Abstract
Poly(lactic-co-glycolic acid) (PLGA) particles carrying antigen and adjuvant is a promising vaccine system which has been shown to stimulate systemic antigen-specific immune responses. In this study, we investigated the relationship of (i) the sizes of PLGA particle and (ii) the presence of cytosine-phosphate-guanine motifs (CpG), with the extent and type of immune response stimulated against Dermatophagoides pteronyssinus-2 (Der p2) antigen. Different sizes of PLGA particles encapsulating CpG were prepared using a double emulsion solvent evaporation method. Mice were vaccinated with Der p2 and different sizes of empty or CpG-loaded PLGA particles. Vaccinated mice were exposed to daily intranasal instillation of Der p2 for 10 days followed by euthanization to estimate leukocyte accumulation in bronchoalveolar lavage (BAL) fluids, antibody profiles, and airway hyperresponsiveness. PLGA particles showed a size-dependent decrease in the proportion of eosinophils found in BAL fluids. Mice vaccinated with the Der p2 coated on 9-μm-sized empty PLGA particles showed increased levels of IgE and IgG1 antibodies as well as increased airway hyperresponsiveness. All sizes of PLGA particles encapsulating CpG prevented airway hyperresponsiveness after Der p2 exposures. Inflammatory responses to Der p2 exposure were significantly reduced when smaller PLGA particles were used for vaccination. In addition, encapsulating CpG in PLGA particles increased IgG2a secretion. This study shows that the size of PLGA particles used for vaccination plays a major role in the prevention of house dust mite-induced allergy and that incorporation of CpG into the PLGA particles preferentially develops a Th1-type immune response.
Keywords: allergy, CpG, Der p2, house dust mite, PLGA, vaccine
INTRODUCTION
In the USA, 84% of residences have detectable levels of house dust mite (HDM) allergens and a quarter of these houses have higher levels of allergens than the proposed limit for asthma (1). Chronic exposure to HDM allergens can lead to lung inflammation characterized by lung eosinophilia and airway obstructions which can trigger asthma attacks in sensitized individuals (2). It has been reported that prolonged exposure to HDM allergens activates dendritic cells (DCs) present in lungs, priming T-helper 2 (Th2) immune responses (3) which consequently promote secretion of proinflammatory cytokines, recruitment of eosinophils to lungs, and B cell stimulation to produce immunoglobulin (Ig) E antibodies (4). Most current therapeutic treatments for asthma target neutralization of inflammatory mediators and relieve local symptoms for only a short duration (5, 6). Recently developed subcutaneous immunotherapy and sublingual immunotherapy to induce HDM-specific long-term tolerance have shown promising results in adults and children but such treatment required regular administration of high doses of HDM allergens for at least 3 years to show satisfactory clinical efficacy (7–9). Absence of a long-term solution to HDM-induced asthma in combination with the dangerous levels of HDM allergens present in households necessitates the requirement for a prophylactic vaccine that would switch the inflammatory immune response induced by HDM allergen to a protective immunity. This vaccine should induce T-helper 1 (Th1) immunity that results in production of interferon-gamma (IFN-γ), interleukin (IL)-12, and IgG2a antibodies (10, 11). This negative regulation of Th2 orchestrated airway inflammation and induction of protective immunity by Th1 cells can reduce pathogenic symptoms associated with allergy (12–14).
Among the various allergen-producing HDM, Dermatophagoides pteronyssinus (Der p) is the most prevalent allergy-causing mite (15, 16). These HDMs produce Der p2, a highly potent allergen that has structural and functional similarities with the immunostimulatory lipopolysaccharide-binding protein, MD2 (17). Serum samples of 79% of patients suffering from asthma, wheezing, and/or rhinitis possessed positive titers for anti-Der p2 IgE antibodies, making it a potential allergen for development of vaccines against HMD allergies (18). However, in an attempt to generate HDM vaccines, subcutaneous injections of Der p2 allergen to mice caused increases in the production of IgE antibodies and Th2-skewed immune responses (19). This necessitated the formulation of a vaccine that involved co-administration of the allergen with adjuvants that promote Th1-biased immune responses in order to effectively induce Der p2-specific Th1-type antibodies that would alleviate lung damage associated with exposure to allergen (20, 21). Unmethylated cytosine-phosphate-guanine motifs–1826 (CpG) is a potent oligodeoxynucleotide used as an adjuvant for polarization of immune responses to the Th1-type (22–24). It is an agonist to Toll-like receptor–9 which activates DCs and B cells to produce Th1-specific cytokines and suppresses Th2-modulated allergic responses (21). Co-administration of CpG-containing immunostimulatory oligodeoxynucleotide (ISS-ODN) with HDM allergen has been shown to decrease eosinophilia and IL-5 production while increasing the production of IFN-γ in nasal lavage fluid (25). In the same study, these responses were significantly improved when ISS-ODN was chemically conjugated with HDM allergen. In a clinical trial for ragweed allergy, peripheral DCs isolated from healthy individuals vaccinated with ragweed allergen conjugated to immunostimulatory oligodeoxyribonucleotide 1018 (Dynavax Technologies, Berkeley, CA) expressed increased levels of Th1 cytokines and decreased levels of Th2 cytokines (26). In a similar murine study, subcutaneous immunization of Balb/c mice with CpG conjugated to cedar pollen allergen was shown to increase the production of allergen-specific IgG2a and secretion of IFN-γ by CD4+ T cells isolated from spleens (27). With the clear demonstration of the importance of CpG at inducing a robust immunity against allergens, these studies also demonstrated that co-delivery of allergen with CpG is essential for stimulating an active Th1-type immune response (28). Chemical conjugation of CpG with allergen, although often successful, is expensive and can lead to structural modification of conjugated molecules changing their immunostimulatory properties. In addition, in vivo spontaneous cleavage of the conjugating bridge between allergen and adjuvant can prevent co-delivery of molecules to the same cell. An alternative co-delivery method is to administer CpG and Der p2 in biodegradable poly(lactic-co-glycolic acid) (PLGA) polymer particles. In addition to co-delivering multiple molecules, many studies have recognized the significance of PLGA particulate vaccines in stimulating robust Th1-type responses as characterized by secretion of IgG2a antibodies (29, 30). Vaccination of mice with antigen-loaded PLGA microparticles and CpG, either co-loaded with antigen or injected as a solution, showed enhanced secretion of IgG2a antibodies with a greater ratio of IgG2a:IgG1 antibodies when compared to mice vaccinated with a mixture of antigen and aluminum hydroxide (31). We have previously reported that PLGA particles encapsulating antigen and CpG can stimulate robust immune responses compared to vaccination of antigen and CpG in solution (32, 33). In addition, we have shown that the magnitude of the immune response generated directly depends on the size of PLGA particles used for immunization (33). While large particles encapsulating antigen with CpG are known to produce high levels of total IgG1 titers, submicron-sized particles containing antigen with CpG have been shown to induce higher ratios of IgG2a to IgG1. To develop prophylactic therapy against allergy-associated lung disorders, induction of high IgG titers and Th1-type immune responses is highly desirable. Th1-polarized immunity could decrease the secretion of IgE antibody and inflammatory damage to lungs upon exposure to allergen (20). Thus, in this study, we sought to determine the effects of the size of PLGA particle vaccines and the influence of CpG on the overall immune response to Der p2-coated PLGA particle vaccines.
MATERIALS AND METHODS
Preparation of CpG-Loaded PLGA Particles
Different sizes of particles were prepared using a modified method described by Joshi et al. (33). Briefly, 3 mg of CpG (Integrated DNA Technologies, Coralville, IA) was dissolved in 75 μL of 1% poly(vinyl alcohol) (PVA; Mowiol® 8–88; MW ∼67,000; Sigma, Allentown, PA). A primary emulsion was prepared by sonication of this solution at 60% output power for 30 s in 2 mL of dichloromethane (DCM) containing 200 mg of PLGA (Resomer® RG 503; viscosity 0.32–0.44 dL/g; MW 24,000–38,000; Boehringer Ingelheim KG, Germany) using a sonic dismembrator (Model FB 120 equipped with an ultrasonic converter probe CL-18; Fisher Scientific, Pittsburgh, PA). To prepare different sizes of PLGA particles, two independent methods were used for the preparation of the secondary emulsion. In method 1, the primary emulsion was emulsified in 1% PVA for 30 s using a sonic dismembrator at 60% output power. In method 2, an Ultra Turrax homogenizer (T 25 basic with 12.7 mm rotor; IKA-werke; Wilmington, NC) at 13,500 rpm/min was used for 30 s to emulsify the primary emulsion in 1% PVA. These secondary emulsions were stirred in a fume hood for 2 h for complete evaporation of DCM. Different sizes of suspended particles were collected by sequential centrifugation of particles at 200 rpm (7×g), 700 rpm (75×g), 4,000 rpm (2,880×g), and 7,000 rpm (6,790×g) for 5 min. Particles were washed with distilled water and lyophilized using FreeZone 4.5 (Labconco Corporation, Kansas City, MO). Particles collected at 700, 4,000, and 7,000 rpm were used for further experiments.
Quantification of CpG Loading into PLGA Particles
Loading of CpG into PLGA particles was estimated by degrading 20 mg of PLGA particles in 1 mL of 0.2 N NaOH for 12 h or until a clear solution was obtained. This solution was then neutralized with 1 N HCl. CpG was quantified using a fluorescence OliGreen® ssDNA quantitation reagent assay kit (Molecular Probes, Eugene, OR). Briefly, in a 96-well plate, 100 μL of 1X working reagent was added to 100 μL of standard CpG solutions of different concentrations and samples with unknown CpG concentrations. The plate was then incubated at room temperature for 5 min in the dark. Fluorescence was measured at λex 444 and λem 520 nm using a SpectraMax® M5 multi-mode microplate reader (Molecular Devices, Sunnyvale, CA). A standard curve generated from the known concentrations of CpG was used to determine the concentrations of CpG in samples.
Loading and percentage encapsulation efficiency (%EE) was calculated according to Eqs. 1 and 2, respectively.
| 1 |
where,
Loading: The amount of CpG in PLGA particles (μg/mg of PLGA particles)
Conc.: Concentration of CpG calculated from Oligreen assay (μg/mL)
Vol.: Volume of neutralized solution of degraded PLGA particles (mL)
Weight of particles: Initial weight of CpG encapsulated PLGA particles used for the assay (mg)
| 2 |
where,
Loading: Loading of CpG as calculated from equation 1 (μg/mg)
WParticles: Amount of CpG encapsulated PLGA particles prepared (mg)
WCpG: Initial amount of CpG used for the preparation (μg)
Characterization of PLGA Particles
The surface morphology of different fractions of particles was studied using Hitachi S-4800 scanning electron microscopy (SEM) (Hitachi High-Technologies, Ontario, Canada). Briefly, a drop of the suspension of lyophilized particles (1–5 mg/mL in deionized water) was plated onto a silicon wafer mounted on a SEM stub using a double stick carbon tape. The suspension was left to dry in air for 1 h. After drying, the silicon wafer was coated with gold-palladium by an argon beam K550 sputter coater (Emitech Ltd., Kent, England). Images were captured using the Hitachi S-4800 SEM at 5 kV accelerating voltage. The average particle sizes (n > or = 100) of different batches were calculated using ImageJ software (US National Institutes of Health, Maryland, USA) as described by Joshi et al. and were confirmed by Zetasizer Nano ZS (Malvern, Worcestershire, UK) measurements (33).
In Vitro Release of CpG from Different Sizes of PLGA Particles
Release kinetics of CpG from different PLGA particle preparations were determined by adding 20 mg of particles from each batch in a glass vial containing 5 mL of phosphate-buffered saline (PBS) heated to 37°C. These vials were capped and placed in a 37°C shaking incubator set at 200 rpm/min. Samples were collected at regular intervals. During the collection of every sample, medium was replenished with fresh PBS and sink conditions were maintained at all times. Samples were analyzed using a fluorescence OliGreen® assay kit as described above.
Animal Models of Der p2-Induced Asthma
Male C3H/HeBFeJ mice (5–6 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME) and provided standard laboratory rodent chow and water ad libitum (34). All animal care, housing, and procedure requirements of the National Institutes of Health Committee on Care and Use of Laboratory Animals were followed. Ninety-six mice were acclimatized for 7 days prior to first vaccination and divided randomly into nine experimental groups as described in Fig. 1. Untreated (sentinels) and PBS-treated (shams) control mice were used as unsensitized controls. Twelve mice per group were used in each experiment, except for the sentinel group (n = 4). A solution of 10 μg LoTox Natural Der p2 (LTN-DP2-1, Indoor Biotechnologists, endotoxin <0.03 EU/μg) in 100 μL PBS was incubated with PLGA particles for 30 min to coat Der p2 on PLGA particles. Mice were vaccinated by subcutaneous (s.c.) injection on day 0 and day 7, under isoflurane anesthesia (using a precision Fortec vaporizer, Cyprane, Keighley, UK) with Der p2 coated on PLGA particles loaded with or without 5 μg CpG. On each of days 14 to 23 (ten doses), mice were exposed to 2.5 μg Der p2 in 50 μL PBS by intranasal instillation during isoflurane anesthesia to induce airway inflammation and hyperresponsiveness. Mice were weighed on day 0, 7, 14, and 24 and observed for any behavioral changes or clinical symptoms. All mice were euthanized on day 24.
Fig. 1.
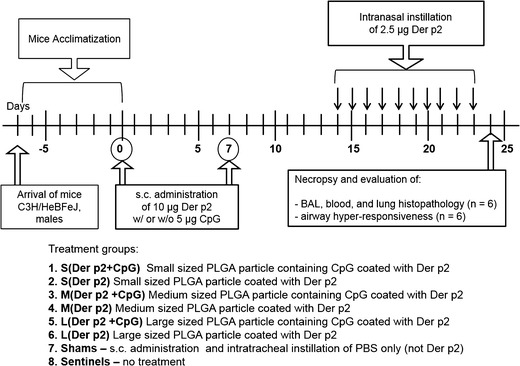
Treatment groups and experimental timeline for investigating effects of size of PLGA particles and presence of CpG in inducing protective immunity against Der p2 allergen. On day 0 and day 7, mice were vaccinated (s.c. injection) with Der p2-coated blank or CpG-loaded microparticles. From day 14 to day 23, mice received ten doses of Der p2 via intranasal instillation
Collection and Processing of Serum and Bronchoalveolar Lavage Fluid
Mice were euthanized with an overdose of isoflurane on day 24. Blood for Igs analysis was collected through cardiac puncture. Bronchoalveolar lavage (BAL) fluid was collected and processed as described by George et al. with minor modifications (35). Briefly, the trachea was exposed; BAL was performed using three doses of 1 mL of sterile saline (0.9% sodium chloride solution; Baxter, Deerfield, IL). BAL fluid was centrifuged at 800×g for 5 min at 4°C, and the supernatant was stored at −80°C for determination of cytokines. Cells in the pellet were resuspended in Hank’s balanced salt solution (Life Technologies, Grand Island, NY) for total cell counts using a hemocytometer. For differential cell counts, cells were spun onto microscope slides at 800×g for 3 min using a Cytospin 4 (Thermo Shandon, Thermo Scientific, Waltham, MA), air-dried, and stained using Protocol® HEMA 3 stain set (Fisher Diagnostics, Pittsburgh, PA), and then, 400 cells per slide were counted to enumerate macrophages, neutrophils, eosinophils, and lymphocytes under an optical microscope (Olympus, Center Valley, PA).
Detection of Serum Der p2-Specific IgE, IgG1, and IgG2a
The presence of anti-Der p2 IgG1, IgE, and IgG2a antibodies in the individual sera were evaluated by ELISA. Corning® 96 Well EIA/RIA Microplates (Corning, Lowell, MA) were coated with 100 μL of LoTox Natural Der p2 (2.5 μg/mL for IgG1 or 5 μg/mL for IgE and IgG2a) in 0.05 M sodium-potassium carbonate buffer (pH 9.6) and incubated overnight at 4°C. After three washes with PBS containing 0.05% v/v Tween® 20 (PBST), the plates were blocked with 150 μL of 1% w/v BSA in PBST for 1 h at room temperature (RT). Serum samples were serially diluted in blocking buffer at ranges from 1:10 to 1:80 for IgE, 1:25 to 1:25,600 for IgG2a, and 1:100 to 1:102,400 for the IgG1 assay. Diluted sera were added to the plates (100 μL/well) and incubated for 2 h at RT. Plates were washed three times and incubated with 100 μL 1/4,000 dilution of HRP-conjugated rat anti-mouse IgG1 or IgG2a or goat anti-mouse IgE (Southern Biotech, Birmingham, AL) for 2 h at RT. Plates were then washed six times with PBST followed by addition of 100 μL/well of TMB substrate (Thermo Scientific, Waltham, MA), and after 15-min incubation at RT, the reaction was stopped by addition of 0.17 N sulfuric acid (100 μL/well). The absorbance (optical density (OD)) was measured at 450 nm in a microplate spectrophotometer (SpectraMax® plus 384, Molecular Devices, Sunnyvale, CA). Because there is no commercially available standard mouse human anti-Der p2 antibodies, the equivalent concentrations of specific-Der p2 IgG1, IgG2a were calculated by comparison with a reference curve generated with a known serum. Results for IgG1 and IgG2a were expressed as ELISA units (U/mL); 1 U/mL was defined as reciprocal value of the serum dilution that gave an OD value of 1. This was always within the linear part of the dilution curve. To ensure reproducibility, a serum sample of known titer was run with each test as a standard.
Histological Analysis
Lungs that were not lavaged were rinsed with saline solution through the heart, perfused via the cannulated trachea, and fixed in 10% zinc formalin (Fisher Scientific, Kalamazoo, MI). Tissues were then paraffin-embedded and 5-μm-thick sections were stained with hematoxylin and eosin (H&E). Lung tissues were evaluated for allergic airway inflammation using light microscopy to study the presence of inflammatory cells infiltrates, perivascular and peribronchiolar inflammation. Severity of perivascular inflammation was quantified by a 4-point scoring system. Briefly, 0 = absence of cell cuffs, 1 = rare to few scattered perivascular inflammatory cell cuffs, 2 = multifocal to moderate numbers of perivascular inflammatory cell cuffs, 3 = large number of diffuse perivascular inflammatory cell cuffs. Total lung inflammation was defined as the sum of perivascular cuffing scores across all slides.
Evaluation of Airway Hyperresponsiveness
Airway hyperresponsiveness (AHR) was assessed on day 24, which was 24 h after the final intranasal instillation of Der p2, using a forced oscillation technique (FlexiVent System, SCIREQ, Montreal, QC, Canada). Mice were anesthetized by an intraperitoneal injection of 90 mg/kg of sodium pentobarbital (Ovation Pharmaceuticals, Inc. Deerfield, IL), and tracheotomy was performed using a tracheal cannula with luer adapter (1.3 mm, length 20 mm, Harvard Apparatus, Holliston, MA). Animals were then connected to a small animal ventilator set at a frequency of 150 breaths/min, a tidal volume of 10 mL/kg and a positive end-expiratory pressure of 2 to 3 cm H2O. Each mouse was challenged with increasing concentrations (3, 10, 30, and 100 mg/mL) of methacholine chloride (ICN Biomedicals, Inc. Solon, OH) aerosol that were generated for 10 s with an in-line nebulizer. Airway resistance was measured using a “snapshot” protocol each 20 s for 5 min, ensuring that measured parameters stabilized. The mean of these 15 values was calculated for each methacholine dose. At the end of the experiment, the animal was disconnected from the ventilator and given an overdose of sodium pentobarbital.
Statistical Analysis
Data were analyzed using statistical and graphing software GraphPad PRISM (GraphPad, San Diego, CA). All assays were compared using one-way analysis of variance (ANOVA) followed by Tukey’s post-test to compare all pairs of treatments. Differences were considered significant at p values that were less than or equal to 0.05. Values given are means ± SEM from at least six animals in each group unless otherwise noted.
RESULTS
Preparation of CpG-Loaded PLGA Particles
The double emulsion solvent evaporation method was used to fabricate CpG-loaded PLGA particles. A modified procedure was used for the preparation of the secondary emulsions. Method 1 and method 2 generated different sizes of PLGA particles which were segregated at different centrifugation speeds into batches of large-, medium-, and small-sized PLGA particles as described in Table I. Method 1, using an Ultra Turrax homogenizer to prepare secondary emulsions, gave a greater fraction of larger-sized particles as compared to the sonic dismembrator used in method 2. SEM images in Fig. 2 show that each batch had spherical particles with a distinct size distribution and smooth morphology. Particle sizes for each batch were calculated using SEM images and are described in Table I. Particle sizes for medium- and small-sized particles were confirmed with dynamic light scattering. De et al. has shown that different sizes of PLGA particles are stable at 4°C for at least a week (36). Lyophilized particles prepared for these studies were stored at −20°C until used.
Table I.
Characterization of a Representative Batch of PLGA Particles Prepared Using Methods 1 and 2
| Batch name | Percentage weight recovered | Collection G force | Size (μm) | Loading of CpG (μg/mg particles) | |
|---|---|---|---|---|---|
| Method 1 | Method 2 | ||||
| Small | 0.0 | 29.6 | 7,000×g | 0.3 ± 0.1 | 2.8 |
| Medium | 4.2 | 62.2 | 4,000×g | 1.0 ± 0.2 | 4.1 |
| Large | 58.4 | 0.0 | 700×g | 9.2 ± 2.1 | 5.1 |
Fig. 2.

Scanning electron micrographs of CpG-loaded i small-, ii medium-, and iii large-sized PLGA particles. Particles are spherical with smooth morphology. Scale bar on lower right represents 5-μm length
Loading and Release Kinetics of PLGA Particles Encapsulating CpG Depends on Particle Size
The loading capacity of CpG was directly proportional to the size of the PLGA particles (see Table I). Encapsulation efficiency for CpG was 22% for method 1 and 22.5% for method 2 as calculated from Eq. 2. Release kinetics of CpG from PLGA particle matrices were assessed in PBS using a 37°C shaking incubator. All sizes of particles demonstrated an initial burst release followed by a sustained release of CpG. The percentage of CpG released during the initial phase depended on the size of particles. Smaller particles, probably due to a larger surface area to volume ratio, showed a higher percentage of burst release of CpG which decreased with increasing particle size. This was followed by sustained release of CpG from every batch of particles as demonstrated in Fig. 3.
Fig. 3.
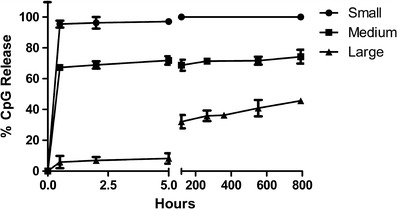
Release study of CpG from different sizes of PLGA particles. Particles were incubated at 37°C in an incubator shaker in PBS. All particles showed burst release followed by sustained release of CpG except small-sized particles that showed 100% release of CpG within 1 h. Values are expressed as mean ± SD (n = 3)
Accumulation of Inflammatory Cells Depends on the Size of PLGA Particles and Presence of CpG
Upon allergen challenge, inflammatory cells can accumulate in the lungs activating downstream inflammatory pathways which can lead to asthma (37). Here, the accumulation of leukocytes was evaluated by studying the phenotype of cells collected from BAL fluids of vaccinated mice on day 24. These mice were intranasally exposed with Der p2 antigen from day 14 to day 23 to induce allergic airway inflammation and hyperresponsiveness which is an established model for human allergic asthma (38, 39). As shown in Fig. 4, accumulation of the total number of cells, primarily eosinophils and lymphocytes, in BAL fluids of mice vaccinated with Der p2 coated on PLGA particles was found to increase with increasing size of PLGA particles. Mice vaccinated with small-sized particles displayed minimal eosinophilia which was similar to shams and sentinels (mice unexposed to Der p2). Figure 4 also demonstrates that the presence of CpG in PLGA particles used for vaccination consistently dampened the eosinophilia and accumulation of lymphocytes in lung tissue of vaccinated mice. When mice were vaccinated with small- or medium-sized microparticles, inclusion of CpG also reduced recruitment of macrophages compared with the mice without CpG (Fig. 4(v)). Mice vaccinated with large particles without CpG exhibited significantly higher influx of inflammatory cells into airways (*p < 0.05, **p < 0.01) as compared to all treatment groups as shown in Fig. 4(i), (ii), and (iii) showing no protection against Der p2 exposures. There was no significant difference among the numbers of neutrophils collected from BAL fluids. Figure 4(vi) compared the fraction of each cell phenotype accumulated in the lungs of vaccinated mice. The fractions of eosinophils collected in lungs were decreased in the presence of CpG and smaller PLGA particles.
Fig. 4.
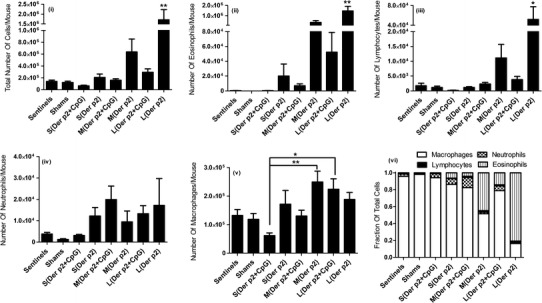
Number of i total cells, ii eosinophils, iii lymphocytes, iv neutrophils, and v macrophages in BAL fluids of vaccinated mice collected on day 24 (n = 6). vi Relative proportions of the different cell types in the BAL fluids of vaccinated mice on day 24. Significant differences were evaluated using one-way ANOVA followed by Tukey’s post-test (*p < 0.05, **p < 0.01). Values are expressed as mean ± SEM
Der p2-Coated PLGA Particles Induces IgG Response and the Presence of CpG Favors Production of IgG2a Antibodies
Serum samples from mice vaccinated with different sizes of PLGA particles with or without CpG were collected on day 24 and measured for the induction of humoral immune responses by evaluating the levels of IgG1, IgG2a, and IgE antibodies specific for Der p2 using ELISA. Mice vaccinated with large particles without CpG showed significantly higher levels of IgG1 antibody (**p < 0.01) than those vaccinated similarly but with CpG as shown in Fig. 5(i). This group of mice had comparatively low levels of IgG2a antibodies compared to PLGA particles with smaller sizes. The presence of CpG enhanced the induction of IgG2a antibodies in sera of mice vaccinated with small- and medium-sized particles as shown in Fig. 5(ii). It has been shown that generation of IgG2a antibody is dependent on cytokines such as IFN-γ which favor the switch of T-helper responses to Th1-type (10). In contrast, secretion of IgG1 and IgE antibodies is supported by IL-4 and IL-5 which primarily promotes Th2-type immune responses (20). Thus, the ratio of IgG2a:IgG1 antibodies measured in serum samples of each treatment group was evaluated in Fig. 5(iii). It was found that the presence of CpG in particles used in vaccinations resulted in increased proportions of IgG2a antibodies compared to mice vaccinated with the corresponding sized particles in the absence of CpG. In addition, ratios of IgG2a:IgG1 antibodies in mice vaccinated with Der p2 coated on small- and medium-sized particles encapsulating CpG were significantly higher (*p < 0.05, **p < 0.01) compared to other treatment groups. This would be expected to induce a blunting of airway inflammation and hyperresponsiveness associated with Der p2 lung exposure. Mice vaccinated with small- and medium-sized PLGA particles showed only small levels of IgE secretion which was not significantly different from mice not exposed to Der p2 (Fig. 5(iv)). In contrast, mice vaccinated with large particles showed significant increases in the levels of IgE antibody detected in the serum (*p < 0.05, **p < 0.01). This clearly demonstrates that decreasing the size of PLGA particles used for vaccination decreases the induction of Th2-polarized antibody responses. This can be enhanced by the inclusion of CpG, but it is the size of the PLGA particles that has the major role in determining the type of Th response.
Fig. 5.
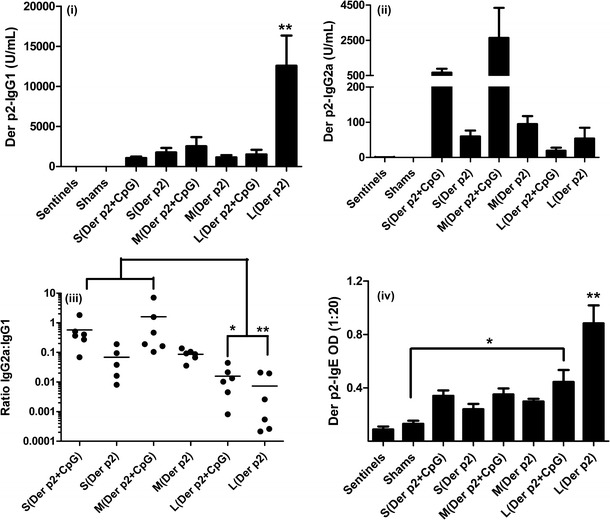
i Anti-Der p2 IgG1, ii anti-Der p2 IgG2a, iii ratio of IgG2a:IgG1 antibodies, and iv anti-Der p2 IgE antibody titers were estimated in serum samples of mice collected through cardiac puncture on day 24 (n = 6). Significant differences were evaluated using one-way ANOVA followed by Tukey’s post-test (*p < 0.05, **p < 0.01). Values are expressed as mean ± SEM
Presence of CpG in Vaccine Diminishes Airway Hyperresponsiveness
Allergic asthma is characterized by an increase in airway hyperresponsiveness (AHR) to nonspecific bronchoconstrictors like methacholine (40). The ability of PLGA particle vaccines to suppress the induction of AHR was evaluated 24 h after ten daily intranasal exposures of Der p2 to vaccinated mice. Mean baseline lung function (in the absence of methacholine challenge) did not differ among all experimental groups. As demonstrated in Fig. 6, mice vaccinated with small-sized particles showed no significant increase in AHR from baseline after increasing the dose of methacholine challenges. In contrast, mice vaccinated with Der p2 coated on large PLGA particles demonstrated the greatest increase in airway resistance after methacholine challenge (10, 30, and 100 mg/mL) which was significantly different from shams and sentinels (**p < 0.01, ***p < 0.001). AHR was also significantly different in mice vaccinated with Der p2-coated medium-sized particles after methacholine challenge at 30 and 100 mg/mL, compared to sentinels and shams (*p < 0.05, ***p < 0.001). Vaccination of mice with CpG encapsulated in PLGA particles significantly suppressed AHR when compared to Der p2-coated medium- and large-sized empty PLGA particles (*p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 6.
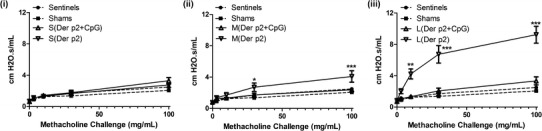
Airway hyperresponsiveness (AHR) of mice vaccinated with Der p2-coated i small-, ii medium-, and iii large-sized PLGA particles with or without CpG (n = 6). Mice were challenged with increasing doses of methacholine on day 24. Significant differences were evaluated using one-way ANOVA followed by Tukey’s post-test (*p < 0.05, **p < 0.01, ***p < 0.001). Data represent mean ± SEM
Presence of CpG Reduces Perivascular Cuffing
Allergy can cause infiltration of inflammatory cells into the peribronchiolar and perivascular connective tissues which were examined by histological analysis. Experimental groups that showed inflammatory cell infiltrates were predominately perivascular in nature with adjacent airways showing a similar, although less intense, inflammatory cell response. As shown in Fig. 7 and consistent with BAL data, the primary lesions caused by perivascular inflammation were predominantly composed of macrophages and eosinophils with fewer neutrophils. Mice vaccinated with Der p2 coated on large- and medium-sized blank PLGA particles showed the most severe perivascular cuffing which was reduced in mice vaccinated with PLGA particles encapsulating CpG. Mice vaccinated with Der p2 coated on small PLGA particles were also free from primary lesions caused by inflammatory cells. These mice along with the mice vaccinated with medium-sized particles containing CpG had only a mild increase in the cellularity of the alveolar septa that was primarily due to increased numbers of mononuclear cells. Overall, using small-sized particles or CpG-containing particles for vaccination can significantly reduce the perivascular cuffing as shown in the graphical insert of Fig. 7.
Fig. 7.
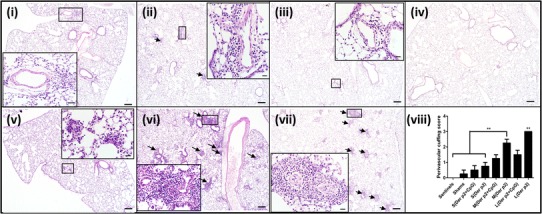
Representative H&E stained lung sections of mice vaccinated with Der p2 coated on i small-, ii medium-, and iii large-sized CpG-containing PLGA particles; v small-, vi medium-, and vii large-sized empty PLGA particles, and iv shams. Lung sections were collected on day 24. Arrows indicate key areas of pathology which were almost exclusively perivascular inflammatory cell infiltrates composed of macrophages and eosinophils and fewer neutrophils which are highlighted in the high resolution image inserts. Scale bar at the lower right corner of each image is 200 μm. Scale bars for high resolution image inserts are 20 μm in length. viii Perivascular cuffing lesion score for lung sections from all mice (n = 6). Significant differences were evaluated using one-way ANOVA followed by Tukey’s post-test (**p < 0.01). Values are expressed as mean ± SEM
DISCUSSION
Asthma caused by HDM leads to inflammatory damage of lungs that is associated with secretion of Th2-dependent anti-Der p2 IgE antibodies, eosinophilic influx to lungs, and airway hyperactivity (4). Currently, there is no permanent treatment for pathological symptoms caused by asthma and most clinicians prescribe temporary therapies for alleviating the inflammatory responses caused by repeated exposure to allergen. Absence of an optimal therapeutic treatment and a high prevalence of asthma in the USA (41) emphasize the need for stimulation of allergen-specific Th1-biased responses to suppress the activation of Th2-driven inflammation. This can be achieved by the careful design of a vaccine system which can stimulate DC, an important modulator for the polarization of Th responses to favor Th1 activation (42). Suzuki et al. have developed transgenic rice carrying murine and human T cell epitopes of Der p1 protein (43). They have shown that mice fed on transgenic rice showed decreased IgE antibody secretion and reduced allergic airway inflammation after allergen exposure when compared to mice fed on the control diet. However, it is difficult to scale up the production of this vaccine and control the standard dose of allergen during vaccination. Recent studies have shown that vaccination of mice using polymer particles encapsulating antigen can stimulate an antigen-specific effector T cell response which is characterized by an increase in cytotoxic T lymphocyte (CTL) activity, IgG2a production, and reduction in IgE secretion (44, 45). Polymer particles offer multiple advantages over soluble vaccine systems. They are stable at 4°C for more than a week (36) and protect the encapsulated antigen from degradation after systemic injection (46, 47). In addition, particle-based vaccines are proposed to mimic bacterial or viral infections which are readily phagocytosed by DCs for antigen presentation to T cells (48). PLGA particles are well-characterized delivery systems which can be used for the co-delivery of allergic antigen and adjuvant to the same DC which in turn can steer immune responses toward a Th1-type (30, 49). The stimulation of allergen-specific Th1-biased immunity can prevent pathological damage associated with allergen exposures. To test this hypothesis, we developed CpG-loaded PLGA particles coated with allergen as a prophylactic vaccine which can prevent AHR associated with pulmonary inflammation on exposure to allergen. In phase I/II clinical trials, HDM-induced allergic rhinoconjunctivitis patients were given subcutaneous vaccinations of HDM extract combined with virus like particles encapsulating CpG. The vaccine showed alleviation of allergy symptoms within 10 weeks but 41% of the patient population suffered from moderate side effects including asthma, pruritus, and hypersensitivity (50). In addition, immunotherapy using HDM extract can induce food allergies to invertebrates and sea foods (51). Thus, in this study, we used purified Der p2 allergen as the antigen for vaccines. In a recent study, we have shown that particle size in a PLGA vaccine system can affect the magnitude of stimulated effector responses (33). This study demonstrated that particle uptake and activation of DCs were increased with decreasing size of PLGA particles encapsulating ovalbumin and CpG. It was also shown that the magnitude of the IgG2a response was highest in mice vaccinated with 300 nm PLGA particles compared to larger particles thereby demonstrating the importance of particle size in generating an appropriate immune response. In the study presented here, we investigated the effect of the size of PLGA particles in generating protective immune responses against Der p2. Vaccinated mice were dosed with ten daily intranasal instillations of Der p2 followed by various diagnostic and immunological assays to determine the efficacy of each vaccination formulation. We found that mice vaccinated with Der p2 coated on small-sized particles encapsulating CpG showed no increase in IgE and IgG1 serum levels after daily Der p2 exposures (Fig. 5). In addition, levels of Th2 cytokines, IL-4, and IL-5, in BAL fluid in this exposure group were below the limit of detection (data not shown) confirming the absence of inflammatory Th2 responses. This was further validated by the discovery that the ratios of IgG2a:IgG1 antibodies were remarkably high for small-sized PLGA particles containing CpG. The immunological responses generated by CpG-loaded PLGA particles coated with Der p2 were in agreement with previously published data showing that PLGA particles of sizes smaller than 1 μm encapsulating ovalbumin and CpG resulted in increased levels of IgG2a antibodies, a higher ratio of IgG2a:IgG1 and enhanced numbers of ovalbumin-specific CD8+ T cells (33). These studies combined suggest that a successful vaccine may be designed by either encapsulating the antigen inside of the particle or by adsorbing the antigen on to the surface of the particle. The shift in Th2-type response to Th1-dominant immunity during early disease has been shown to prevent allergic inflammatory responses and lung remodeling (52, 53). In this study, analysis of BAL fluids (Fig. 4) and lung histopathology (Fig. 7) of vaccinated mice exposed to Der p2 antigen demonstrated that antigen-specific immunity generated in mice vaccinated with small PLGA particles coated with Der p2 prevented pulmonary influx of leukocytes. Similarly, no significant increase in airway resistance was observed with small PLGA particles when challenged with increasing doses of methacholine. Multiple DNA-based vaccine systems have also reported induction of allergen-specific IgG2a responses and reduced eosinophilia following vaccination (54, 55). However, these responses are restricted by the ability of the plasmid DNA constructs to reach a threshold antigen expression after vaccination. Our data showed that blank or CpG-loaded small-sized particles coated with Der p2 generate a robust immune response which can prevent strong allergic responses to Der p2 exposures. Mice vaccinated with medium- and large-sized empty PLGA particles coated with Der p2 exhibited airway remodeling and increased AHR upon Der p2 exposures when compared to sentinels. On encapsulation of CpG in these particles, AHR (Fig. 6) and eosinophilia (Fig. 4 (ii)) were significantly reduced compared to medium- and large-sized empty PLGA particles. These results demonstrate that incorporation of CpG can significantly improve efficacy of the vaccine.
CONCLUSION
This is the first study that comprehensively evaluates the effect of size of PLGA particles and presence of CpG in generating a vaccine against HDM. We have clearly demonstrated that the size of PLGA particles used for the subcutaneous vaccination of mice against Der p2-induced asthma has a significant impact on the efficacy of vaccine. With a decrease in the size of particle vaccines, airway hyperresponsiveness and eosinophilia accumulation in lungs were decreased after Der p2 exposures. This was accompanied with an increase in the secretion of Der p2-specific IgG2a antibodies. Although larger-sized blank particle vaccines failed to protect against lung damage and inflammation induced by Der p2 exposures, encapsulation of CpG in large-sized particle vaccines successfully steered the Th2-type response to Th1-dominant immunity. Thus, the combined use of smaller-sized PLGA particle vaccines with CpG can significantly alleviate the asthmatic response induced by HDM allergens.
Acknowledgments
The authors gratefully acknowledge support from the National Institute of Environmental Health Sciences-funded Environmental Health Sciences Research Center (NIH P30 ES005605). Other sources of support include the American Cancer Society (RSG-09-015-01-CDD), the National Cancer Institute (NIH 1R21CA13345-01/1R21CA128414-01A2/UI Mayo Clinic Lymphoma SPORE), and the Lyle and Sharon Bighley Professorship. We thank Sean Geary for expert reading of the manuscript.
Footnotes
Vijaya B. Joshi, Andrea Adamcakova-Dodd, and Xuefang Jing contributed equally to this work.
Contributor Information
Peter S. Thorne, Phone: (319) 335-4216, Email: peter-thorne@uiowa.edu
Aliasger K. Salem, Phone: (319) 335-8810, Email: aliasger-salem@uiowa.edu
References
- 1.Arbes SJ, Jr, Cohn RD, Yin M, Muilenberg ML, Burge HA, Friedman W, et al. House dust mite allergen in US beds: results from the First National Survey of lead and allergens in housing. J Allergy Clin Immunol. 2003;111(2):408–14. doi: 10.1067/mai.2003.16. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care. 2004;169(3):378–85. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 5.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108(2):E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129(1):48–59. doi: 10.1016/j.jaci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Incorvaia C, Di Rienzo A, Celani C, Makri E, Frati F. Treating allergic rhinitis by sublingual immunotherapy: a review. Ann Ist Super Sanita. 2012;48(2):172–6. doi: 10.4415/ANN_12_02_10. [DOI] [PubMed] [Google Scholar]
- 8.Eifan AO, Akkoc T, Yildiz A, Keles S, Ozdemir C, Bahceciler NN, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy. 2010;40(6):922–32. doi: 10.1111/j.1365-2222.2009.03448.x. [DOI] [PubMed] [Google Scholar]
- 9.Eifan AO, Calderon MA, Durham SR. Allergen immunotherapy for house dust mite: clinical efficacy and immunological mechanisms in allergic rhinitis and asthma. Expert Opin Biol Ther. 2013;13(11):1543–56. doi: 10.1517/14712598.2013.844226. [DOI] [PubMed] [Google Scholar]
- 10.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 11.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 12.Wohlleben G, Erb KJ. Atopic disorders: a vaccine around the corner? Trends Immunol. 2001;22(11):618–26. doi: 10.1016/S1471-4906(01)02055-5. [DOI] [PubMed] [Google Scholar]
- 13.Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–96 e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 14.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Arlian LG, Morgan MS, Neal JS. Dust mite allergens: ecology and distribution. Curr Allergy Asthma Rep. 2002;2(5):401–11. doi: 10.1007/s11882-002-0074-2. [DOI] [PubMed] [Google Scholar]
- 16.Kidon MI, Chiang WC, Liew WK, Ong TC, Tiong YS, Wong KN, et al. Mite component-specific IgE repertoire and phenotypes of allergic disease in childhood: the tropical perspective. Pediatr Allergy Immunol. 2011;22(2):202–10. doi: 10.1111/j.1399-3038.2010.01094.x. [DOI] [PubMed] [Google Scholar]
- 17.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trombone APF, Tobias KRC, Ferriani VPL, Schuurman J, Aalberse RC, Smith AM, et al. Use of a chimeric ELISA to investigate immunoglobulin E antibody responses to Der p 1 and Der p 2 in mite-allergic patients with asthma, wheezing and/or rhinitis. Clin Exp Allergy. 2002;32(9):1323–8. doi: 10.1046/j.1365-2745.2002.01455.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan LK, Huang CH, Kuo IC, Liew LM, Chua KY. Intramuscular immunization with DNA construct containing Der p 2 and signal peptide sequences primed strong IgE production. Vaccine. 2006;24(29–30):5762–71. doi: 10.1016/j.vaccine.2006.04.064. [DOI] [PubMed] [Google Scholar]
- 20.Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW, et al. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol. 2001;166(1):207–17. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 21.Kline JN, Krieg AM. Toll-Like receptor 9 activation with CpG oligodeoxynucleotides for asthma therapy. Drug News Perspect. 2008;21(8):434–9. doi: 10.1358/dnp.2008.21.8.1272133. [DOI] [PubMed] [Google Scholar]
- 22.Fonseca DE, Kline JN. Use of CpG oligonucleotides in treatment of asthma and allergic disease. Adv Drug Deliv Rev. 2009;61(3):256–62. doi: 10.1016/j.addr.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Pulsawat P, Pitakpolrat P, Prompetchara E, Kaewamatawong T, Techakriengkrai N, Sirivichayakul S, et al. Optimization of a Der p 2-based prophylactic DNA vaccine against house dust mite allergy. Immunol Lett. 2013;151(1–2):23–30. doi: 10.1016/j.imlet.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Heeg K, Zimmermann S. CpG DNA as a Th1 trigger. Int Arch Allergy Immunol. 2000;121(2):87–97. doi: 10.1159/000024303. [DOI] [PubMed] [Google Scholar]
- 25.Mo JH, Park SW, Rhee CS, Takabayashi K, Lee SS, Quan SH, et al. Suppression of allergic response by CpG motif oligodeoxynucleotide-house-dust mite conjugate in animal model of allergic rhinitis. Am J Rhinol. 2006;20(2):212–8. [PubMed] [Google Scholar]
- 26.Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol. 2004;113(6):1144–51. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Kaburaki Y, Fujimura T, Kurata K, Masuda K, Toda M, Yasueda H, et al. Induction of Th1 immune responses to Japanese cedar pollen allergen (Cry j 1) in mice immunized with Cry j 1 conjugated with CpG oligodeoxynucleotide. Comp Immunol Microbiol Infect Dis. 2011;34(2):157–61. doi: 10.1016/j.cimid.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of T-helper type 2 cell and airway eosinophilia by transmucosal coadministration of antigen and oligodeoxynucleotides containing CpG motifs. Am J Respir Cell Mol. 2000;22(2):176–82. doi: 10.1165/ajrcmb.22.2.3772. [DOI] [PubMed] [Google Scholar]
- 29.Gomez JMM, Fischer S, Csaba N, Kundig TM, Merkle HP, Gander B, et al. A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm Res. 2007;24(10):1927–35. doi: 10.1007/s11095-007-9318-0. [DOI] [PubMed] [Google Scholar]
- 30.Scholl I, Kopp T, Bohle B, Jensen-Jarolim E. Biodegradable PLGA particles for improved systemic and mucosal treatment of Type I allergy. Immunol Allergy Clin N Am. 2006;26(2):349–64. doi: 10.1016/j.iac.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Martinez Gomez JM, Fischer S, Csaba N, Kundig TM, Merkle HP, Gander B, et al. A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm Res. 2007;24(10):1927–35. doi: 10.1007/s11095-007-9318-0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. J Immunother. 2007;30(5):469–78. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]
- 33.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS J. 2013;15(1):85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorne PS, McCray PB, Howe TS, O'Neill MA. Early-onset inflammatory responses in vivo to adenoviral vectors in the presence or absence of lipopolysaccharide-induced inflammation. Am J Respir Cell Mol. 1999;20(6):1155–64. doi: 10.1165/ajrcmb.20.6.3632. [DOI] [PubMed] [Google Scholar]
- 35.George CL, White ML, Kulhankova K, Mahajan A, Thorne PS, Snyder JM, et al. Early exposure to a nonhygienic environment alters pulmonary immunity and allergic responses. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L512–22. doi: 10.1152/ajplung.00278.2005. [DOI] [PubMed] [Google Scholar]
- 36.De S, Robinson DH. Particle size and temperature effect on the physical stability of PLGA nanospheres and microspheres containing Bodipy. AAPS PharmSciTech. 2004;5(4):e53. doi: 10.1208/pt050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32(9):402–11. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Yang Q, Wang P, Luo L, Chen Z, Liao B, et al. Derp2-mutant gene vaccine inhibits airway inflammation and up-regulates Toll-like receptor 9 in an allergic asthmatic mouse model. Asian Pac J Allergy Immunol. 2010;28(4):287–93. [PubMed] [Google Scholar]
- 39.Daan de Boer J, Roelofs JJ, de Vos AF, de Beer R, Schouten M, Hommes TJ, et al. Lipopolysaccharide inhibits Th2 lung inflammation induced by house dust mite allergens in mice. Am J Respir Cell Mol. 2013;48(3):382–9. doi: 10.1165/rcmb.2012-0331OC. [DOI] [PubMed] [Google Scholar]
- 40.Sumino K, Sugar EA, Irvin CG, Kaminsky DA, Shade D, Wei CY, et al. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol. 2012;130(1):69–75 e6. doi: 10.1016/j.jaci.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 42.Bharadwaj AS, Bewtra AK, Agrawal DK. Dendritic cells in allergic airway inflammation. Can J Physiol Pharmacol. 2007;85(7):686–99. doi: 10.1139/Y07-062. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, et al. Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol J. 2011;9(9):982–90. doi: 10.1111/j.1467-7652.2011.00613.x. [DOI] [PubMed] [Google Scholar]
- 44.Kündig TM, Senti G, Schnetzler G, Wolf C, Prinz Vavricka BM, Fulurija A, et al. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J Allergy Clin Immunol. 2006;117(6):1470–6. doi: 10.1016/j.jaci.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Standley SM, Mende I, Goh SL, Kwon YJ, Beaudette TT, Engleman EG, et al. Incorporation of CpG oligonucleotide ligand into protein-loaded particle vaccines promotes antigen-specific CD8 T-cell immunity. Bioconjug Chem. 2007;18(1):77–83. doi: 10.1021/bc060165i. [DOI] [PubMed] [Google Scholar]
- 46.O'Hagan DT, Rappuoli R. Novel approaches to pediatric vaccine delivery. Adv Drug Deliv Rev. 2006;58(1):29–51. doi: 10.1016/j.addr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Shen J, Burgess DJ. Drugs for long acting injections and implants. In: Wright JC, Burgess DJ, editors. Long acting injections and implants. New York: Springer; 2012. p. 73–92.
- 48.Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63(10–11):943–55. doi: 10.1016/j.addr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Scholl I, Weissenbock A, Forster-Waldl E, Untersmayr E, Walter F, Willheim M, et al. Allergen-loaded biodegradable poly (D, L-lactic-co-glycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy. 2004;34(2):315–21. doi: 10.1111/j.1365-2222.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 50.Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Müller P, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009;39(4):562–70. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 51.van Ree R, Antonicelli L, Akkerdaas JH, Garritani MS, Aalberse RC, Bonifazi F. Possible induction of food allergy during mite immunotherapy. Allergy. 1996;51(2):108–13. doi: 10.1111/j.1398-9995.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 52.Bosnjak B, Stelzmueller B, Erb KJ, Epstein MM. Treatment of allergic asthma: modulation of Th2 cells and their responses. Respir Res. 2011;12:114. doi: 10.1186/1465-9921-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang MLK, Powell CVE. Childhood asthma as an allergic disease: rationale for the development of future treatment. Eur J Pediatr. 2001;160(12):696–704. doi: 10.1007/s004310100809. [DOI] [PubMed] [Google Scholar]
- 54.Pulsawat P, Pitakpolrat P, Prompetchara E, Kaewamatawong T, Techakriengkrai N, Sirivichayakul S, et al. Optimization of a Der p 2-based prophylactic DNA vaccine against house dust mite allergy. Immunol Lett. 2013;151(1–2):23–30. doi: 10.1016/j.imlet.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Huang G, Hu B, Song Y, Shi Y. Induction of immune tolerance in asthmatic mice by vaccination with DNA encoding an allergen-cytotoxic T lymphocyte-associated antigen 4 combination. Clin Vaccine Immunol: CVI. 2011;18(5):807–14. doi: 10.1128/CVI.00434-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


