Abstract
Dendrimers are synthetic macromolecules composed of repetitive layers of branching units that emerge from a central core. They are characterized by a tunable size and precise number of peripheral groups which determine their physicochemical properties and function. Their high multivalency, functional surface, and globular architecture with diameters in the nanometer scale makes them ideal candidates for a wide range of applications. Gallic acid-triethylene glycol (GATG) dendrimers have attracted our attention as a promising platform in the biomedical field because of their high tunability and versatility. The presence of terminal azides in GATG dendrimers and poly(ethylene glycol) (PEG)-dendritic block copolymers allows their efficient functionalization with a variety of ligands of biomedical relevance including anionic and cationic groups, carbohydrates, peptides, or imaging agents. The resulting functionalized dendrimers have found application in drug and gene delivery, as antiviral agents and for the treatment of neurodegenerative diseases, in diagnosis and as tools to study multivalent carbohydrate recognition and dendrimer dynamics. Herein, we present an account on the preparation and recent applications of GATG dendrimers in these fields.
KEY WORDS: block copolymer, dendrimer, drug delivery, multivalency, NMR
INTRODUCTION
Dendrimers are synthetic tree-like macromolecules composed of repetitive layers of branching units that emerge from a central core (Fig. 1). They are synthesized in a controlled iterative fashion through generations with nil dispersity, precise molecular weight, and discrete properties (2–4). Their high functional surface, globular architecture in the nanometer scale, and inherent multivalency make them ideal candidates for a wide range of applications, from bio- and nanotechnology (5–9) to catalysis and materials science (10–12). The first reports on dendrimers were published independently in the late 1970s and early 1980s of the last century by the groups of Vögtle (13), Newkome (14), and Tomalia (15). Since then, over a hundred dendritic architectures have been described in the search of improved and novel properties. Among them, recognized dendritic families include polyamidoamine (PAMAM) (16), polypropylene imine (PPI) (17), and others based on polyamide (14), polyether (18), polyester (19,20), and phosphorous-based (21) scaffolds.
Fig. 1.
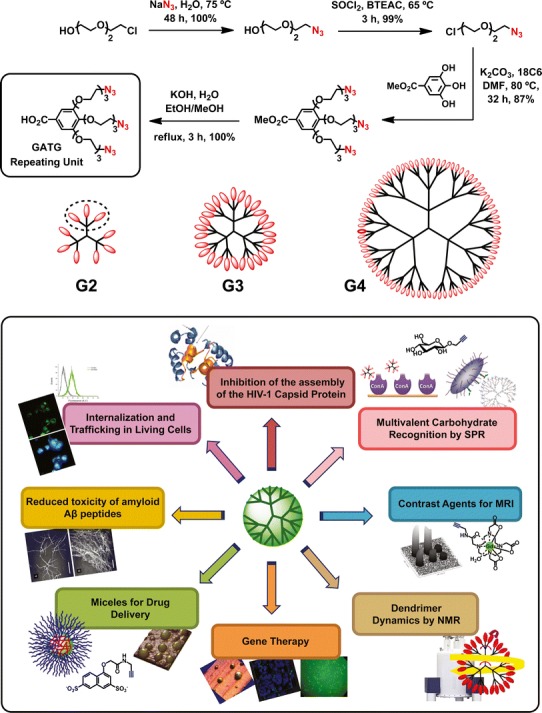
Structure, synthesis of the repeating unit, and applications of GATG dendrimers. Reprinted with permission from reference (1)
Recently, we have turned our attention to the gallic acid-triethylene glycol (GATG) dendritic family as a promising platform in the biomedical field (Fig. 1). GATG dendrimers, first described by the group of Roy (22–24), are composed of a repeating unit carrying a gallic acid core and hydrophilic triethylene glycol arms with terminal azide groups. Advantage of the azides is taken for the dendritic generation growth, easily accomplished by a reduction/amide coupling sequence, as well as for the dendrimer decoration by means of the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) (25–29) as demonstrated by our group. As part of our effort to develop the biomedical applications of GATG, we have also described the incorporation of the FDA-approved poly(ethylene glycol) (PEG) at the focal point of dendritic wedges to render PEG-GATG block copolymers with increased solubility and stealth properties. PEG-dendritic block copolymers constitute interesting hybrid structures where differences in the solubility properties of the blocks can be exploited in the preparation of micelles and other nanostructures of biomedical interest (30–32).
Herein, we present an account of our journey with GATG that focuses on recent examples in the fields of drug and gene delivery, diagnosis, and antiviral activity, as well as the use of GATG as tools to study biological processes and the dynamics of dendrimers.
SYNTHESIS OF GATG DENDRIMERS AND PEGYLATED BLOCK COPOLYMERS
GATG dendrimers ([Gn]-N3, where n is the generation number) are synthesized divergently from a repeating unit shown in Fig. 1, following a straightforward azide reduction/amide coupling sequence. Initial reports by the group of Roy in the 1990s described GATG sialodendrimers and dendronized chitosans up to the second generation (G2) as promising microbicides (22–24). Nevertheless, the synthesis of the repeating unit (four steps from triethylene glycol, 23% overall yield) proved to be a hurdle in accessing large quantities and higher G of these dendrimers, which finally hampered their subsequent development.
Aware of these limitations, in 2006, our research group described an improved preparation of this repeating unit from commercially available chlorotriethylene glycol in 77% overall yield (33). By observing green chemistry principles (atom economy, safety, waste reduction), this synthetic route has been further developed for the cost-effective production of the repeating unit in batches larger than 100 g in excellent overall yield (86%) and purity (Fig. 1) (1). With an easy and scalable access to the repeating unit, the preparation of GATG dendrimers and PEG-dendritic block copolymers has been efficiently achieved in large quantities up to G4 (243 peripheral azides) (33–35). The PEGylated block copolymers are synthesized following a “chain first” approach where the PEG is initially incorporated at the focal point of a GATG repeating unit and then the higher G obtained divergently. Noteworthy, PEG facilitates the purification steps in the synthesis of the block copolymers thanks to its properties as a soluble polymeric support (36). As mentioned, the surface functionalization of GATG dendrimers and block copolymers by CuAAC with unprotected ligands (carbohydrates, anionic and cationic moieties, peptides, imaging agents) has proceeded straightforward in our hands, allowing the preparation of a variety of functional dendritic structures of biomedical interest that will be presented in the following sections.
CELLULAR INTERNALIZATION OF GATG DENDRIMERS
Dendrimers have traditionally found great attention in targeted drug delivery, particularly for cancer therapy and diagnosis (6,7). The high number of functional groups on their periphery allows the simultaneous incorporation of bioactive molecules, cytostatic drugs, diagnostic probes, and targeting ligands. In addition, the combination of high water solubility and hydrophobic cores is well suited for the covalent attachment or physical encapsulation of large payloads of therapeutic molecules. Cellular uptake of dendrimer-based drug delivery systems has proved significantly higher than that of linear polymeric carriers (37,38), which can be explained on the basis of their compact nanosized architecture in solution.
In order to explore the versatility of PEG-GATG block copolymers as tools in the biomedical field, we have analyzed in collaboration with Albertazzi their cellular internalization and intracellular fate (39), both properties of particular interest in drug delivery. To this aim, a PEGylated block copolymer of third generation (PEG-[G3]-N3) was functionalized via CuAAC with different peripheral groups, including neutral and charged moieties, biologically active carbohydrates, and peptides. All dendritic structures were labeled with fluorescein isothiocyanate (FITC), and the effect of the surface functionalization on cell-uptake and intracellular trafficking was studied by confocal microscopy in HeLa cells. It was observed that, while cationic PEG-[G3]-NH3+ showed a strong internalization consistent with its ability to bind cell membranes through ionic interactions, anionic PEG-[G3]-OSO3− displayed only a weak internalization because of its lower affinity for cell membranes. This behavior was even more evident for a neutral acetylated PEG-[G3]-NHAc that exhibited no internalization at all. In addition, the intracellular final fate of PEG-[G3]-NH3+ was tracked, revealing colocalization with lysosomes. The effect of the surface functionalization with biologically relevant ligands, such as lactose and a cyclic arginylglycylaspartic acid (RGD) peptide, was also investigated (39). Confocal microscopy and flow cytometry assays showed significant cellular uptake in HepG2 cells for dendritic structures decorated with lactose and in HeLa cells for those containing the RGD sequence. Based on these results, a prototype of drug carrier capable of selectively entering cells and specifically releasing its payload in the acidic lysosomal environment was designed from PEG-[G3]-NH3+. With this aim, a coumarin dye was bound through a pH-sensitive hydrazone linker to the dendritic platform (Fig. 2). The performance of this system was evaluated with a double fluorescent-labeling allowing for simultaneous monitoring of the localization of the dendritic carrier (FITC) and the coumarin dye as a model cargo molecule (aminomethylcoumarin (AMCA)-hydrazide). Fluorescence experiments at short incubation times showed high colocalization between FITC and AMCA in the endolysosomal system. However, reduced colocalization and increased fluorescence intensity due to AMCA could be observed at longer incubation times (Fig. 2), which are consistent with a lysosomal hydrolysis of the hydrazone linkers and subsequent release of AMCA to the cytoplasm. This GATG-based conjugate accomplishes the main requirements for a successful drug delivery system (cell internalization, intracellular release, endosomal escape) and represents a promising proof-of-principle for further applications of GATG dendrimers and block copolymers in drug delivery.
Fig. 2.
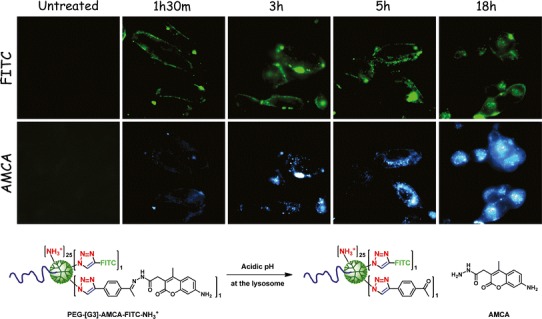
Schematic structure of PEG-[G3]-AMCA-FITC-NH3 +. Cell-uptake and intracellular trafficking in HeLa cells: fluorescent signals from FITC (carrier) and AMCA (model cargo). Reprinted with permission from reference (39)
GATG NANOSTRUCTURES FOR DRUG AND GENE DELIVERY
PEG-GATG block copolymers are especially suited for drug and gene delivery applications. For instance, they have been used in the preparation of polyion complex (PIC) micelles. PIC micelles are smart delivery systems originally described by the groups of Kataoka and Kabanov (40,41) that are formed by electrostatic interaction between oppositely charged polyions. Similarly to classical polymeric micelles, PIC micelles have a core–shell structure with a core of ionic blocks surrounded by a neutral hydrophilic corona, typically of PEG. Properties such as their small size, electrical neutrality, and narrow size distribution make these systems highly attractive for drug delivery applications (42,43).
Our research group has reported the preparation of nanosized PIC micelles from an anionic PEG-GATG block copolymer of G3 decorated with 27 peripheral sulfates and poly-l-lysine (PLL) as a model polymer of opposite charge (Fig. 3) (44). Notably, these micelles displayed enhanced stability toward ionic strength compared to conventional PIC micelles from linear copolymers, a fact has been ascribed to the more rigid dendritic architecture. These micelles are envisioned as attractive delivery systems for low-molecular-weight drugs, proteins, nucleic acids, and imaging agents. In another example, making use of the developed CuAAC conditions for the anionic decoration of GATG dendrimers, carboxylates have been introduced onto the dendritic periphery of PEG-GATG copolymers, which has allowed the preparation of related pH-sensitive PIC micelles with potential applications in cancer therapy (45).
Fig. 3.
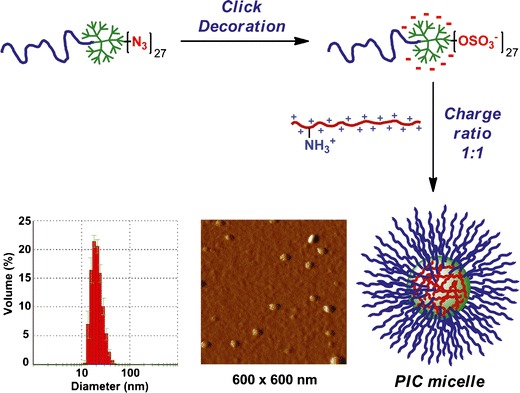
Schematic representation of the formation of PIC micelles from an anionic PEG-GATG block copolymer and PLL. DLS histogram and tapping-mode AFM image of the micelles. Reprinted with permission from reference (44)
Cationic synthetic carriers have been widely assayed in the last decade as an alternative to viral vectors for the delivery of nucleic acids (46). Among them, cationic dendrimers with the ability to electrostatically interact with negatively charged nucleic acids have received special attention (8,47). The complexes, named dendriplexes, obtained from commercially available PAMAM and PPI dendrimers, have been by far the most investigated ones. Unfortunately, some limitations have arisen, mainly associated to the excess of positive charge necessary to efficiently complex the genetic material, which results in aggregation with blood components and cytotoxicity. One strategy to overcome these limitations has been the use of PEGylated cationic block copolymers to mask the positive charge (48,49). Similarly to PIC micelles, the positively charged dendritic block interacts with the negatively charged nucleic acid forming an inner core surrounded by a hydrophilic PEG corona. The obtained dendriplexes are sterically stabilized and present lower z-potential, reduced cytotoxicity, and increased circulation times.
In light of these results, cationic GATG dendrimers and their block copolymers appeared to be excellent candidates for gene delivery applications. Amine-decorated GATG scaffolds are easily obtained by reduction of the terminal azides. This results in a high positive charge in physiological media and so in the ability to condense and protect nucleic acids. In addition, the hydrophobic nature of the gallic acid was envisioned to enhance the cellular uptake and transfection efficiency of the dendriplexes. In collaboration with the group of Alonso, we have recently evaluated the ability of amino-functionalized GATG dendrimers and block copolymers to complex plasmid DNA (pDNA) (34,50). As a result of an analysis of the influence of G, N/P ratio, and the presence/absence of PEG on the dendriplex size and z-potential, we have proposed these dendriplexes as core–shell nanostructures with sterically induced stoichiometry. A single pDNA condensed at the core surrounded by a shell of dendrimers with a stoichiometry determined by the core/dendrimer relative size: the higher the dendrimer G, the fewer the dendrimers that can be accommodated on the dendriplex surface (Fig. 4). Interestingly, in the case of PEG-dendritic block copolymers, this implies the possibility of tuning the PEG density on the dendriplex surface, which may be of interest to control the stealth properties for specific gene therapy applications.
Fig. 4.
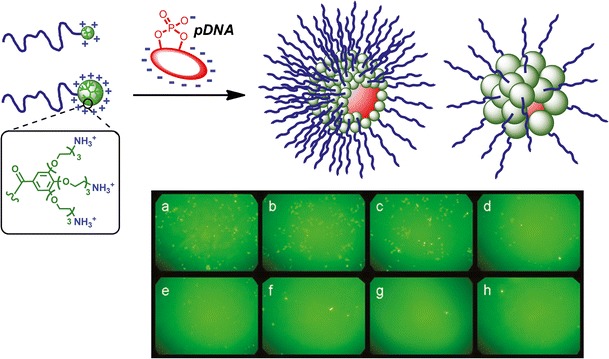
Schematic representation of dendriplexes prepared from a plasmid DNA (pDNA) and two generations of PEG-GATG block copolymers as nanostructures with core–shell stoichiometry. Expression of EGFP in HEK-293T cells transfected with dendriplexes prepared with increasing molar ratios of PEG-[G3]-NH2/[G3]-NH2: a 0% (solely G3), b 0.5%, c 1%, d 5%, e 10%, f 20%, g 50%, and h 100% (solely PEG-G3). Reprinted with permission from reference (34) and (50)
The stability, cytotoxicity, and interaction with blood components of the dendriplexes were studied, along with their ability to transfect mammalian cells (50). It was revealed that the dendriplexes formed from GATG dendrimers are stable, biocompatible, and do protect pDNA from degradation. More importantly, dendriplexes were effectively internalized by HEK-293T cells, which were successfully transfected. It was also observed that PEGylation remarkably influences the properties of the dendriplexes. As previously seen in other nanostructures, PEG improves the biocompatibility at the cost of a reduced cellular uptake (51). Our results highlighted that the PEGylation degree of nanostructures should be carefully adjusted in order to obtain an optimized stealth formulation without compromising the transfection efficiency. A straightforward approach for modulating the density of the PEG shell that ensured a successful transfection was developed by employing mixtures of GATG dendrimers and PEGylated copolymers for the preparation of the dendriplexes (Fig. 4). Further investigations, including in vivo studies, are planned to draw definite conclusions on the efficacy of this mixed stealth formulation in animal models.
GATG DENDRIMERS AS DRUGS: INTERACTIONS WITH PROTEINS AND PEPTIDES
Besides the aforementioned application of dendrimers as drug carriers, they can also act as drugs by themselves (9). For instance, several dendritic structures have shown promising antimicrobial and antibacterial activity that pave their way as alternatives to conventional antibiotics (52–54). Anionic dendrimers with anti-inflammatory properties (55,56) or as agents for the multiplication of human natural killer cells (57) have been also described. The use of dendrimers as inhibitors of viral infections has been investigated as well, in particular, against the human immunodeficiency (HIV) and herpes simplex viruses (HSV-1 and HSV-2) (58–60). VivaGel™, a l-lysine-based dendritic microbicide decorated with anionic groups, undoubtedly constitutes the most relevant example. It has been evaluated in phase II clinical trials as a vaginal gel for preventing/reducing transmission of HIV and genital herpes (61). There are also reports on the use of dendrimers that dissolve prion-protein aggregates and hamper fibril formation of prion and β-amyloid (Aβ) peptides (62,63).
In this context, our group together with those of Velázquez-Campoy and Neira were encouraged to explore the ability of GATG dendrimers to interact with HIV-1. We hypothesized that if dendrimers can destabilize the tertiary structure of proteins, they could also disrupt the quaternary structure of the capsid protein CA of HIV-1 and hamper its assembly to form the viral capsid. Thus, CA has recently emerged as a promising target for the development of new anti-HIV drugs based on its critical role during HIV morphogenesis (64). Our results demonstrate that G1 GATG dendrimers bind to the C-terminal domain of CA (CTD) with a dissociation constant in the micromolar range, as shown by isothermal titration calorimetry (ITC) (65). The affinity of some of the dendrimers for CTD was similar to that of synthetic peptides binding the dimerization region and of comparable magnitude to the homodimerization affinity of both CTD and CA. More importantly, a G1 dendrimer decorated by CuAAC with peripheral benzoate groups ([G1]-CO2Na) was able to hamper the assembly of the HIV capsid in vitro (Fig. 5), in what represents the first example of a dendrimer as a lead compound for the development of anti-HIV drugs targeting the capsid assembly.
Fig. 5.
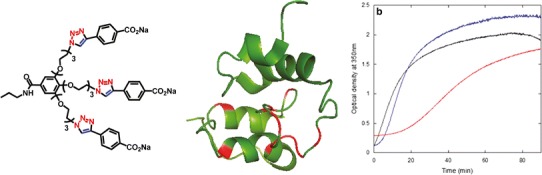
Left structure of [G1]-CO2Na. Center binding of GATG dendrimers to monomeric CTDW184A by NMR (residues in red change their peak intensities in the presence of GATG). Right inhibitory activity of [G1]-CO2Na on the in vitro assembly of CA. Oligomerization of CA in the absence (black line) or presence of [G1]-CO2Na at a 5-fold (blue line) and 10-fold molar excess (red line). Reprinted with permission from reference (65)
Nanomedicine has shown great potential for the treatment of many central nervous disorders, such as brain cancer, epilepsy, Alzheimer’s, or Parkinson’s diseases. Among the different nanostructures employed for this purpose, dendrimers have been intensively investigated in neurodegenerative processes, especially Alzheimer’s disease (66). According to the amyloid cascade hypothesis, amyloid peptide aggregation is closely related to the onset and development of Alzheimer’s disease. Since Aβ peptide oligomers intermediate in the assembly of fibrils are more neurotoxic than the end products, novel strategies aimed to reduce their toxic effects by affecting the aggregation process are of much relevance (62,67,68). Encouraged by the promising properties of GATG dendrimers as inhibitors of the dimerization of CA, we envisaged GATG interfering in the formation of amyloid fibrils. In a joint effort with the group of Klajnert, a morpholine-decorated GATG dendrimer ([G3]-Mor) was identified to effectively accelerate the formation of amyloid fibrils from a Aβ 1–28 peptide (thioflavin T assay, CD, transmission electron microscopy; Fig. 6) (69). Interestingly, when the cytotoxicity of the Aβ and the pair Aβ/[G3]-Mor was monitored at different stages of the aggregation process, it was observed that [G3]-Mor significantly reduced the toxicity of the peptide, most likely by speeding up the fibril formation and lowering the concentration of the toxic prefibrillar forms in the system.
Fig. 6.
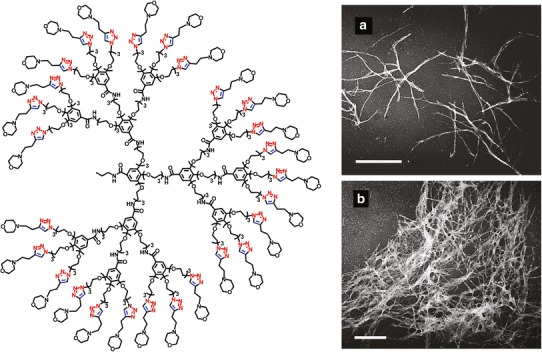
Left structure of [G3]-Mor. Right electron micrographs of Aβ 1–28 at the end of an aggregation process in the absence (a) and the presence of 1 μM [G3]-Mor (b). The length of the bar equals to 200 nm. Reprinted with permission from reference (69)
GATG GLYCODENDRIMERS AS TOOLS TO STUDY MULTIVALENT CARBOHYDRATE RECOGNITION
Carbohydrates cover a large spectrum of bioactivities from energy source and structural roles to others crucial for the development, growth, function, or survival of organisms. From bacteria to mammals, cells are coated with sugars as first points of contact with their environment. Thus, they are in a position to modulate a plethora of biological processes including cell–cell recognition, fertilization, pathogen invasion, and toxin and hormone mediation. Moreover, strong evidence suggests that carbohydrates are key diagnostic and prognostic indicators as well as therapeutic targets (70). In addition, the clustered arrangement of carbohydrates on the cell surface enables their multivalent interaction in global processes characterized by affinities and specificities much higher than monovalent interactions (71,72). This fact has prompted the development of synthetic multivalent glycoconjugates (linear polymers, micelles, nanoparticles, nanotubes, dendrimers) with the ability to promote/inhibit biological events (73). Since carbohydrate recognition is commonly mediated by proteins (lectins), the development of more efficient diagnostic and therapeutic tools relies on a better understanding of the carbohydrate–lectin interaction. However, the complexity of the binding mechanisms associated with multivalency (intermolecular crosslinking, chelation, statistical rebinding) makes them very difficult to measure experimentally. As a consequence, binding data are frequently extracted from indirect competitive methods in solution where only relative affinities are obtained (73). In addition, these experimental designs usually represent rough models for mimicking surface-based interactions as underestimate the multivalency derived from the lectin clustering.
With the aim of gaining insight into the fundamental mechanisms of multivalent carbohydrate recognition, we have synthesized GATG glycodendrimers by CuAAC from unprotected alkynated saccharides (Fig. 7) (33,35). These were foreseen as nanotools of precise size and multivalency for mechanistic studies by surface plasmon resonance (SPR). We have compared the outcome of carbohydrate-lectin binding studies in solution (via competitive experiments) and surface-bound direct experiments (with immobilized lectins on a chip surface) (74,75). To that end, we selected the lectin Concanavalin A (Con A) and four generations of glycodendrimers carrying 3–81 mannose/glucose residues ([Gn]-Man and [Gn]-Glc). Solution experiments demonstrated the importance of multivalency in carbohydrate recognition, with affinity increases being observed from the monosaccharide to G2. The lack of further affinity enhancements at higher G, however, contrasts with the surface-bound experiments. This different outcome not only stresses the relevance of the experimental design for soluble vs surface-bound lectins but also that higher dendrimer G not necessarily result in higher affinities in solution.
Fig. 7.
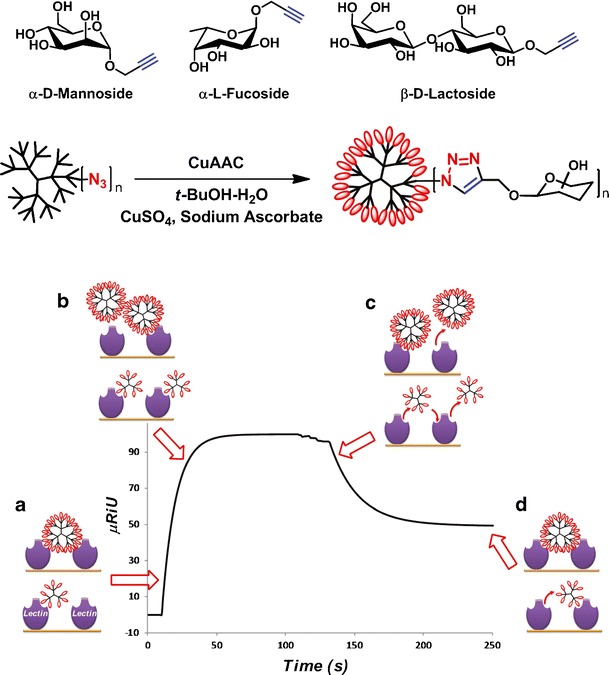
Synthesis of GATG glycodendrimers and schematic representation of the dynamic binding heterogeneity of surface-bound experiments between lectin clusters and glycodendrimers: a Initial binding of glycodendrimers to the lectin cluster with potential stabilization via chelate mechanism depending on glycodendrimer size and lectin cluster density. b At longer association times, competition between dendrimers for lectin complexation increases, promoting monovalent interactions primarily stabilized via rebinding effects. c An initial fast dissociation of glycodendrimers bound with low affinity is followed by d a slower dissociation due to stabilization by rebinding and potential chelate effects. Reprinted with permission from references (33) and (74)
In the surface-bound experiments, a complex binding profile was disclosed with two limiting binding modes, a low-affinity mode associated with dendrimers binding the lectin surface monovalently, and a high-affinity mode associated with dendrimers with higher functional valency. SPR studies also revealed the dynamic nature of the binding mechanisms, with contributions depending not only on the glycoconjugate multivalency and lectin cluster density, but also on the local concentration of glycoconjugates in the proximity of the lectin cluster, which is a time-dependent factor (Fig. 7). As a result, an original SPR protocol was designed to gather kinetic and thermodynamic information on the interaction by analyzing the early association and late dissociation phases of the sensorgrams, areas where the low analyte concentration nearby the receptor surface favors the highest affinity binding modes. In addition, it was concluded that for surface-bound experiments, the density of receptors should be carefully selected to mimic as much as possible the biological environment if relevant quantitative information is desired beyond a list of relative affinities.
GATG AS CONTRAST AGENTS FOR MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) uses a strong magnetic field and radio frequency pulses to obtain internal images of organs and lesions. Differences in contrast of the images reflect the rate at which excited protons of water molecules return to the equilibrium state (relaxation times, T) (76). Although it is possible to obtain high-quality images by manipulation of pulse sequences, high contrast is better achieved by adding exogenous contrast agents that, upon coordination to water, accelerate relaxation. Contrast agents in the clinic are based on paramagnetic ions such as gadolinium (Gd3+) complexed to low-molecular-weight ligands (e.g., DTPA, DOTA, DO3A) (77). These complexes present high relaxivity and adequate biocompatibility, stability, and solubility. However, because of their small size, they suffer from rapid excretion (high doses necessary) and passive distribution into the interstitial space. The use of macromolecular contrast agents is envisioned to provide longer circulation times in the bloodstream (acquisition windows) and selective diffusion through angiogenic tissue, along with an increased relaxivity per Gd (78). Among the macromolecular contrast agents, dendrimers are especially appealing because of their monodisperse nature and absolute control of their size. Their branched structure imparts rigidity and a high density of functional groups for the multivalent display of Gd and other synergistically integrated agents for therapy and diagnosis. In addition, the pharmacokinetics and pharmacodynamics of dendrimers, their permeability, excretion routes, and recognition by the reticulo-endothelial system can be controlled by generation (78–80). The synthesis of dendritic contrast agents has been conventionally achieved in a stepwise fashion through postlabeling approaches, which involve the incorporation of suitable ligands onto the macromolecular scaffold, followed by complexation to the desired metal ion. Unfortunately, these strategies suffer from incomplete functionalization that results in mixtures of polydisperse compounds and reduced relaxivity. To solve this inconvenience, we have turned our attention to a prelabeling approach using Gd complexes and CuAAC as an efficient coupling technology. This way, the complete functionalization of three generations of PEG-GATG block copolymers was demonstrated with an alkynated Gd-DO3A complex (Fig. 8) (81). The resulting monodisperse macromolecular contrast agents, incorporating up to 27 Gd ions at the periphery, display molecular relaxivities that increase with G up to values in the range of Gadomer-17 (82), a polylysine-based dendritic contrast agents bearing 24 DO3A-Gd chelates and considered as a reference in the field. The analysis of the pharmacokinetic properties of this new family of PEG-dendritic contrast agents was studied in mice using a C6 glioma model (Fig. 8). After intravenous injection of the contrast agents, T1-weighted images showed similar increments of signal intensity and kinetic profiles to Gadomer-17 (with maximum intensities of 4 min after injection), which reveals them as a promising platform for the development of dendritic contrast agents for MRI. The experimental simplicity of this CuAAC-based prelabeling approach should be of relevance for the preparation of alternative macromolecular metal complexes for applications in chemical exchange saturation transfer MRI, fluorescence imaging, and radiolabeling.
Fig. 8.
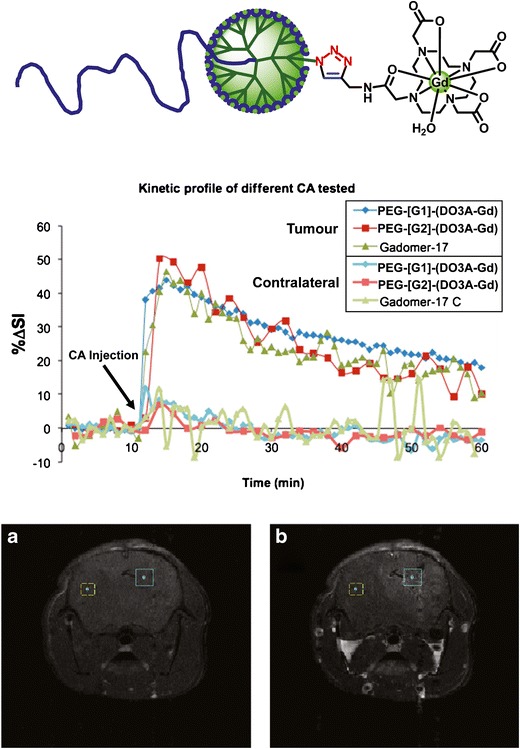
Schematic structure of PEG-GATG contrast agents for MRI. Normalized increase in signal intensity (ΔSI) in tumor and contralateral hemisphere. T 1-weighted images of a mouse brain before (a) and after (b) administration of PEG-[G2]-(DO3A-Gd). Squares in images show tumor (right) and contralateral hemisphere (left) regions analyzed. Reprinted with permission from reference (81)
DENDRIMER DYNAMICS
The flexibility of the chemical bonds within dendrimers determines their internal dynamics, hydrodynamic size, and topological localization of the external groups, all of relevance for pharmacological properties such as biodistribution and surface accessibility. In spite of initial controversies about the dendritic conformation, shape, and packing, a consensus has recently emerged on dendrimers as flexible macromolecular structures with a dense core and fluctuating repeating unit groups (83).
Nuclear magnetic resonance (NMR) is a powerful tool to study the dynamics of macromolecules at an atomic level (84). Information is usually extracted by measuring longitudinal (T1) and transverse (T2) relaxation times and Nuclear Overhauser effect (NOE). It is especially suited for the analysis of dendrimers since their repetitive nature offers the opportunity to probe different layers and G. Because of our interest in developing the bioapplications of GATG dendrimers, we decided to analyze their dynamical properties. With this aim, we initially performed a 1H NMR relaxation study in CDCl3 (35). Increasing T1 and T2 values were observed on going from the core to the periphery that, according to the theoretical variation of relaxation times with the correlation time (τ; Fig. 9) (86), were interpreted as a radial increase of dynamics in the same direction (congested core protons surrounded by more flexible external nuclei). This dynamical picture was later confirmed under more relevant aqueous conditions by a quantitative 13C relaxation study in collaboration with the group of Widmalm (87).
Fig. 9.
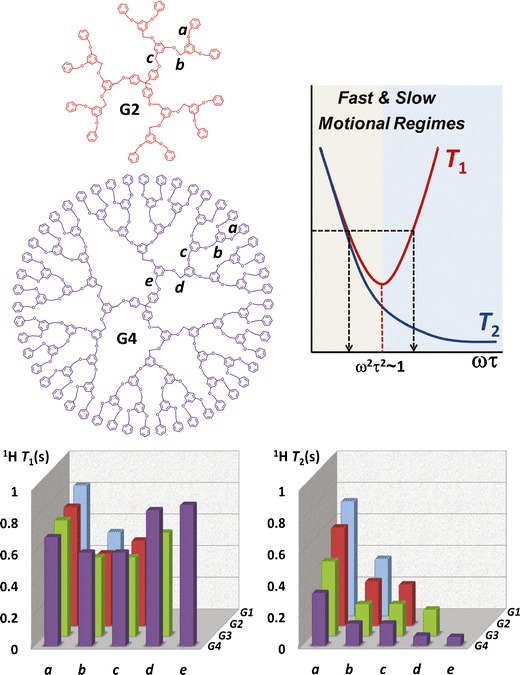
Top left panel structures of poly(aryl ether) dendrimers. Top right panel schematic representation of the theoretical dependence of T 1 and T 2 on correlation time (τ). Bottom panel 1H T 1 and T 2 for the benzylic protons of G1–G4 poly(aryl ether) dendrimers (CDCl3, 500 MHz, 298 K). Reprinted with permission from reference (85)
In general, for NMR studies on the dynamics of macromolecules, quantitative modeling from 13C relaxation is preferred to 1H. However, lengthy 13C experiments and the necessity of recording various parameters at different magnetic fields have limited such approach in dendrimers to a single report apart from ours (87,88). Conversely, a great deal of information has been extracted by qualitative interpretation of 1H and/or 13C relaxation. These studies have, nevertheless, afforded conflicting results on the relative dynamics between the dendritic core and the periphery. With the aim of throwing light on this controversy, we have recently performed a comprehensive relaxation study (1H, 13C; various magnetic fields and temperatures) of Fréchet-type poly(aryl ether) dendrimers (Fig. 9) (18), as an example of a dendritic family where conflicting relative dynamics between core and periphery have been reported by 1H T1 relaxation (89,90) and by alternative techniques (91,92). As a result of this work (85), it was revealed that NMR relaxation in dendrimers has been often misinterpreted in terms of dynamics. Dendrimers show slower dynamics at internal layers and display internal nuclei with T2 values shorter than the periphery, but T1 values that can be either shorter or larger depending on their position in the fast or slow motional regimes (Fig. 9). Accordingly, only the recording of T1 data at various temperatures (alternatively, T2 or NOE at one temperature) can ensure the correct interpretation of dendrimer dynamics. The large number of dendritic families, other than poly(aryl ether), where dynamics have been evaluated on the basis of T1 data at one temperature urges necessity of revisiting previous NMR relaxation studies.
The fact that dendrimers obey a dense core model with increasing T2 values from the core to the periphery has been more recently exploited in our laboratory in T2-edited NMR experiments (93) for the stepwise filtering of the internal nuclei (94). The resulting filtered spectra benefit from reduced signal overlapping, which facilitates NMR assignment and characterization (Fig. 10). This filtering strategy has been applied to various dendritic families, nuclei (1H, 13C, 31P), and 2D experiments (COSY and HSQC), and is envisaged to aid structural characterization and end-group analysis in related dendritic structures, including block, dendronized, and hyperbranched polymers functionalized with drugs, active targeting moieties, and other labels.
Fig. 10.
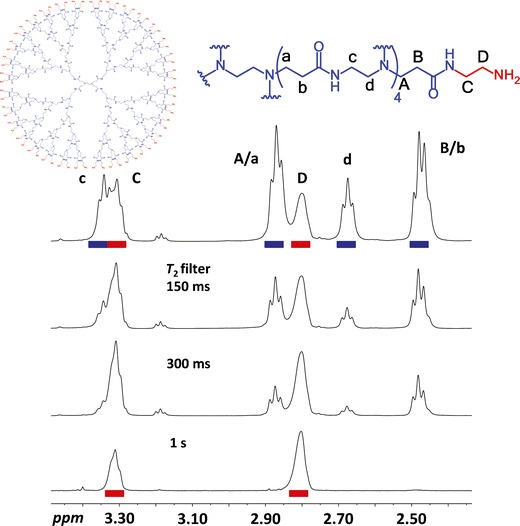
Structure of G4 PAMAM and 1H T 2-filtered NMR spectra (500 MHz, CDCl3, 298 K). Increasing filters between 150 ms and 1 s resulted in a spectrum showing only the most peripheral protons, which remained partially hidden in the original spectrum. Reprinted with permission from reference (94)
CONCLUSIONS
GATG dendrimers and their PEGylated block copolymers represent promising macromolecular scaffolds for a plethora of biomedical applications. Key features in GATG are a high tunability and versatility. The presence of terminal azides in GATG allows their efficient functionalization with a variety of ligands of biomedical relevance. Depending on the desired application, GATG dendritic scaffolds have been easily decorated in a single step with anionic or cationic groups, carbohydrates, peptides, or imaging agents. The resulting functionalized dendrimers have found application as nanotools to study multivalent interactions, as building blocks for the preparation of polymeric micelles and dendriplexes for gene delivery, as antiviral drugs or agents for the treatment of neurodegenerative diseases, and as contrast agents for MRI. In addition, the analysis of GATG dynamics by NMR relaxation has prompted a fundamental study on dendrimer dynamics. As a result, profound differences between the relaxation behavior of dendrimers and linear polymers were revealed that have been exploited in the filtering of NMR spectra to facilitate signal assignment and characterization.
As a concluding remark, we would like to highlight that despite the promising results of GATG dendrimers in the biomedical field, there are still fascinating puzzles ahead of us to be solved where GATG might play a role. Examples of current challenges faced by this dendritic family in our laboratory are the development of nanosystems able to tackle unresolved problems in drug delivery and to avoid the limitations derived from the potential Cu contamination after CuAAC. Regarding the first objective, advantage can be taken of the modularity of GATG dendrimers. Indeed, by making discrete structural changes, such as varying the length of the PEG chain, the dendritic generation or the hydrophobicity at the periphery, the solubility, and dynamical properties of GATG can be effectively tuned. As for the second challenge, we are currently engaged in the development of Cu-free approaches that circumvents the generation of reactive oxygen species, also avoiding the use of large linkers like strained cycloalkynes. Finally, we are motivated to transfer GATG dendrimers to in vivo situations where parameters such as, biodegradability, immunogenicity, or bioaccumulation, among others, will have to be carefully evaluated to validate their clinical potential.
Acknowledgments
The authors wish to acknowledge past and present lab members who have contributed to the development of dendrimers in our group. This work was financially supported by the Spanish Government (CTQ2009-10963, CTQ2012-34790, CTQ2009-14146-C02-02, CTQ2012-33436) and the Xunta de Galicia (10CSA209021PR and CN2011/037).
Conflict of Interest
The authors declare that they have no competing interests.
Abbreviations
- AMCA
Aminomethylcoumarin
- CA
HIV capsid protein
- CTD
C-Terminal domain
- Con A
Concanavalin A
- CuAAC
Cu(I)-catalyzed azide-alkyne cycloaddition
- DTPA
Diethylenetriaminepentaacetate
- DOTA
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
- DO3A
Tri-tert-butyl 1,4,7,10-tetraazacyclododecane-1,4,7-triacetate
- EGFP
Enhanced green fluorescent protein
- FDA
Food and Drug Administration
- FITC
Fluorescein isothiocyanate
- GATG
Gallic acid-triethylene glycol
- Gn
Dendrimer generation, n denotes the generation number
- Glc
Glucose
- HEK293T
Human embryonic kidney cell line 293T
- HIV
Human immunodeficiency virus
- HPMA
N-(2-hydroxypropyl)methacrylamide
- HSV
Herpes simplex virus
- ITC
Isothermal titration calorimetry
- Man
Mannose
- Mor
Morpholine
- MRI
Magnetic resonance imaging
- NOE
Nuclear Overhauser effect
- PAMAM
Polyamidoamine
- PEG
Poly(ethylene glycol)
- PPI
Polypropylene imine
- PIC
Polyion complex
- RGD
Arginylglycylaspartic acid
- SPR
Surface plasmon resonance
Footnotes
Ana Sousa-Herves and Ramon Novoa-Carballal contributed equally to this work.
References
- 1.Amaral SP, Fernandez-Villamarin M, Correa J, Riguera R, Fernandez-Megia E. Efficient multigram synthesis of the repeating unit of gallic acid-triethylene glycol dendrimers. Org Lett. 2011;13(17):4522–5. doi: 10.1021/ol201677k. [DOI] [PubMed] [Google Scholar]
- 2.Fréchet JMJ, Tomalia DA, editors. Dendrimers and other dendritic polymers. New York: Jonh Wiley & Sons; 2001
- 3.Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci. 2005;30(3–4):294–324. doi: 10.1016/j.progpolymsci.2005.01.007. [DOI] [Google Scholar]
- 4.Newkome GR, Moorefield CN, Vögtle F. Dendrimers and dendrons: concepts, syntheses, applications. Wiley-VCH: Weinheim; 2001. [Google Scholar]
- 5.Mintzer MA, Grinstaff MW. Biomedical applications of dendrimers: a tutorial. Chem Soc Rev. 2011;40(1):173–90. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 6.Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem Rev. 2009;109(1):49–87. doi: 10.1021/cr068212n. [DOI] [PubMed] [Google Scholar]
- 7.Medina SH, El-Sayed MEH. Dendrimers as carriers for delivery of chemotherapeutic agents. Chem Rev. 2009;109(7):3141–57. doi: 10.1021/cr900174j. [DOI] [PubMed] [Google Scholar]
- 8.Dufès C, Uchegbu IF, Schätzlein AG. Dendrimers in gene delivery. Adv Drug Delivery Rev. 2005;57(15):2177–202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Rolland O, Turrin C-O, Caminade A-M, Majoral J-P. Dendrimers and nanomedicine: multivalency in action. New J Chem. 2009;33(9):1809–24. doi: 10.1039/b901054h. [DOI] [Google Scholar]
- 10.Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110(4):1857–959. doi: 10.1021/cr900327d. [DOI] [PubMed] [Google Scholar]
- 11.Reek JNH, Arévalo S, van Heerbeek R, Kamer PCJ, van Leeuwen PWNM. Dendrimers in catalysis. In: Bruce CG, Helmut K, editors. Advances in catalysis: Academic Press; 2006. p. 71–151.
- 12.Rosen BM, Wilson CJ, Wilson DA, Peterca M, Imam MR, Percec V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem Rev. 2009;109(11):6275–540. doi: 10.1021/cr900157q. [DOI] [PubMed] [Google Scholar]
- 13.Buhleier E, Wehner W, Vogtle F. Synthesis. 1978;2:155–8. doi: 10.1055/s-1978-24702. [DOI] [Google Scholar]
- 14.Newkome GR, Yao Z, Baker GR, Gupta VK. Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-arborol. J Org Chem. 1985;50(11):2003–4. doi: 10.1021/jo00211a052. [DOI] [Google Scholar]
- 15.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–32. doi: 10.1295/polymj.17.117. [DOI] [Google Scholar]
- 16.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–36. doi: 10.1016/S1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 17.de Brabander-vandenBerg EMM, Meijer EW. Poly(propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. Angew Chem, Int Ed. 1993;32(9):1308–11. doi: 10.1002/anie.199313081. [DOI] [Google Scholar]
- 18.Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112(21):7638–47. doi: 10.1021/ja00177a027. [DOI] [Google Scholar]
- 19.Carnahan MA, Grinstaff MW. Synthesis of generational polyester dendrimers derived from glycerol and succinic or adipic acid. Macromolecules. 2005;39(2):609–16. doi: 10.1021/ma0518407. [DOI] [Google Scholar]
- 20.Ihre H, Padilla De Jesús OL, Fréchet JMJ. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J Am Chem Soc. 2001;123(25):5908–17. doi: 10.1021/ja010524e. [DOI] [PubMed] [Google Scholar]
- 21.Majoral J-P, Caminade A-M. Dendrimers containing heteroatoms (Si, P, B, Ge, or Bi) Chem Rev. 1999;99(3):845–80. doi: 10.1021/cr970414j. [DOI] [PubMed] [Google Scholar]
- 22.Sashiwa H, Shigemasa Y, Roy R. Chemical modification of chitosan. 10.1 synthesis of dendronized chitosan–sialic acid hybrid using convergent grafting of preassembled dendrons built on gallic acid and tri(ethylene glycol) backbone. Macromolecules. 2001;34(12):3905–9. doi: 10.1021/ma001832k. [DOI] [Google Scholar]
- 23.Meunier SJ, Wu Q, Wang S-N, Roy R. Synthesis of hyperbranched glycodendrimers incorporating α-thiosialosides based on a gallic acid core. Can J Chem. 1997;75(11):1472–82. doi: 10.1139/v97-177. [DOI] [Google Scholar]
- 24.Roy R, Park WKC, Wu Q, Wang S-N. Synthesis of hyper-branched dendritic lactosides. Tetrahedron Lett. 1995;36(25):4377–80. doi: 10.1016/0040-4039(95)00817-V. [DOI] [Google Scholar]
- 25.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem, Int Ed. 2002;41(14):2596–9. [DOI] [PubMed]
- 26.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–64. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 27.Meldal M, Tornøe CW. Cu-catalyzed azide–alkyne cycloaddition. Chem Rev. 2008;108(8):2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 28.Lallana E, Sousa-Herves A, Fernandez-Trillo F, Riguera R, Fernandez-Megia E. Click chemistry for drug delivery nanosystems. Pharm Res. 2012;29(1):1–34. doi: 10.1007/s11095-011-0568-5. [DOI] [PubMed] [Google Scholar]
- 29.Lallana E, Fernandez-Trillo F, Sousa-Herves A, Riguera R, Fernandez-Megia E. Click chemistry with polymers, dendrimers, and hydrogels for drug delivery. Pharm Res. 2012;29(4):902–21. doi: 10.1007/s11095-012-0683-y. [DOI] [PubMed] [Google Scholar]
- 30.Wurm F, Frey H. Linear-dendritic block copolymers: the state of the art and exciting perspectives. Prog Polym Sci. 2011;36(1):1–52. doi: 10.1016/j.progpolymsci.2010.07.009. [DOI] [Google Scholar]
- 31.Gitsov I. Hybrid linear dendritic macromolecules: from synthesis to applications. J Polym Sci, Part A: Polym Chem. 2008;46(16):5295–314. doi: 10.1002/pola.22828. [DOI] [Google Scholar]
- 32.Sousa-Herves A, Riguera R, Fernandez-Megia E. PEG-dendritic block copolymers for biomedical applications. New J Chem. 2012;36:205–10. doi: 10.1039/c2nj20849k. [DOI] [Google Scholar]
- 33.Fernandez-Megia E, Correa J, Rodríguez-Meizoso I, Riguera R. A click approach to unprotected glycodendrimers. Macromolecules. 2006;39(6):2113–20. doi: 10.1021/ma052448w. [DOI] [Google Scholar]
- 34.Raviña M, de la Fuente M, Correa J, Sousa-Herves A, Pinto J, Fernandez-Megia E, et al. Core–shell dendriplexes with sterically induced stoichiometry for gene delivery. Macromolecules. 2010;43(17):6953–61. doi: 10.1021/ma100785m. [DOI] [Google Scholar]
- 35.Fernandez-Megia E, Correa J, Riguera R. Clickable PEG-dendritic block copolymers. Biomacromolecules. 2006;7(11):3104–11. doi: 10.1021/bm060580d. [DOI] [PubMed] [Google Scholar]
- 36.Gravert DJ, Janda KD. Organic synthesis on soluble polymer supports: liquid-phase methodologies. Chem Rev. 1997;97(2):489–510. doi: 10.1021/cr960064l. [DOI] [PubMed] [Google Scholar]
- 37.Jelínková M, Strohalm J, Etrych T, Ulbrich K, Říhová B. Starlike vs classic macromolecular prodrugs: two different antibody-targeted HPMA copolymers of doxorubicin studied in vitro and in vivo as potential anticancer drugs. Pharm Res. 2003;20(10):1558–64. doi: 10.1023/A:1026170830782. [DOI] [PubMed] [Google Scholar]
- 38.Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, et al. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem. 2006;17(6):1464–72. doi: 10.1021/bc060240p. [DOI] [PubMed] [Google Scholar]
- 39.Albertazzi L, Fernandez-Villamarin M, Riguera R, Fernandez-Megia E. Peripheral functionalization of dendrimers regulates internalization and intracellular trafficking in living cells. Bioconjugate Chem. 2012;23(5):1059–68. doi: 10.1021/bc300079h. [DOI] [PubMed] [Google Scholar]
- 40.Harada A, Kataoka K. Formation of polyion complex micelles in an aqueous milieu from a pair of oppositely-charged block copolymers with poly(ethylene glycol) segments. Macromolecules. 1995;28(15):5294–9. doi: 10.1021/ma00119a019. [DOI] [Google Scholar]
- 41.Kabanov AV, Bronich TK, Kabanov VA, Yu K, Eisenberg A. Soluble stoichiometric complexes from poly(N-ethyl-4-vinylpyridinium) cations and poly(ethylene oxide)-block-polymethacrylate anions. Macromolecules. 1996;29(21):6797–802. doi: 10.1021/ma960120k. [DOI] [Google Scholar]
- 42.Lee Y, Kataoka K. Biosignal-sensitive polyion complex micelles for the delivery of biopharmaceuticals. Soft Matter. 2009;5:3810–7. doi: 10.1039/b909934d. [DOI] [Google Scholar]
- 43.Miyata K, Christie RJ, Kataoka K. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71(3):227–34. doi: 10.1016/j.reactfunctpolym.2010.10.009. [DOI] [Google Scholar]
- 44.Sousa-Herves A, Fernandez-Megia E, Riguera R. Synthesis and supramolecular assembly of clicked anionic dendritic polymers into polyion complex micelles. Chem Commun. 2008;27:3136–8. doi: 10.1039/b805208e. [DOI] [PubMed] [Google Scholar]
- 45.Sousa-Herves A, Riguera R, Fernandez-Megia E. The pH-sensitive dendritic polymeric micelles as drug delivery systems. PCT Int Appl (2010) WO 2010018286 A1 20100218.
- 46.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Rocchi P, Peng L. Dendrimers as non-viral vectors for siRNA delivery. New J Chem. 2012;36(2):256–63. doi: 10.1039/c1nj20408d. [DOI] [Google Scholar]
- 48.Wood KC, Little SR, Langer R, Hammond PT. A family of hierarchically self-assembling linear-dendritic hybrid polymers for highly efficient targeted gene delivery. Angew Chem, Int Ed. 2005;44(41):6704–8. doi: 10.1002/anie.200502152. [DOI] [PubMed] [Google Scholar]
- 49.Choi JS, Joo DK, Kim CH, Kim K, Park JS. Synthesis of a barbell-like triblock copolymer, poly(l-lysine) dendrimer-block-poly(ethylene glycol)-block-poly(l-lysine) dendrimer, and its self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(3):474–80. doi: 10.1021/ja9931473. [DOI] [Google Scholar]
- 50.de la Fuente M, Ravina M, Sousa-Herves A, Correa J, Riguera R, Fernandez-Megia E, et al. Exploring the efficiency of gallic acid-based dendrimers and their block copolymers with PEG as gene carriers. Nanomedicine (Lond) 2012;7(11):1667–81. doi: 10.2217/nnm.12.51. [DOI] [PubMed] [Google Scholar]
- 51.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83(3):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 52.Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Quaternary ammonium functionalized poly(propylene imine) dendrimers as effective antimicrobials: structure–activity studies. Biomacromolecules. 2000;1(3):473–80. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 53.Meyers SR, Juhn FS, Griset AP, Luman NR, Grinstaff MW. Anionic amphiphilic dendrimers as antibacterial agents. J Am Chem Soc. 2008;130(44):14444–5. doi: 10.1021/ja806912a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ortega P, Copa-Patino JL, Munoz-Fernandez MA, Soliveri J, Gomez R, de la Mata FJ. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org Biomol Chem. 2008;6(18):3264–9. doi: 10.1039/b809569h. [DOI] [PubMed] [Google Scholar]
- 55.Dernedde J, Rausch A, Weinhart M, Enders S, Tauber R, Licha K, et al. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci U S A. 2010;107(46):19679–84. doi: 10.1073/pnas.1003103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayder M, Poupot M, Baron M, Nigon D, Turrin C-O, Caminade A-M, et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci Transl Med. 2011;3(81):81–35. doi: 10.1126/scitranslmed.3002212. [DOI] [PubMed] [Google Scholar]
- 57.Griffe L, Poupot M, Marchand P, Maraval A, Turrin C-O, Rolland O, et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew Chem, Int Ed. 2007;46(14):2523–6. doi: 10.1002/anie.200604651. [DOI] [PubMed] [Google Scholar]
- 58.Chonco L, Pion M, Vacas E, Rasines B, Maly M, Serramía MJ, et al. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J Control Release. 2012;161(3):949–58. doi: 10.1016/j.jconrel.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 59.Jimenez JL, Pion M, Mata FJ, Gomez R, Munoz E, Leal M, et al. Dendrimers as topical microbicides with activity against HIV. New J Chem. 2012;36(2):299–309. doi: 10.1039/c1nj20396g. [DOI] [Google Scholar]
- 60.Blanzat M, Turrin C-O, Aubertin A-M, Couturier-Vidal C, Caminade A-M, Majoral J-P, et al. Dendritic catanionic assemblies: in vitro anti-HIV activity of phosphorus-containing dendrimers bearing Galβ1cer analogues. ChemBioChem. 2005;6(12):2207–13. doi: 10.1002/cbic.200500203. [DOI] [PubMed] [Google Scholar]
- 61.Rupp R, Rosentha SL, Stanberry LR. VivaGel™ (SPL7013 Gel): a candidate dendrimer—microbicide for the prevention of HIV and HSV infection. Int J Nanomed. 2007;4:561–6. [PMC free article] [PubMed] [Google Scholar]
- 62.Supattapone S, Nguyen H-OB, Cohen FE, Prusiner SB, Scott MR. Elimination of prions by branched polyamines and implications for therapeutics. Proc Natl Acad Sci U S A. 1999;96(25):14529–34. doi: 10.1073/pnas.96.25.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klajnert B, Cangiotti M, Calici S, Majoral JP, Caminade AM, Cladera J, et al. EPR study of the interactions between dendrimers and peptides involved in Alzheimer’s and prion diseases. Macromol Biosci. 2007;7(8):1065–74. doi: 10.1002/mabi.200700049. [DOI] [PubMed] [Google Scholar]
- 64.Neira JL. The capsid protein of human immunodeficiency virus: designing inhibitors of capsid assembly. FEBS J. 2009;276(21):6110–7. doi: 10.1111/j.1742-4658.2009.07314.x. [DOI] [PubMed] [Google Scholar]
- 65.Doménech R, Abian O, Bocanegra R, Correa J, Sousa-Herves A, Riguera R, et al. Dendrimers as potential inhibitors of the dimerization of the capsid protein of HIV-1. Biomacromolecules. 2010;11(8):2069–78. doi: 10.1021/bm100432x. [DOI] [PubMed] [Google Scholar]
- 66.Xu L, Zhang H, Wu Y. Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chem Neurosci. 2013;5(1):2–13. doi: 10.1021/cn400182z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hindo SS, Mancino AM, Braymer JJ, Liu Y, Vivekanandan S, Ramamoorthy A, et al. Small molecule modulators of copper-induced Aβ aggregation. J Am Chem Soc. 2009;131(46):16663–5. doi: 10.1021/ja907045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo SI, Yang M, Brender JR, Subramanian V, Sun K, Joo NE, et al. Inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins. Angew Chem, Int Ed. 2011;50(22):5110–5. doi: 10.1002/anie.201007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klajnert B, Wasiak T, Ionov M, Fernandez-Villamarin M, Sousa-Herves A, Correa J, et al. Dendrimers reduce toxicity of Aβ 1–28 peptide during aggregation and accelerate fibril formation. Nanomedicine (New York, NY, U S) 2012;8:1372–8. doi: 10.1016/j.nano.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Pashkuleva I, Reis RL. Sugars: burden or biomaterials of the future? J Mater Chem. 2010;20(40):8803–18. doi: 10.1039/c0jm01605e. [DOI] [Google Scholar]
- 71.Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, et al. Multivalency as a chemical organization and action principle. Angew Chem, Int Ed. 2012;51(42):10472–98. doi: 10.1002/anie.201201114. [DOI] [PubMed] [Google Scholar]
- 72.Kiessling LL, Young T, Gruber TD, Mortell KH. Multivalency in protein–carbohydrate recognition. In: Fraser-Reid B, Tatsuta K, Thiem J, editors. Glycoscience. Heidelberg: Springer Berlin; 2008. pp. 2483–523. [Google Scholar]
- 73.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem Rev. 2002;102(2):555–78. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 74.Munoz EM, Correa J, Riguera R, Fernandez-Megia E. Real-time evaluation of binding mechanisms in multivalent interactions: a surface plasmon resonance kinetic approach. J Am Chem Soc. 2013;135(16):5966–9. doi: 10.1021/ja400951g. [DOI] [PubMed] [Google Scholar]
- 75.Munoz EM, Correa J, Fernandez-Megia E, Riguera R. Probing the relevance of lectin clustering for the reliable evaluation of multivalent carbohydrate recognition. J Am Chem Soc. 2009;131(49):17765–7. doi: 10.1021/ja9074826. [DOI] [PubMed] [Google Scholar]
- 76.Terreno E, Castelli DD, Viale A, Aime S. Challenges for molecular magnetic resonance imaging. Chem Rev. 2010;110(5):3019–42. doi: 10.1021/cr100025t. [DOI] [PubMed] [Google Scholar]
- 77.Geraldes CFGC, Laurent S. Classification and basic properties of contrast agents for magnetic resonance imaging. Contrast Media Mol Imaging. 2009;4(1):1–23. doi: 10.1002/cmmi.265. [DOI] [PubMed] [Google Scholar]
- 78.Villaraza AJ, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110(5):2921–59. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5–6):171–85. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 80.Louie A. Multimodality imaging probes: design and challenges. Chem Rev. 2010;110(5):3146–95. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernández-Trillo F, Pacheco-Torres J, Correa J, Ballesteros P, Lopez-Larrubia P, Cerdán S, et al. Dendritic MRI contrast agents: an efficient prelabeling approach based on CuAAC. Biomacromolecules. 2011;12(8):2902–7. doi: 10.1021/bm2004466. [DOI] [PubMed] [Google Scholar]
- 82.Dong Q, Hurst DR, Weinmann HJ, Chenevert TL, Londy FJ, Prince MR. Magnetic resonance angiography with gadomer-17: an animal study. Investig Radiol. 1998;33(9):699–708. doi: 10.1097/00004424-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 83.Matthias Ballauff CNL. Dendrimers in solution: insight from theory and simulation. Angew Chem, Int Ed. 2004;43(23):2998–3020. [DOI] [PubMed]
- 84.Palmer AG. NMR characterization of the dynamics of biomacromolecules. Chem Rev. 2004;104(8):3623–40. doi: 10.1021/cr030413t. [DOI] [PubMed] [Google Scholar]
- 85.Pinto LF, Correa J, Martin-Pastor M, Riguera R, Fernandez-Megia E. The dynamics of dendrimers by NMR relaxation: interpretation pitfalls. J Am Chem Soc. 2013;135(5):1972–7. doi: 10.1021/ja311908n. [DOI] [PubMed] [Google Scholar]
- 86.Kowalewski J, Maeler L, Editors. Nuclear spin relaxation in liquids: theory, experiments, and applications: CRC Press; 2006.
- 87.Novoa-Carballal R, Säwén E, Fernandez-Megia E, Correa J, Riguera R, Widmalm G. The dynamics of GATG glycodendrimers by NMR diffusion and quantitative 13C relaxation. Phys Chem Chem Phys. 2010;12(25):6587–9. doi: 10.1039/c003645p. [DOI] [PubMed] [Google Scholar]
- 88.Meltzer AD, Tirrell DA, Jones AA, Inglefield PT, Hedstrand DM, Tomalia DA. Chain dynamics in poly(amidoamine) dendrimers: a study of carbon-13 NMR relaxation parameters. Macromolecules. 1992;25(18):4541–8. doi: 10.1021/ma00044a013. [DOI] [Google Scholar]
- 89.Hecht S, Fréchet JMJ. An alternative synthetic approach toward dendritic macromolecules: novel benzene-core dendrimers via alkyne cyclotrimerization. J Am Chem Soc. 1999;121(16):4084–5. doi: 10.1021/ja9842215. [DOI] [Google Scholar]
- 90.Kimata S-I, Jiang D-L, Aida T. Morphology-dependent luminescence properties of poly(benzyl ether) dendrimers. J Polym Sci, Part A: Polym Chem. 2003;41(22):3524–30. doi: 10.1002/pola.10830. [DOI] [Google Scholar]
- 91.Mourey TH, Turner SR, Rubinstein M, Frechet JMJ, Hawker CJ, Wooley KL. Unique behavior of dendritic macromolecules: intrinsic viscosity of polyether dendrimers. Macromolecules. 1992;25(9):2401–6. doi: 10.1021/ma00035a017. [DOI] [Google Scholar]
- 92.Wooley KL, Klug CA, Tasaki K, Schaefer J. Shapes of dendrimers from rotational-echo double-resonance NMR. J Am Chem Soc. 1997;119(1):53–8. doi: 10.1021/ja962285e. [DOI] [Google Scholar]
- 93.Novoa-Carballal R, Fernandez-Megia E, Jimenez C, Riguera R. NMR methods for unravelling the spectra of complex mixtures. Nat Prod Rep. 2011;28(1):78–98. doi: 10.1039/c005320c. [DOI] [PubMed] [Google Scholar]
- 94.Pinto LF, Riguera R, Fernandez-Megia E. Stepwise filtering of the internal layers of dendrimers by transverse-relaxation-edited NMR. J Am Chem Soc. 2013;135(31):11513–6. doi: 10.1021/ja4059348. [DOI] [PubMed] [Google Scholar]


