Abstract
The highly conserved part of the nucleotide-binding domain of the hsp70 gene family was amplified from the soil diplopod Tachypodoiulus niger (Julidae, Diplopoda). Genomic DNA yielded 701, 549 and 540 bp sequences, whereas cDNA from heat shocked animals produced only one distinct fragment of 543 bp. The sequences could be classified as a 70 kDa heat shock protein (hsp70), the corresponding 70 kDa heat shock cognate (hsc70) and a glucose-related hsp70 homologue (grp78). Comparisons of genomic and cDNA sequences of hsc70 identified two introns within the consensus sequence. Generally, stress-70 expression levels were low, which hampered successful RT-PCR and subsequent subcloning. Following experimental heat shock, however, the spliced hsc70 was amplified predominantly, instead of its inducible homologue hsp70. This finding suggests that microevolution in this soil-dwelling arthropod is directed towards low constitutive stress-70 levels and that the capacity for stress-70 induction presumably is limited. hsc70, albeit having introns, apparently is inducible and contributes to the stress-70 response.
Keywords: Stress response, Soil invertebrate, Arthropods, Heat shock proteins, Heat shock cognates, Glucose-related proteins, Biomarker
Introduction
The heat shock response is a general cellular mechanism that helps to protect organisms from the harmful effects of exposure to various kinds of environmental stress (Lindquist 1986). It is essentially based on an increased expression of inducible heat shock proteins (hsps), also designated as stress proteins. By binding to uncoiled and thus denatured polypeptide chains, they prevent them from aggregation and assist in the proper refolding of misfolded proteins (Gething and Sambrook 1992; Mayer and Bukau 2005). In addition to the stress inducible hsp70, the constitutively expressed heat shock cognates (hscs) also play an important role under unstressed conditions (Bukau and Horwich 1998; Richter et al. 2010).
The significance of hsps within the cellular stress response and their ability to integrate overall adverse effects on protein integrity also make them informative biomarkers for tracking environmental pollution (Sanders 1990; De Pomerai 1996; Kammenga et al. 2000; Nadeau et al. 2001). Among the numerous classes of stress proteins, the stress-70 (hsp70) family is in this respect far best investigated. The potential of stress-70 proteins as biomarkers in physiological ecology and ecotoxicology arises from (1) their induction by the presence of damaged and malfolded proteins (Edington et al. 1989), which may occur as a result of proteotoxicity, (2) their induced expression as a response to a wide variety of stressors (Tomanek and Sanford 2003; Clark and Peck 2009) including heavy metals, UV light, xenobiotics and even multiple interacting toxic substances (Sanders 1993), as well as (3) their occurrence in literally all eukaryotic organisms along with their high degree of sequence conservation (Morris et al. 2013). Both the abundance of stress-70 mRNA and the accumulation of stress-70 proteins can be employed for measuring the organisms’ response to environmental stress at different levels of sensitivity to either concentration and/or time of exposure to pollutants (Köhler et al. 1998). However, depending on the particular traits, tissue function and developmental stage of the species in question, as well as, of course, the type of stressor and the tolerance towards it variations in the basal stress-70 level and the induction of hsp70 are to be expected (e.g. Eckwert and Köhler 1997; Arts et al. 2004). Particularly, the interdependency between adaptations to stressful environmental conditions and the constitutive hsp70 expression and the potential for induction of hsp70 may be complex and variable according to the ecophysiological context. The species under study may also differ in stress tolerance and/or adaptation to rather stable environments (Kültz 2003). Indeed, for each species and ecological context, the benefits of protection by the stress response need to be balanced against the associated trade-offs, notably in terms of metabolic costs (Sørensen et al. 2003). The costs and benefits of the complex heat shock response may best be addressed by simultaneous analyses of gene expression and the corresponding protein levels, as well as the contribution of different isoforms to the overall stress-70 response (Morris et al. 2013).
Despite their great potential as biomarker of environmental pollution little is known about stress-70 proteins in soil invertebrates (Köhler et al. 1992; Kammenga et al. 2000). Diplopods, for example, are hardly studied (Zanger et al. 1996) although they contribute significantly to the detritivorous soil fauna, which reduces litter and thereby, helps to further decompose organic matter by microorganisms. Any perturbation of the soil biota by pollution may affect decomposition processes and nutrient cycles (Sylvain and Wall 2011). Albeit an ever growing number of sequences in GenBank and research work using hsp70 gene expression as a molecular marker in nematodes (Guisbert et al. 2013), lumbricides (Chen et al. 2011), slugs and snails (Köhler et al. 1998; Reuner et al. 2008), and collembolans (Bahrndorff et al. 2009; Waagner et al. 2012) information about stress genes of the soil fauna is still relatively scarce.
The premise to any quantitative stress gene expression study is the availability of at least a partial hsp70 sequence. Accordingly, the objective of this study was to characterize the nucleotide sequence of a highly conserved region of the 70 kDa heat shock protein in the diplopod species Tachypodoiulus niger (Leach 1815) (Julidae).
The approach employs degenerate primers targeting a conserved region of the hsp70 family genes (Bahrndorff et al. 2009) that were developed previously for the netted slug Deroceras reticulatum (Köhler et al. 1998).
Results and discussion
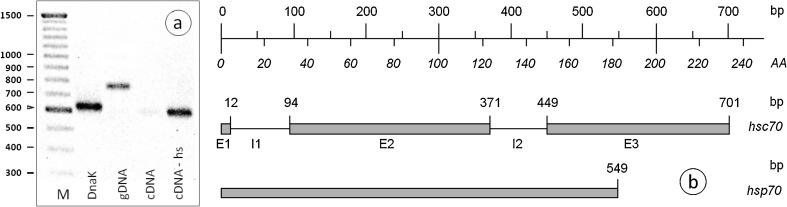
Previously designed oligonucleotides for polymerase chain reaction (PCR) amplification of hsp70 in the netted slug D. reticulatum (Köhler et al. 1998) were applied for the amplification of hsp70 targets of the millipede T. niger either from genomic DNA (gDNA) or from reverse transcribed mRNA. Depending on the template DNA, two major products of different length were obtained. The longer product amplified exclusively from gDNA consisted of 701 bp (excluding primers), the shorter PCR product of 543 (excluding primers) was obtained from cDNA of heat-shocked individuals. Minor PCR products corresponding to the shorter sequences could also be obtained as faint or very faint bands after electrophoresis when targeting cDNA of untreated individuals or gDNA, respectively (Table 1, Fig. 1). DNA sequencing revealed (1) two genomic sequences of 701 and 549 bp that subsequent BLAST searches suggested as being the hsc70 gene for the longer sequence and as hsp70 for the shorter one; (2) a short cDNA sequence with high similarity to the 70 kDa heat shock cognate and (3) another short cDNA sequence identified as the gene coding for glucose regulated protein grp78 (Table 1). The respective DNA sequences can be retrieved from EMBL/GenBank (accession numbers are provided in Table 1). On the one hand, these results confirm the universal applicability of these degenerate oligonucleotides (see also Schill et al. 2004; Reuner et al. 2008). Particularly, the targeted, highly conserved consensus region codes for the N-terminal nucleotide-binding domain of the hsp70 proteins (Conserved Domain Database, http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml; (Marcheler-Bauer et al. 2011), which regulates chaperone activity by ATP hydrolysis (Wisniewska et al. 2010). This domain is considered to be less variable than the also well-conserved peptide-binding domain (Reddy et al. 2010). On the other hand, the degenerate primers amplify various isoforms, and thus, primers specific to the different isoforms need to be designed on the basis of the sequences obtained with degenerate primers (e.g. as in Bahrndorff et al. 2009).
Table 1.
PCR products of the nucleotide binding domain of the partial hsc70 and hsp70 genes in the diplopod Tachypodoiulus niger
| Accession no. | Sequence similarity | Degenerate primers | Template | PCR product (bp) |
|---|---|---|---|---|
| AM502906 | hsc70 | Fw: 5′ATYAAYGARCCIACKGCIGCIGCWATTGCITATGG3′
Rev: 5′GAYGARGCWGTTGCITAYGGIGCWGCIGTICARGC3′ |
mRNA | 543 |
| AM502907 | gDNA | 701 | ||
| AM502908 | hsp70 | gDNA | 549 | |
| AM502909 | grp78 | gDNA | 540 |
Animals were collected from a beech forest stand, kept at 16 °C at a 12 h/12 h light/dark photoperiod in transparent plastic boxes and fed with partially decayed leaves of the Common Hazel (Corylus avellana). Heat shock was carried out in a heating cabinet for 1 h at 30 °C followed by 15 min at 40 °C. Total genomic DNA (gDNA) was isolated after removal of the alimentary tract using a modified method of Zhang and Hewitt (Zhang and Hewitt 1998). The total RNA was isolated using an invertebrate RNA isolation kit (Peqlab, Erlangen, Germany). A reverse transcription was carried out with SuperScript® II RT and oligo(dT)12–18-primer (Invitrogen Life Technologies, Darmstadt, Germany). Degenerate primers and PCR programs (3 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 56 °C and 1.5 min at 72 °C followed by a final 10 min at 72 °C) were adopted from Köhler et al. (Köhler et al. 1998) and slightly modified when necessary. PCR products were purified by agarose gel electrophoresis and subsequently cloned into the pCR4-TOPO-vector (Invitrogen, Groningen, The Netherlands). The plasmid DNA was isolated from positive clones with the E.Z.N.A. plasmid miniprep kit II (Peqlab). A custom sequencing was carried out in both directions and confirmed twice by Seqlab, Sequencing Laboratories (Göttingen, Germany). The BLAST searches (Altschul et al. 1990) were used for sequence identification. Alignments of the hsp70 sequences were carried out using ALIGN program (Smith and Waterman 1981)
Fig. 1.
a PCR products of Tachypodoiulus niger revealing a fragment of about 760 bp derived from genomic DNA (gDNA) and a prominent product of about 610 bp for transcripts derived from previously heat shocked animals (cDNA-hs). A faint band was also obtained from transcripts of animals that had not been subjected to heat shock (cDNA). M = 100 bp DNA-ladder. The prokaryotic hsp70 homologue DnaK + (plasmid pUHE21-2Δ12fd) was used as a positive control (DnaK); b predicted intron/exon structure of the nucleotide-binding domain of the partial hsc70 and hsp70 genes in the diplopod T. niger
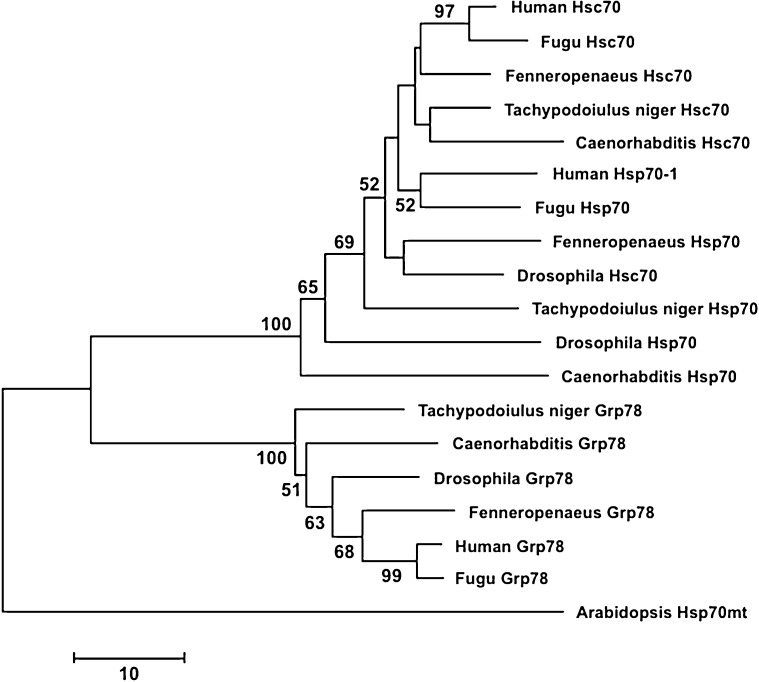
The deduced amino acid sequences of the T. niger hsp70s (Fig. 2) were aligned to hsp70 sequences from 18 other species retrieved from GenBank (Fig. 3). The network obtained with a neighbour joining analysis illustrates the basic split into two major groups of sequences (Fig. 3). The cytoplasmic hsp70 and hsc70 sequences are found in one group, while the grp78 sequences of the endoplasmic reticulum form a separate clade. The obtained network reflect that there is even on the level of just a short 188 AA alignment sufficient distinction for discriminating between cognate and inducible forms of hsp70 and other members of the family, such as grp78.
Fig. 2.
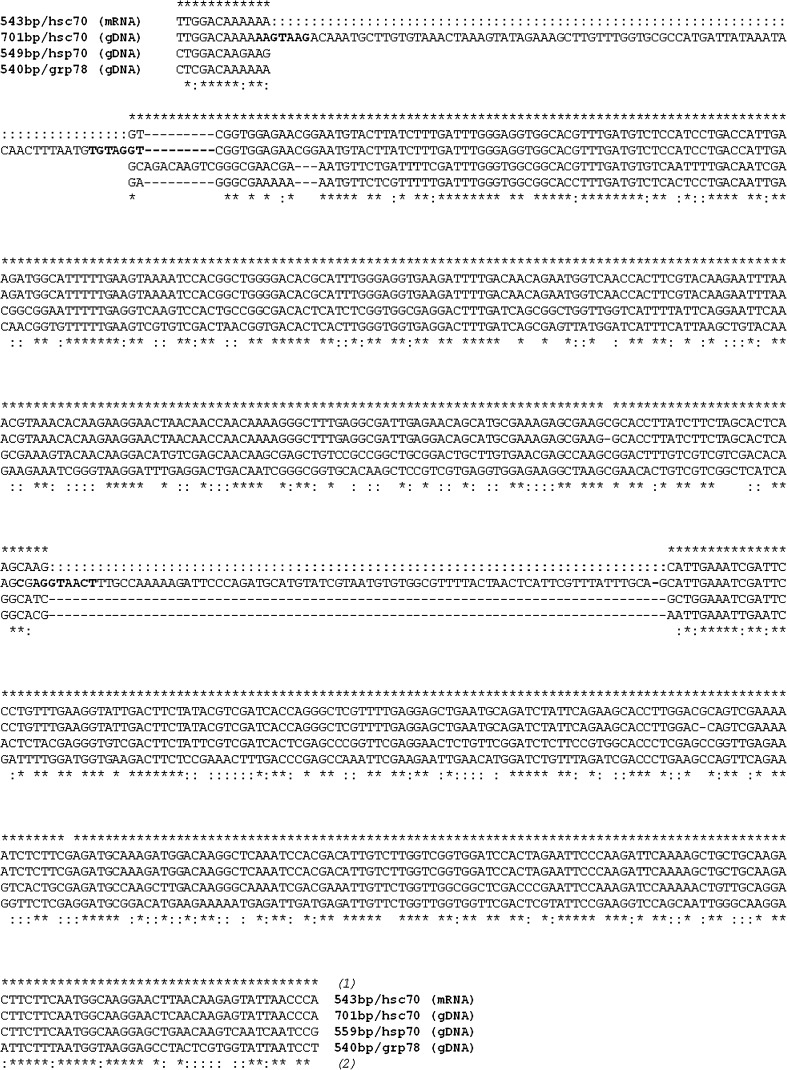
Alignment of the three partial nucleotide sequences from the stress-70 gene nucleotide-binding region for hsc70 (AM502906, AM502907), hsp70 (AM502908) and grp78 (AM502909) of Tachypodoiulus niger. Asterisks indicate (1) homology between hsc70 mRNA and hsc70 gDNA and (2) homology over all sequences (* identity over all four and : over three sequences); bold letters indicate splice sequences after Mount (1982)
Fig. 3.
Neighbour-joining network illustrating the clustering of hsp70 family sequences. The predicted protein sequences of 18 hsp70s homologues that mostly represent model organisms (Caenorhabditis elegans: hsp70 [NP_492485], hsc70 [NP_503068], grp78 [NP_495536]; Drosophila melanogaster: hsp70 [NP_731651], hsc70 [NP_524356], grp78 [NP_727563]; Fenneropenaeus chinensis: hsp70 [ACN38704], hsc70 [AAW71958], grp78 [ABM92447]; Takifugu rubripes: hsp70 [CAA69894], hsc70 [XP_003966054], grp78 [XP_003965205]; and Homo sapiens: hsp70 [AAD21816], hsc70 [NP_006588], grp78 [NP_005338]) were compiled in a 188 AA long alignment with the predicted protein sequences from Tachypodoiulus niger (hsp70 [CAM7362], hsc70 [CAM73262], grp78 [CAM73265]). Analyses were carried out using MEGA 5.2.1 (Tamura et al. 2011). The network is based on the number of sequence differences with pairwise deletion of gaps, and bootstrap support was estimated from 1 000 replicates. The bootstrap support was estimated from 100 replicates; only values ≥50 are indicated. The mitochondrial hsp70 from Arabidopsis thaliana [NP_195504] served as an out-group. (Accession numbers in brackets.)
The hsc70 gene represented the major PCR product when targeting gDNA with high stringency. When lowering the annealing temperature, the shorter 549 bp fragment corresponding to hsp70 could also be obtained, but only as a faint band. A PCR product of similar size was amplified from cDNA, but this required a specific induction of the hsp70 gene family by experimental heat shock. In fact, reverse transcription of hsp70 mRNA may be difficult to obtain if the number of transcripts to produce sufficient amount of cDNA is low. Accordingly, RT-PCR of the constitutive expression of hsp70/hsc70 appeared to be rather low. Only after heat shock a considerable gene expression was effectuated that resulted in a PCR product of similarly strong intensity as the one of the positive control DnaK. Intriguingly, from this cDNA product obtained from heat-shocked animals, the inducible isoform of hsp70 could not be amplified by PCR, but an intron-free cognate gene transcript was always obtained instead. Just as the longer 701 bp sequence, this 543 bp cDNA product almost perfectly matched cognates of the hsp70 family. Moreover, both fragments showed 99.8 % sequence identity when removing two putative indels of 81 and 77 bp. In fact, multiple sequence alignment provided evidence for at least one intron in the hsc70 gene of T. niger. However, consensus sequences for the 5′ and 3′ splice sites according to Mount (Mount 1982) were found at positions 10–17 and 89–95 defining a first intron, and at positions 369–377 and possibly 444–450 defining a second intron. The canonical GT-AG 5′ and 3′ splice junctions render the presence of two introns plausible as does the alignment with sequences from other soil invertebrates obtained using the same methodology. The two indels together account for most of the length differences between the two PCR products, which therefore most likely represent the same hsc70 gene.
Historically stress-inducible hsp70s were distinguished from their constitutively expressed hsc70 homologues. In contrast to the stress-induced hsps, cognates are known to contain introns (e.g. Snutch et al. 1988; La Rosa et al. 1990). Mammalian hsc70, for example, contains eight introns (Hunt et al. 1999; Daugaard et al. 2007). However, the number and distribution of introns reported in the literature is highly variable as is the inducibility of the respective genes. For a number of plant model-organisms, the nucleotide binding domain is interrupted by a single intron only, the position of which appears to be conserved (Reddy et al. 2010). In various animal taxa, such as, for example, decapod crustaceans, insects and fish, introns of different size were found in the 5′ UTR, in the nucleotide binding and in the peptide binding domain (e.g. Ali et al. 2003; Karouna-Renier et al. 2003; Jiao et al. 2004; Leignel et al. 2007; Ming et al. 2010). Also intronless hsc70 were reported (Liu et al. 2004). Hence, the presence of two introns in the partial sequence belonging to the nucleotide-binding domain is conceivable, and more intervening sequences are likely to be present in the entire hsc70 gene.
Heat shock typically induces intronless hsp70. In T. niger, however, severe temporary heat shock appeared to induce considerable expression of the stress-70 cognate since hsc70 was the only product obtained with RT-PCR. We thus conclude that the intron-containing cognate form represents the stoichiometrically most important stress-70 gene in T. niger under these experimental heat shock conditions, although it needs to be acknowledged that degenerate primers are not apt to distinguish between the different isoforms. This appears intriguing because it would contradict the concept of intron-containing chaperones not being induced by stress conditions such as elevated temperature, heavy metal exposure or the impact of chemicals (Yost and Lindquist 1988). This hypothesis is mainly based on the notion that splicing of pre-mRNA itself is sensitive to stress (e.g. Bond 1988; Winter et al. 1988; Yost and Lindquist 1988). Accordingly, processing of the mature hsps may be impaired under stress conditions, thus leading to non-functional hsps, which in turn would hamper an effective stress response. In contrast, intron-free genes can be rapidly expressed, which is a prerequisite for an efficient response to exogenous stress (Feder and Krebs 1998). Despite such theoretical considerations, cognate stress genes that can be induced by heat shock were reported earlier (Rochester et al. 1986; Muller et al. 1992; Sconzo et al. 1992; Marcucci et al. 1995). A critical issue may be seen in the definition of induction, which varies in the literature and may range from a three to a hundred times increase in expression (Sorger 1991). Furthermore, variable numbers of hsp70 genes have been reported, ranging from a handful of hsp70s to a couple of dozens in plants or invertebrates. Presumably, gene duplications allow for generally higher expression levels and may enable a fine-tuned regulation of specific isoforms that may respond to a particular stress by specific response elements in the promoter region (e.g. Zhang et al., 2012). Thus, the number of hsp70 copies and the variability in isoforms may account for different levels of inducibility depending on the particular species in question and in response to specific environmental stressors. Eventually, this could reflect different evolutionary strategies and adaptation to the specific ecological context. Species occurring in variable environments may have evolved a higher potential for induction (Feder and Krebs 1998) than those colonizing more stable habitats (reviewed in Clark and Peck 2009; Coleman et al. 1995). The latter may also be true for the diplopod T. niger, a member of the soil fauna that prefers a woodland habitat, characterised by only minor temperature variation in the upper soil horizons. It is well known that these animals avoid higher temperatures by moving into deeper soil horizons or into the inner parts of decayed wooden trunks, where they meet their temperature optimum. Accordingly, selection for specialised, highly inducible isoforms might have been weak.
Conclusions
The potential of gene expression as a highly sensitive biomarker has been widely discussed and successfully applied for stress-70 genes (e.g. Köhler et al. 1998; Schill et al. 2004; Reuner et al. 2008; Bahrndorff et al. 2009). Degenerate primers targeting the highly conserved nucleotide-binding domain of the hsp70 gene proved useful in amplifying stress protein genes in various animal taxa and subsequent determination of hsp70 nucleotide sequences. This is particularly useful for non-model organisms for which little or no genetic information is available. The obtained consensus region may then serve as a basis for further analysis of the hsp70 family sequences in the respective species by RACE-PCR. Subsequently, primer pairs for highly specific stress gene probes can be constructed and employed in real-time RT-qPCR. By examining the inducibility of different homologues, the stress response may be examined in a differentiated manner thereby better reflecting its complexity. Eventually, the ecophysiological context of a particular species needs to be taken into consideration with regard to its ability for the stress-70 response, as, apparently, the characteristics of induction are highly dependent on the species and the stress conditions it encounters in its environment.
Acknowledgments
We gratefully acknowledge the support by D. Ammermann, N.K. Jacob, M. Knigge, T. Monsinjon and R. Paxton. The authors are also grateful to M. Bulant for assistance with the phylogenetic analysis. This study received funding from the German Research Council (Grant No. Ko 1978/2-1/2).
References
- Ali K, Dorgai L, Abraham M, Hermesz E. Tissue- and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun. 2003;307:503–509. doi: 10.1016/S0006-291X(03)01206-3. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arts M-J, Schill RO, Knigge T, Eckwert H, Kammenga JE, Köhler H-R. Stress proteins (hsp70, hsp60) induced in isopods and nematodes by field exposure to metals in a gradient near Avonmouth, UK. Ecotoxicology. 2004;13:739–755. doi: 10.1007/s10646-003-4473-5. [DOI] [PubMed] [Google Scholar]
- Bahrndorff S, Mariën J, Loeschke V, Ellers J. Dynamics of heat-induced thermal stress resistance and Hsp70 expression in the springtail, Orchesella cincta. Funct Ecol. 2009;23:233–239. doi: 10.1111/j.1365-2435.2009.01541.x. [DOI] [Google Scholar]
- Bond U. Heat shock but not other stress inducers leads to the disruption of a sub-set of snRNPs and inhibition of in vitro splicing in HeLa cells. EMBO J. 1988;7:3509–3518. doi: 10.1002/j.1460-2075.1988.tb03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/S0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Chen C, Xue S, Zhou Q, Xie X. Multilevel ecotoxicity assessment of polycyclic musk in the earthworm Eisenia fetida using traditional and molecular endpoints. Ecotoxicology. 2011;20:1949–1958. doi: 10.1007/s10646-011-0735-9. [DOI] [PubMed] [Google Scholar]
- Clark MS, Peck LS. Triggers of the HSP70 stress response: environmental responses and laboratory manipulation in an Antarctic marine invertebrate (Nacella concinna) Cell Stress Chaperones. 2009;14:649–660. doi: 10.1007/s12192-009-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JS, Heckathorn SA, Hallberg RL. Heat-shock proteins and thermotolerance: linking molecular and ecological perspectives. Trends Ecol Evol. 1995;10:305–306. doi: 10.1016/S0169-5347(00)89112-0. [DOI] [PubMed] [Google Scholar]
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- De Pomerai DI (1996) Heat schock proteins as biomarkers of pollution. Human and Exp. Toxicol 15:279–285 [DOI] [PubMed]
- Eckwert H, Köhler H-R. The indicative value of the hsp70 stress response as a marker for metal effects in Oniscus asellus (Isopoda) field populations: variability between populations from metal polluted and uncontaminated sites. Appl Soil Ecol. 1997;6:275–282. doi: 10.1016/S0929-1393(97)00020-6. [DOI] [Google Scholar]
- Edington BV, Whelan SA, Highttower LE. Inhibition of heat shock (stress) protein induction by deuterium oxide and glycerol: Additional support for the abnormal protein hypothesis of induction. J Cell Physiol. 1989;139:219–228. doi: 10.1002/jcp.1041390202. [DOI] [PubMed] [Google Scholar]
- Feder ME, Krebs RA. Natural and genetic engeneering of the heat-shock protein hsp70 in Drosophila melanogaster: consequences for thermotolerance. Am Zool. 1998;38:503–517. [Google Scholar]
- Gething M-J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Guisbert E, Czyz DM, Richter K, McMullen PD, Morimoto RI. Identification of a tissue-selective heat shock response regulatory network. PLoS Genet. 2013;9:18. doi: 10.1371/journal.pgen.1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CR, Parsian AJ, Goswami PC, Kozak CA. Characterization and expression of the mouse Hsc70 gene. Biochim Biophys Acta. 1999;19:315–325. doi: 10.1016/S0167-4781(98)00285-1. [DOI] [PubMed] [Google Scholar]
- Jiao C, Wang Z, Li F, Zhang C, Xiang J. Cloning, sequencing and expression analysis of cDNA encoding a constitutive heat shock protein 70 (HSC70) in Fenneropenaeus chinensis. Chin Sci Bull. 2004;49:2385–2393. [Google Scholar]
- Kammenga JE, Dallinger R, Donker MH, Kohler H-R, Simonsen V, Triebskorn R, Weeks JM. Biomarkers in terrestrial invertebrates for ecotoxicological soil risk assessment. Rev Environ Contam Toxicol. 2000;164:93–147. [PubMed] [Google Scholar]
- Karouna-Renier NK, Yang WJ, Rao KR. Cloning and characterization of a 70 kDa heat shock cognate gene (HSC70) from two species of Chironomus. Insect Mol Biol. 2003;12:19–26. doi: 10.1046/j.1365-2583.2003.00383.x. [DOI] [PubMed] [Google Scholar]
- Köhler H-R, Triebskorn R, Stocker W, Kloetzel PM, Alberti G. The 70 kD heat shock protein (hsp 70) in soil invertebrates: a possible tool for monitoring environmental toxicants. Arch Environ Contam Toxicol. 1992;22:334–338. doi: 10.1007/BF00212095. [DOI] [PubMed] [Google Scholar]
- Köhler H-R, Belitz B, Eckwert H, Adam R, Rahman B, Trontelj P. Validation of hsp70 stress gene expression as a marker of metal effects in Deroceras reticulatum (Pulmonata): Correlation with demographic parameters. Environ Toxicol Chem. 1998;17:2246–2253. doi: 10.1897/1551-5028(1998)017<2246:VOHSGE>2.3.CO;2. [DOI] [Google Scholar]
- Kültz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitious function. J Exp Biol. 2003;206:3119–3124. doi: 10.1242/jeb.00549. [DOI] [PubMed] [Google Scholar]
- La Rosa M, Sconzo G, Giudice G, Roccheri MC, Di Carlo M. Sequence of a sea urchin hsp70 gene and its 5′ flanking region. Gene. 1990;96:295–300. doi: 10.1016/0378-1119(90)90267-U. [DOI] [PubMed] [Google Scholar]
- Leignel V, Cibois M, Moreau B, Chenais B. Identification of new subgroup of HSP70 in Bythograeidae (hydrothermal crabs) and Xanthidae. Gene. 2007;396:84–92. doi: 10.1016/j.gene.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang WJ, Zhu XJ, Karouna-Renier NK, Rao RK. Molecular cloning and expression of two HSP70 genes in the prawn, Macrobrachium rosenbergii. Cell Stress Chaperones. 2004;9:313–323. doi: 10.1379/CSC-40R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheler-Bauer A, Shennan L, Anderson JB, Chitasz F, Derbyshire MK, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225–D229. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci R, Stefani P, Gomes SL. A unique intron-containing hsp70 gene induced by heat shock and during sporulation in the aquatic fungus Blastocladiella emersonii. Gene. 1995;152:19–26. doi: 10.1016/0378-1119(95)00645-M. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming J, Xie J, Xu P, Liu W, Ge X, Liu B, He Y, Cheng Y, Zhou Q, Pan L. Molecular cloning and expression of two HSP70 genes in the Wuchang bream (Megalobrama amblycephala Yih) Fish Shellfish Immunol. 2010;28:407–418. doi: 10.1016/j.fsi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Morris JP, Thatje S, Hauton C. The use of stress-70 proteins in physiology: a re-appraisal. Mol Ecol. 2013;22:1494–1502. doi: 10.1111/mec.12216. [DOI] [PubMed] [Google Scholar]
- Mount SM. A catalogue of splice junction sequences. Nucleic Acids Res. 1982;10:459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FW, Igloi GL, Beck CF. Structure of a gene encoding heat-shock protein HSP70 from the unicellular alga Chlamydomonas reinhardtii. Gene. 1992;111:165–173. doi: 10.1016/0378-1119(92)90684-H. [DOI] [PubMed] [Google Scholar]
- Nadeau D, Corneau S, Plante I, Morrow G, Tanguay RM. Evaluation for Hsp70 as a biomarker of effect of pollutants on the earthworm Lumbricus terrestris. Cell Stress Chaperones. 2001;6:153–163. doi: 10.1379/1466-1268(2001)006<0153:EFHAAB>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PS, Mallikarjuna G, Kaul T, Chakradhar T, Mishra RN, Sopory SK, Reddy MK. Molecular cloning and characterization of gene encoding for cytoplasmic Hsc70 from Pennisetum glaucum may play a protective role against abiotic stresses. Mol Genet Genomics. 2010;283:243–254. doi: 10.1007/s00438-010-0518-7. [DOI] [PubMed] [Google Scholar]
- Reuner A, Brümmer F, Schill RO. Heat shock proteins (Hsp70) and water content in the estivating Mediterranean Grunt Snail (Cantareus apertus) Comp Biochem Physiol B. 2008;151:28–31. doi: 10.1016/j.cbpb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rochester DE, Winer JA, Shah DM. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986;5:451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders BM. Stress proteins: potential as multitiered biomarkers. Boca Raton, USA: Lewis Publishers, CRC Press; 1990. [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Crit Rev Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Schill RO, Steinbruck GH, Kohler H-R. Stress gene (hsp70) sequences and quantitative expression in Milnesium tardigradum (Tardigrada) during active and cryptobiotic stages. J Exp Biol. 2004;207:1607–1613. doi: 10.1242/jeb.00935. [DOI] [PubMed] [Google Scholar]
- Sconzo G, Scardina G, Ferraro MG. Characterization of a new member of the sea urchin Paracentrotus lividus hsp70 gene family and its expression. Gene. 1992;121:353–358. doi: 10.1016/0378-1119(92)90143-D. [DOI] [PubMed] [Google Scholar]
- Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Heschl MF, Baillie DL. The Caenorhabditis elegans hsp70 gene family: a molecular genetic characterization. Gene. 1988;64:241–255. doi: 10.1016/0378-1119(88)90339-3. [DOI] [PubMed] [Google Scholar]
- Sørensen J, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Sorger P (1991) Heat shock factor and the heat shock response. Cell 65:363–366 [DOI] [PubMed]
- Sylvain ZA, Wall DH. Linking soil biodiversity and vegetation: implications for a changing planet. Am J Bot. 2011;98:517–527. doi: 10.3732/ajb.1000305. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Petersen N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek L, Sanford E. Heat-shock protein 70 (Hsp70) as a biochemical stress indicator: an experimental field test in two congeneric intertidal gastropods (genus: Tegula) Biol Bull. 2003;205:276–284. doi: 10.2307/1543291. [DOI] [PubMed] [Google Scholar]
- Waagner D, Bayley M, Marïen J, Holmstrup M, Ellers J, Roelfs D. Ecological and molecular consequences of prolonged drought and subsequent rehydration in Fosomia candida. J Insect Physiol. 2012;58:130–137. doi: 10.1016/j.jinsphys.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Winter J, Wright R, Duck N, Gasser C, Fraley R, Shah D. The inhibition of petunia hsp70 mRNA processing during CdC12 stress. Mol Gen Genet. 1988;211:315–319. doi: 10.1007/BF00330609. [DOI] [Google Scholar]
- Wisniewska M, Karlberg T, Lehtio L, Johansson I, Kotenyova T, Moche M, Schuler H. Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70-2, HSPA6/Hsp70B’, and HSPA5/BiP/GRP78. PLoS One. 2010;5:0008625. doi: 10.1371/journal.pone.0008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ, Lindquist S. Translation of unspliced transcripts after heat shock. Science. 1988;242:1544–1548. doi: 10.1126/science.3201243. [DOI] [PubMed] [Google Scholar]
- Zanger M, Alberti G, Kuhn M, Köhler H-R (1996) The stress −70 protein family in diplopods: induction and characterization. J. Comp. Physiol. B 156:622–627
- Zhang D-Z, Hewitt GM. Special DNA extraction methods for some animal species. London, UK: Chapman & Hall; 1998. [Google Scholar]
- Zhang G, Fang X, Guo X, Li L, Luo R, Xu F et al. (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490(7418):49–54 [DOI] [PubMed]