNS immune activation, and well-documented occurrence of symptomatic peripheral nerve disorders. These disorders are poorly understood, but are likely immune-modulated, and may improve after this early dynamic period of immune response initiation. Therefore, it is plausible that PHI might be characterized by a higher prevalence of peripheral neuropathy than early chronic HIV infection.
Plasma HIV RNA and CD4+ T cell count associate with and are predictive of the development and severity of HIV-associated peripheral neuropathy in chronic infection28,29. In contrast, we found no difference in these factors between PN and NPN PHI subjects, suggesting that they may not be crucial mediators of PNS involvement in early infection. Additionally, HIV transcripts and proteins have been detected in DRG neurons and surrounding satellite cells in subjects with peripheral neuropathy and HIV-DSP30 However, it is generally believed that HIV replication in peripheral nerves is scarce. CSF HIV RNA, CSF total protein levels (a measurement of all detected CSF proteins indicative of blood brain barrier, BBB, integrity), and CSF:blood albumin ratios (a highly specific marker of BBB integrity) were similar between PN and NPN subjects, suggesting that the viral burden measurable within the nervous system and BBB penetrance may not contribute to neuropathy during PHI.
Amplified immune activation characterized our subjects with signs of peripheral neuropathy. CSF markers have been previously implicated in advanced stages of HIV neurological disease progression: CSF neopterin rises along with CNS HIV disease severity and decreases following ART31; levels of IP-10 positively correlate with the presence of HIV-associated dementia (HAD)32; and elevated MCP-1 is detected in the brain and CSF of subjects with HIV-encephalitis and HAD33,34. Our results support the premise that immune activation is crucial to peripheral neurological dysfunction in HIV. The chemoattractants MCP-1 and IP-10 likely escalate disease through augmentation of viral replication in already-infected cells, and enhance local inflammation by lymphocyte and monocyte recruitment35,36. With increased monocyte activation and macrophage presence, HIV cellular transmission perpetuates, and infection leads to macrophage priming, induction of pro-inflammatory cytokines and TNF-α, leading to neuronal dysfunction. Furthermore, in rat models, endothelial cells that supply DRGs are highly fenestrated, suggesting that this area of the PNS may be particularly vulnerable to toxic effects of circulating activated monocytes and pro-inflammatory cytokines37.
Our findings of increased CNS inflammation in the presence of peripheral neuropathy may be explained through alternative models: HIV concurrently infects both the CNS and PNS, and our detected associations are coincidental, or, alternatively, peripheral neuropathy induces secondary changes within the CNS. In support of the former, studies of SIV-infected macaques at 12-weeks post inoculation have detected increased monocyte infiltration of DRGs, as well as decreased DRG neuronal density and conduction velocity in the absence of neuritis or damage to myelinated peripheral nerves38. Thus, though there may be an association between central and peripheral immune activation, the presence of CNS and PNS lesions may not correlate. Alternatively, in HIV-DSP, macrophage secretion of pro-inflammatory molecules within the nerve may lead to CNS immune activation. In support of this, perineural application of the HIV envelope glycoprotein gp120 to rat sciatic nerves leads to TNF-α expression in the DRG and glial cells in the spinal cord39. Pro-inflammatory cytokines may promote a paracellular route for HIV-1 across the BBB, facilitating HIV infection of the CNS40. MCP-1 contributes to blood spinal cord barrier permeability following peripheral nerve injury, and individuals with mutant MCP-1 genotypes have increased risk of HAD and accelerated disease progression41,42. Therefore, a cycle of immune activation and subsequent overproduction of pro-inflammatory cytokines and chemokines may allow for further cell trafficking from the periphery into the CNS to create further inflammation and neuronal damage.
In our participants, flow cytometry analysis of T lymphocyte activation demonstrated elevated percentages of CD38+/HLA-DR+ CD8+ T lymphocytes in the CSF of PN subjects. HLA-DR and CD38 expression on CD8+ T lymphocytes correlates with clinical stages of HIV disease, with simultaneous expression of both increasing in symptomatic disease43. MHC class II molecule HLA-DR expression increases in CD8+ T lymphocytes upon HIV seroconversion and remains stable, while expression of CD38 levels on CD8+ lymphocytes increases throughout disease and is thought to be a predictor of AIDS progression and death44,45. The percentage of CD38+ and HLA-DR+ CD8+ T lymphocytes rises progressively with advancing HIV disease in pre-ART subjects46, while ART decreases the level of blood CD38+/HLA-DR+ co-expression on CD8+ T lymphocytes, even in subjects only partially responsive to treatment47. Our findings suggest that increased migration to or accumulation of ‘activated’ phenotype CD8+ T lymphocytes within the CSF pathologically associates with peripheral neuropathy. This is the first study to examine T lymphocyte activation in the CNS compartment in relation to clinical neurological disease in HIV. Whether these findings indicate that peripheral neuropathy is associated with processes accelerating disease progression within the nervous system, or another mechanism of injury warrants further study.
We examined the PN group using more stringent criteria of both signs and symptoms of peripheral neuropathy, identifying 13 SPN subjects. When compared with our NPN group, elevations in inflammatory markers of CSF neopterin, CSF MCP-1, CSF IP-10, and blood neopterin were noted. The lack of association between presence of SPN and monocyte and T lymphocyte activation levels could be due to lower samples in this subgroup, reducing our power to detect significant differences from NPN. The consistency in elevation of inflammatory markers in the setting of symptomatic disease supports the explanation that peripheral neuropathy is mediated in part by an inflammatory response. Animal studies have demonstrated elevated pro-inflammatory cytokines in rats experiencing sustained allodynia and hyperalgesia following intrathecal and perineural gp-120 administration, which is thought to be neurotoxic and lowers the excitation threshold48–50. This aberrant immune response may lead to neuronal hyperexcitability, creating exaggerated pain states that explain symptomatic peripheral neuropathy seen in early infection.
Limitations of this study include the cohort homogeneity, since all subjects were ART-naïve men who have sex with men (MSM) with PHI, within a focused range of age and education. Although this limits generalizability, it allows for the examination of the effects of early HIV with reduced confounders such as potential toxicities of ART. Furthermore, though we did not perform electromyography and nerve conduction studies (EMG/NCS) in our subjects to electrophysiologically characterize neuropathy, our diagnostic criteria have been used previously in studies examining HIV peripheral neuropathy51,28. Importantly, potential confounders to the analysis need to be considered, and the PN subjects had elevated rates of alcohol abuse compared to NPN subjects. Previous studies have shown that age52, alcohol abuse53, and ethnicity54 are risk factors for peripheral neuropathy. Our adjusted analyses suggest that though these factors may contribute to and be confounders to the finding of peripheral neuropathy, in the context of elevated CNS and systemic immune activation, they are unlikely to entirely explain our finding of a notable relationship between immune activation and the presence of peripheral neuropathy. Peripheral neuropathy may also develop in the setting of nutritional deficiencies55. Although we did not measure vitamin levels in subjects, none had advanced HIV infection, food insecurity, signs of wasting, or low BMI. Thus, the presence of vitamin deficiency contributing to nutritional neuropathy seems doubtful. Finally, the results of this study are limited to the timeframe of PHI. Longitudinal follow up of our cohort is underway to study the natural history of peripheral neuropathy in early infection and to investigate whether neuropathy during early infection is transient or progressive.
Previous studies have examined the prevalence of peripheral neuropathy in HIV-infected individuals on ART, in subjects with longstanding, chronic HIV infection, or AIDS. This is the first study to examine the prevalence of peripheral neuropathy among ART-naïve individuals within the first months after HIV acquisition. We found a high rate of peripheral neuropathy among PHI subjects that correlated with increased levels of systemic and intrathecal pro-inflammatory markers. Recognition of PHI as an essential period of immune activation associated with peripheral neuropathy and neurological disorders may provide rationale for more aggressive screening for recent HIV infection, and early institution of ART and possibly immunomodulatory therapy during this early eriod.
Acknowledgments
We sincerely thank our study volunteers for participating in this study. We also thank the staff at the University of California-San Francisco Options project and Magnet for their invaluable assistance with subject referrals. We thank the National Institutes of Health for grant support, including: R01 MH081772, K23 MH074466, P30 AI027763, P01 AI071713, T32 MH090847, and UL1 TR000004.
Footnotes
The results of this study were presented at the Conference for Retroviruses and Opportunistic Infections in Seattle, Washington, USA, March 5–8, 2012.
Conflicts of Interest and Sources of support:
The current study is not industry sponsored. Dr. Ho receives research royalties from Santa Cruz Biotechnology, Inc. Dr. Price has received speaker honoraria and support for meeting travel from Abbott Labs. He is also a consultant to Merck and Co. No activities were related to this study. Dr. Robertson consults for Abbott labs and ViiV/GSK. No activities were related to this study. Dr. Spudich has received support for travel to a scientific meeting and a speaker honorarium from AbbVie. The remaining authors report no disclosures.
References
- 1.Hogan C, Wilkins E. Neurological complications in HIV. Clin Med. 2011;11:571–575. doi: 10.7861/clinmedicine.11-6-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Power C, Boissé L, Rourke S, et al. NeuroAIDS: an evolving epidemic. Can J Neurol Sci. 2009;36:285–295. doi: 10.1017/s0317167100007009. [DOI] [PubMed] [Google Scholar]
- 3.Schifitto G, McDermott MP, McArthur JC, et al. Incidence of and risk factors for HIVassociated distal sensory polyneuropathy. Neurology. 2002;58:1764–1768. doi: 10.1212/wnl.58.12.1764. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson KR, Nakasujja N, Wong M, et al. Pattern of neuropsychological performance among HIV positive patients in Uganda. BMC Neurol. 2007;7:8. doi: 10.1186/1471-2377-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De la Monte SM, Gabuzda DH, Ho DD, et al. Peripheral neuropathy in the acquired immunodeficiency syndrome. Ann Neurol. 1988;23:485–492. doi: 10.1002/ana.410230510. [DOI] [PubMed] [Google Scholar]
- 7.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 8.Pardo CA, Mcarthur JC, Griffin JW. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;27:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- 9.Nagano I, Shapshak P, Yoshioka M, et al. Increased NADPH-diaphorase reactivity and cytokine expression in dorsal root ganglia in acquired immunodeficiency syndrome. J Neurol Sci. 1996;136:117–128. doi: 10.1016/0022-510x(95)00317-u. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka M, Shapshak P, Srivastava aK, et al. Expression of HIV-1 and interleukin-6 in lumbosacral dorsal root ganglia of patients with AIDS. Neurology. 1994;44:1120–1130. doi: 10.1212/wnl.44.6.1120. [DOI] [PubMed] [Google Scholar]
- 11.Piette AM, Tusseau F, Vignon D, et al. Acute neuropathy coincident with seroconversion for anti-LAV/HTLV-III. Lancet. 1986;1:852. doi: 10.1016/s0140-6736(86)90956-6. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg L, Malmvall BE, Svennerholm L, et al. Guillain-Barré syndrome as an early manifestation of HIV central nervous system infection. Scand J Infect Dis. 1986;18(6):591–592. doi: 10.3109/00365548609021668. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese LH, Proffitt MR, Levin KH, et al. Acute infection with the human immunodeficiency virus (HIV) associated with acute brachial neuritis. Ann Intern Med. 1987;107:849–851. doi: 10.7326/0003-4819-107-6-849. [DOI] [PubMed] [Google Scholar]
- 14.Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spudich S, Gisslen M, Hagberg L, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–760. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tambussi G, Gori A, Capiluppi B, et al. Neurological symptoms during primary human immunodeficiency virus (HIV) infection correlate with high levels of HIV RNA in cerebrospinal fluid. Clin Infect Dis. 2000;30:962–965. doi: 10.1086/313810. [DOI] [PubMed] [Google Scholar]
- 17.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007;21:19–48. doi: 10.1016/j.idc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Lindback S, Thorstensson R, Karlsson AC, et al. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. AIDS. 2000 Jul;:2333–9. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- 19.Little SJ, Frost SDW, Wong JK, et al. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J Virol. 2008;82:5510–5518. doi: 10.1128/JVI.02579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immuno Def Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dahl V, Lee E, Peterson J, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–1945. doi: 10.1093/infdis/jir667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton P, Poly H, Gonnaud P, et al. Acute meningoradiculitis concomitant with seroconversion to human immunodeficiency virus type 1. Res Virol. 1990;141:427–433. doi: 10.1016/0923-2516(90)90043-i. [DOI] [PubMed] [Google Scholar]
- 23.Castellanos F, Mallada J, Ricart C, et al. Ataxic neuropathy associated with human immunodeficiency virus seroconversion. Archives of Neurology. 1993;51:236. doi: 10.1001/archneur.1994.00540150022010. [DOI] [PubMed] [Google Scholar]
- 24.Rizzuto N, Cavallaro T, Monaco S, et al. Role of HIV in the pathogenesis of distal symmetrical peripheral neuropathy. Acta Neuropathol. 1995;90:244–250. doi: 10.1007/BF00296507. [DOI] [PubMed] [Google Scholar]
- 25.Shacklett BL, Cox Ca, Wilkens DT, et al. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis. 2004;189:2202–2212. doi: 10.1086/421244. [DOI] [PubMed] [Google Scholar]
- 26.Price RW, Epstein LG, Becker JT, et al. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69:1781–1788. doi: 10.1212/01.wnl.0000278457.55877.eb. [DOI] [PubMed] [Google Scholar]
- 27.Barohn MC, Gronseth GS, LeForce BR, et al. Peripheral nervous system involvement in a large cohort of human immunodeficiency virus – infected individuals. Arch Neurol. 1993;50:167–171. doi: 10.1001/archneur.1993.00540020045016. [DOI] [PubMed] [Google Scholar]
- 28.Childs EA, Lyles RH, Selnes OA, et al. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 29.Simpson DM, Haidich AB, Schifitto G, et al. Severity of HIV-associated neuropathy is associated with plasma HIV-1 RNA levels. AIDS. 2002;16:407–412. doi: 10.1097/00002030-200202150-00012. [DOI] [PubMed] [Google Scholar]
- 30.Brannagan TH, Nuovo GJ, Hays AP, et al. Human immunodeficiency virus infection of dorsal root ganglion neurons detected by polymerase chain reaction in situ hybridization. Ann Neurol. 1997 Sep;42(3):368–72. doi: 10.1002/ana.410420315. [DOI] [PubMed] [Google Scholar]
- 31.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb SA, Sporer B, Lahrtz F, et al. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 33.Kelder W, McArthur JC, Nance-Sproson T, et al. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 34.Ragin AB, Wu Y, Ochs R, et al. Biomarkers of neurological status in HIV infection: A three year study. Proteomics Clin Appl. 2011;4:295–303. doi: 10.1002/prca.200900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss JM, Nath A, Major EO, et al. HIV-1 tat induces monocyte chemoattractant protein 1 mediated monocyte transmigration. J Immunol. 1999;63:2953–2959. [PubMed] [Google Scholar]
- 36.Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005;168:154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez-Andrade JM, Herrera MB, Ghilardi JR, et al. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 2008;4:10. doi: 10.1186/1744-8069-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laast VA, Shim B, Johanek, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng W, Ouyang H, Zheng X, et al. Glial TNF-α in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Mol Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiala M, Looney DJ, Stins M, et al. TNF-α opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 41.Echeverry S, Shi XQ, Rivest S, et al. Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31:10819–10828. doi: 10.1523/JNEUROSCI.1642-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonsaelez E, Rovin BH, Sen L, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–14800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kestens L, Vanham G, Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Cumberland WG, Hultin LE, et al. CD8+ T lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Def Syndr Hum Retrovirol. 1998;18:332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Giorgi JV, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS cohort study. Clin Immunol Immunopathol. 1989;52:10–18. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 46.Levacher M, Hulstaert F, Tallet S, et al. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–382. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouscarat F, Levacher M, Landman R, et al. Changes in blood CD8+ lymphocyte activation status and plasma HIV RNA levels during antiretroviral therapy. AIDS. 1998;12:1267–1273. doi: 10.1097/00002030-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Milligan ED, O’Connor KA, Nguyen KT, et al. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- 50.Oh SB, Tran PB, Gillard, et al. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2011;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schifitto G, McDermott MP, McArthur JC, et al. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology. 2005;64:842–848. doi: 10.1212/01.WNL.0000152981.32057.BB. [DOI] [PubMed] [Google Scholar]
- 52.Watters MR, Poff PW, Shiramizu BT, et al. Symptomatic distal sensory polyneuropathy in HIV after age 50. Neurology. 2004;62:1378–1383. doi: 10.1212/01.wnl.0000120622.91018.ea. [DOI] [PubMed] [Google Scholar]
- 53.Monforte R, Estruch R, Valls-Sole J, et al. Autonomic and peripheral neuropathies in patients with chronic alcoholism. Arch Neurol. 1995;52:45–51. doi: 10.1001/archneur.1995.00540250049012. [DOI] [PubMed] [Google Scholar]
- 54.Evans SR, Ellis RJ, Chen H, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar N. Nutritional neuropathies. Peripheral Neuropathies. 2007;25:209–255. doi: 10.1016/j.ncl.2006.11.001. [DOI] [PubMed] [Google Scholar]


