Abstract
Heat-shock protein 90 (HSP90) is a highly conserved molecular chaperone found in all species except for Archaea, which is required not only for stress tolerance but also for normal development. Recently, it was reported that HSP83, one member of the cytosolic HSP90 family, contributes to oogenesis and responds to heat resistance in Tribolium castaneum. Here, a novel ER-based HSP90 gene, Tchsp90, has been identified in T. castaneum. Phylogenetic analysis showed that hsp90s and hsp83s evolved separately from a common ancestor but that hsp90s originated earlier. Quantitative real-time polymerase chain reaction illustrated that Tchsp90 is expressed in all developmental stages and is highly expressed at early pupa and late adult stages. Tchsp90 was upregulated in response to heat stress but not to cold stress. Laval RNAi revealed that Tchsp90 is important for larval/pupal development. Meanwhile, parental RNAi indicated that it completely inhibited female fecundity and partially inhibited male fertility once Tchsp90 was knocked down and that it will further shorten the lifespan of T. castaneum. These results suggest that Tchsp90 is essential for development, lifespan, and reproduction in T. castaneum in addition to its response to heat stress.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0487-y) contains supplementary material, which is available to authorized users.
Keywords: HSP90, Heat stress, Development, Reproduction, Lifespan, Tribolium castaneum
Introduction
Heat-shock proteins (HSPs) are highly conserved chaperones typically expressed in response to environmental stress factors and were identified from Drosophila melanogaster in 1964 (Ritossa and Vonborstel 1964). Usually, HSPs can be classified into several families, such as HSP110, HSP90, HSP70, and small HSPs (sHSPs) on the basis of their molecular weight and the homology of their amino acid sequences (Csermely et al. 1998; Lindquist and Craig 1988). Heat-shock protein 90 (HSP90) was discovered in all species except in Archaea, and the molecular weight of this family ranges from 82 to 94 kDa in different organisms (Csermely et al. 1998; Kim et al. 1998; Large et al. 2009). They consist of the following conserved domains: N-terminal ATP-binding domain, the charged linker domain, middle-domain, and the C-terminal dimerization domain (Marzec et al. 2012; Meyer et al. 2003; Street et al. 2011).
However, there are variant isoforms of HSP90 that exist among eukaryotes. For example, in D. melanogaster and Caenorhabditis elegans, only one cytosolic HSP90 homolog exists, and two closely related isoforms exist in mammals as well as in yeast cytosol, which are differentially regulated in tissues and by various forms of cellular stress, while there are three isoforms in zebra fish (Haslbeck et al. 2012; Johnson 2012; Katherine et al. 1989; Krone et al. 1997). Beyond these cytosolic HSP90 proteins, there is another major HSP90 group, which is the ER-based HSP90 homolog (also called as 94-kDa glucose-regulated protein, GRP94, gp96, or endoplasmin), which shows high sequence similarity with cytosolic HSP90 homolog, except that it has an additional signal peptide in its N terminus and ER-targeting motif KDEL in its C terminus (Marzec et al. 2012; Pelham 1989). Moreover, mitochondria, chloroplast HSP90 homologs, and even HTPG (high-temperature protein G in bacterial cytosol) have been discovered lately, although these three homologs show less sequence similarity with the previous two members. Meanwhile, mitochondria and chloroplast HSP90 function in specific cell organs, and HTPG is specific for prokaryotes (Chen et al. 2006).
Current research has mostly focused on the vital role that cytosolic HSP90 plays against environmental stress such as heat, cold, toxins, and invading pathogens (Boher et al. 2012; Colinet et al. 2010; Kaiser et al. 2011; Liu et al. 2004) and in its development as a chaperone of steroid hormone receptors and signaling kinases in assisting other proteins in folding and maturation with its co-chaperone (Richter and Buchner 2001; Theodoraki and Caplan 2012). However, relatively little is known regarding the chaperone biology of ER-based HSP90. While ER-based HSP90 is an endoplasmic reticulum stress protein of the heat-shock protein (HSP) 90 family with an ER retention signal and has high levels in secretory tissues (Johnson 2012). The upregulation of ER-based Hsp90 is used as a hallmark of responses to ER stress (Eletto et al. 2010). Recent studies have demonstrated ER-based HSP90 is involved in maturation of proteins destined for cell surface display or export and plays a vital role in both the adaptive and innate immune systems (Marzec et al. 2012; Tramentozzi et al. 2011; Yang et al. 2007). Moreover, it is also required during the early developmental stage in C. elegans, fly, and mouse (Marzec et al. 2012; Maynard et al. 2010; Wanderling et al. 2007). And, in fly, this HSP90 orthologous protein mutant larvae has pronounced defects in its midgut epithelium (Marzec et al. 2012; Maynard et al. 2010).
Very recently, like the other model species, cytosolic HSP90 was renamed as HSP83 based on its molecular weight and was identified in a recently emerged model insect, red flour beetle Tribolium castaneum. It has been shown that hsp83 contributes to oogenesis, compound eye development, and response to heat resistance in the T. castaneum (Knorr and Vilcinskas 2011, Xu et al. 2009; 2010a). However, no information as to the role of the hsp90 (the homologue of ER-based hsp90 in T. castaneum) gene in T. castaneum, either for stress response or for insect development, is available to date. Therefore, based on their molecular weight, we refer to cytosolic HSP90 and ER-based HSP90 as HSP83 and HSP90, respectively, for convenience. In this study, we have cloned cDNA of the Tchsp90 gene and phylogenetic analysis has revealed that Tchsp90 and Tchsp83 evolved separately from a common ancestor and Tchsp90 originated earlier than Tchsp83. In order to understand the function of Tchsp90, we further investigated whether this molecule responds to heat or cold stresses. Moreover, an RNAi experiment has illustrated that Tchsp90 plays vital roles in the development, reproduction, and lifespan in T. castaneum.
Materials and methods
Experimental animals
The Georgia-1 (GA-1) strain of T. castaneum was reared at 30 °C and 40 % relative humidity in 5 % yeasted flour under standard conditions (Haliscak and Beeman 1983; Li et al. 2011).
Identification and cloning of hsp90 gene in T. castaneum
The Tchsp90 gene was identified by searching Beetlebase (http://www.beetlebase.org/). Based on the predicted sequence, we designed the primers to amplify the full-length of Tchsp90 cDNA. The primers used for polymerase chain reaction (PCR)-based cloning are listed in Table 1. cDNA from the late larva was used as the template for cloning, and PCR products were cloned into the pEASY-T3 Cloning Vector (TaKaRa) and sequenced in the Majorbio of Shanghai.
Table 1.
Oligo-nucleotide primers used in this study
| Primer | Direction (3′–5′) | Fragment length (bp) | Sequence | Remarks |
|---|---|---|---|---|
| hsp90-L1 | Forward | 2349 | ATGAAGCAGTTGATTGTTTTAGCG | Whole ORF of hsp90 |
| hsp90-R1 | Reverse | TTACAACTCGTCATGATCCCCA | ||
| hsp90-L2 | Forward | 303 | CACACGAGCAGATGAATCTATTG | hsp90 transcriptional level |
| hsp90-R2 | Reverse | CAAGCGGATCTTATCTAAAGCGT | ||
| hsp90-L3 | Forward | 511 | TAATACGACTCACTATAGGGTTAAAAGAACTGCGCGACAAAGC | dsRNA of hsp90 |
| hsp90-R3 | Reverse | TAATACGACTCACTATAGGGCACAATGGAAAAGCTTGACGAATC | ||
| P1 | GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN | 3′RACE cDNA synthesis | ||
| P2 | Reverse | GCGAGCACAGAATTAATACGACT | 3′RACE | |
| P3 | Reverse | CGCGGATCCGAATTAATACGACTCACTATAGG | ||
| hsp83-L | Forward | 324 | CTTCCAGACTCGATTCAGGCAAA | hsp83 transcriptional level |
| hsp83-R | Reverse | GTGAAGCTACCACCAGCTGA | ||
| rps3 | Forward | 260 | TCAAATTGATCGGAGGTTTG | Internal control transcriptional level |
| rps3 | Reverse | GTCCCACGGCAACATAATCT |
Sequences and phylogenetic analyses
To identify candidate HSP83 and HSP90 genes (cytosolic HSP90 and ER-Based HSP90 isoforms) from other organisms, BLASTP and TBLASTN were used to search the following databases: FlyBase (http://flybase.org/), BeeBase (http://hymenopteragenome.org/beebase/), Silkworm Genome Database (http://silkworm.genomics.org.cn/), AphidBase (http://www.aphidbase.com/aphidbase), VectorBase (http://www.vectorbase.org/index.php), BeetleBase (http://www.beetlebase.org/), and NCBI (http://www.nibi.nlm.nih.gov/). The E value for evaluating all sequences in homology search is 10−6. These candidate genes were further examined by reciprocal Blast in NCBI to ensure they are real HSP90. A total of 21 hsp90 homologues and 31 hsp83 homologues were derived from 21 species which have completed genomic data from invertebrate to vertebrate animals for subsequent analysis (Electronic supplementary material Table S1). The gene structural prediction was analyzed online using PredictProtein (http://www.predictprotein.org/) and InterProScan (http://www.ebi.ac.uk/Tools/InterProScan/). Multiple sequence alignments of the amino acid sequences were performed online using ClustalW2 from the website (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The alignments were viewed and edited with the BOXSHADE software (http://www.ch.embnet.org/software/BOX_form.html). Phylogenetic trees were constructed using MEGA 5.0 software with the neighbor-joining and maximum likelihood (ML) methods (Tamura et al. 2011). One thousand bootstrap tests were performed, and values lower than 50 % are not shown.
Quantitative real-time RT-PCR
Total RNAs from pools of three individuals were extracted at each of the following developmental stages: early eggs (EE, 1 day old), late eggs (LE, 3 days old), early larvae (EL, 1 day old), late larvae (LL, last-instar larvae), early pupae (EP, 1 day old), late pupae (LP, 5 days old), early adults (EA, 1 day old), and late adults (LA, 1 week old) with the RNAiso Plus reagent (TaKaRa). Reverse transcription was performed using 1 μg total RNA. PCR amplification was performed under the following conditions: one cycle of 94 °C for 5 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s; and one cycle of 72 °C for 7 min. The T. castaneum ribosomal protein S3 (rps3, GenBank accession numbers CB335975) gene served as an internal control and was run for 28 cycles under the same conditions (Arakane et al. 2008). The primers are listed in Table 1.
RNA interference
Double-stranded RNAs (dsRNA) was designed on nucleotides 199–710 (511 bp) of the CDS region of the Tchsp90, and these regions span exons 3–4. The nucleotide sequence identity between Tchsp90 and Tchsp83 in the targeted region is only 59.5 %, while the longest stretch of identical matching sequence for the region is only 14 bp. About 200 ng of dsRNA in 200 nl solution was injected into last-instar larvae and early pupae (Arakane et al. 2005; Tomoyasu and Denell 2004). On the sixth day after dsRNA injection, the insects were used to detect whether RNA interference is effective and whether it shows cross interference between Tchsp90 and Tchsp83. Afterwards, these insects were reared in the same conditions mentioned above, and the developmental status was observed.
Behavior analysis
Larval injections were followed by the observation of the noticeable morphological defects and mortality. Three biological replications were carried out for the experiments with 25 beetles/replication/group. Mortality occurring less than 5 days after injection was attributed to injection injury rather than to target transcript knockdown. In general, mortality due to injection damage was <10 % (Begum et al. 2009). Pupal injections, which did not cause any detectable defects in adult eclosion, were regarded as parental RNAi experiments (Bucher et al. 2002) and were followed by the assessment of oviposition rates and progeny survival. Individuals in two replications of RNAi were utilized for single-pair matings (10 to 15 pairs/replication, respectively). Three-day ovipositions (13-day- to 16-day-old females) were collected, counted, and held for hatch-rate measurement, and their development was observed. Numbers of offspring were counted 15 days after the eggs were collected. Negative controls consisted of injections of either vermilion dsRNA or an equal volume of buffer only (Begum et al. 2009). The data analysis was done using STATISTIC version 10.0 by one-way ANOVA.
Stress treatment
To examine whether Tchsp90 is a response to thermal and cold stress, 24 late larvae for each group were reared at 45 °C and 4 °C condition. Six insects for each time point were collected after the insects were treated with 45 °C and 4 °C for 1, 2, 4, and 12 h, respectively. In total, three biological replications were carried out for these experiments. Thereafter, the expression level of Tchsp90 was measured.
For a starvation tolerance assay, parental RNAi was carried out. Once the wild-type and Tchsp90 RNAi insects were treated under starvation conditions, the expression level of Tchsp90 and survival rates were investigated. Two biological replications were carried out for the experiments, and 25 beetles were used in each group.
Results
Identification of hsp90 gene from T. castaneum
According to the known D. melanogaster cytosolic and ER-based hsp90s sequences, we mined out two candidates from T. castaneum genome and named them as Tchsp83 and Tchsp90, respectively, based on their predicted molecular weights. Tchsp90 was identified in T. castaneum for the first time and is located on chromosome 9 of the T. castaneum genome. Tchsp90 contains a 2,349 bp open reading frame (ORF) interrupted by three introns, which produced a putative protein of 782 amino acids with the predicted molecular mass of 89.5 kDa. The 3′ non-translated region of the Tchsp90 is 115 bp and contains a typical motif polyadenylation signal (accession number: KF922652). TcHSP90 is highly conserved with the homologs of HSP90 from Acromyrmex echinatior (accession number: EGI61481.1) and D. melanogaster (accession number: CG1242) with 72.8 % and 73.3 % identity, respectively. HSP90 shares 48.8 %–99.5 % identity across animals; HSP83 shares 47.1 %–99.3 %, but HSP90 and HSP83 share 24.6 %–43.3 % identity across animals (Electronic supplementary material Table S2, Table S3, and Table S4). However, TcHSP90 only showed 41.6 % amino sequence identity with TcHSP83, although both subfamilies of HSP90 and HSP83 contain four conserved domains and an ATP binding domain (Fig. 1). For TcHSP90, beside these domains, there is an extra ∼20aa with Gln-rich signal peptide that only exists in the N-terminal of HSP90 not in that of HSP83. Moreover, the C terminus of HSP90 has a conserved HDEL motif which is an endoplasmic reticulum targeting sequence, while HSP83 has an MEEVD motif (Fig. 1).
Fig. 1.
Alignment of the amino acid sequences of HSP90s and HSP83s. The signal peptide was marked with black box. The first 50 amino acids of HSP90 (GRP94) are distinct from the N terminus of HSP83 in both length and sequence. The ATP-bind domain located in N terminus both HSP90 and HSP83. The C-terminal conserved signature sequences of HSP83 were marked with asterisk while the HSP90 were marked with number sign. The HSP90 sequences derived from D. melanogaster (DmHSP90, CG5520); Homo sapiens (HsHSP90, CAI64497.1); T. castaneum (TcHSP90,TC012185), and the HSP83s derived from D. melanogaster (DmHSP83, CG1242); H. sapiens (HsHSP83-1,NP-005339.3; HsHSP83-2,NP-031381.2); T. castaneum (TcHSP83,TC014606)
Phylogenetic analysis
In order to understand the evolutionary history of HSP83 and HSP90, 21 genome sequenced invertebrates and vertebrates were selected. A total of 34 hsp83 homologs and 21 hsp90 homologs were derived from the genome database of these organisms. Phylogenic analysis revealed that HSP83s and HSP90s possessed the same origin and diverged before the differentiation of invertebrates and vertebrates (Fig. 2 and electronic supplementary material Fig. S1). Thereafter, another major duplication occurred before the differentiation of vertebrates and which led to the fact that vertebrates have two copies of HSP83, except that Danio rerio has three HSP83s which are located on chromosome 20, and these three HSP83 share 79.2 % identity. On the other hand, invertebrates have only one HSP83 except that Apis mellifera and Acyrthosiphon pisum have two HSP83s derived from species-specific gene duplication. The two HSP83s of A. mellifera are located on chromosome 1 and chromosome 7 separately, and share 82.1 % amino acid identity with each other. The two HSP83s of A. pisum are located on scaffold GL349748 and GL349707 with 87.1 % amino acid identity. However, each species has only one highly conserved hsp90 gene in the group based on their genome sequences.
Fig. 2.
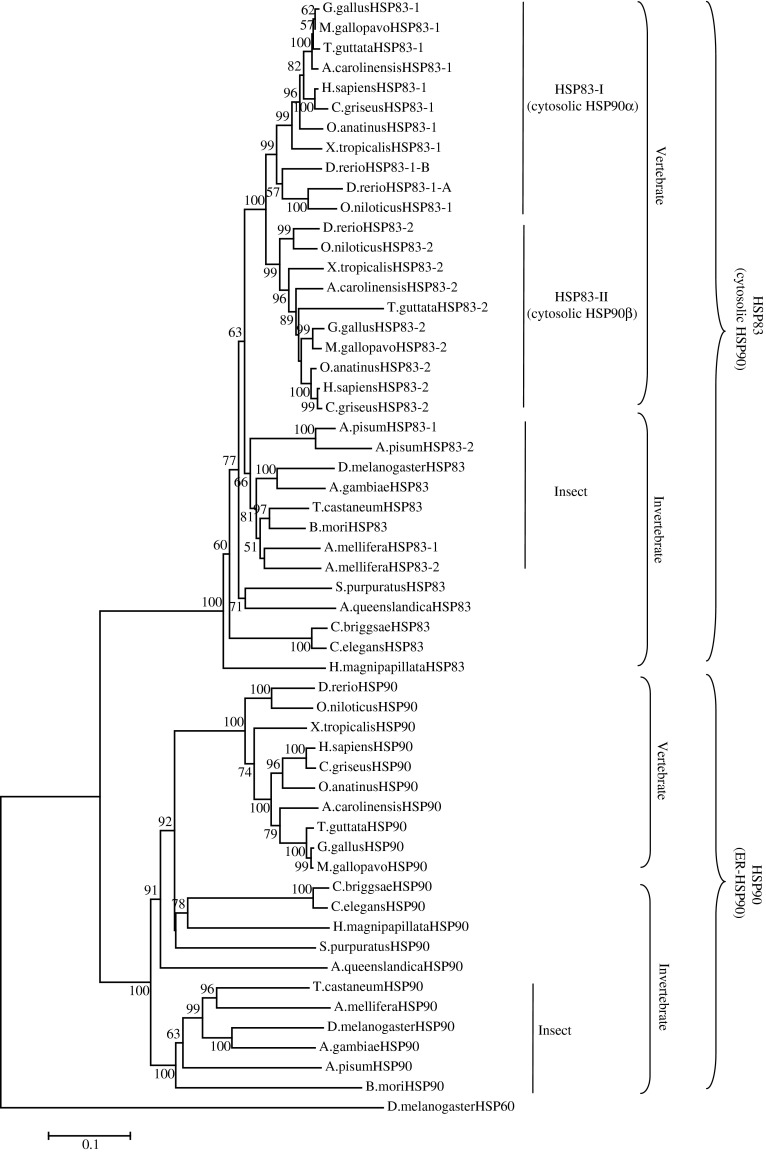
Phylogenetic analysis of HSP90s and HSP83s. Bootstrap values below 50 were removed from the tree. The ML tree is rooted by the D. melanogaster HSP60. The details of the gene information which used in the analysis were descript in Electronic supplementary material Table S1
Expression pattern of Tchsp90
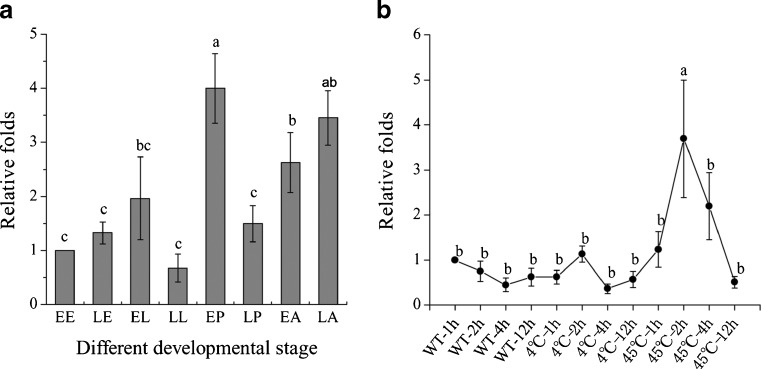
The expression patterns of Tchsp90 gene were investigated by quantitative real-time polymerase chain reaction (qRT-PCR)using the rps3 gene for normalization. Tchsp90 transcript abundance was highly expressed at the early stage for each developmental stage and formed three expression peaks from larvae to adults except for the embryo stage, while it was least expressed at the later larval stage and was expressed most at the early pupal stage (Fig. 3a).
Fig. 3.
The expression pattern of Tchsp90 in different developmental stages and under cold and heat treatment. a Quantitative real-time PCR analysis of Tchsp90 mRNA relative transcript levels were normalized to ribosomal protein s3 (rps3) in the same cDNA samples. Different development stages are EE early egg (1-day-old); LE late egg (3-day-old); EL early larval (1-day-old); LL late larval (7-day-old); EP early pupae (1-day-old); LP late pupae (6-day-old); EA early adult (1-day-old); LA late adult (7-day-old). b The expression of hsp90 in cold (4 °C) and heat (45 °C) treatment. The lowercase letters were analyzed by HSD multiple compartment. Error bars represent standard deviations among three biological replications
Response to stresses
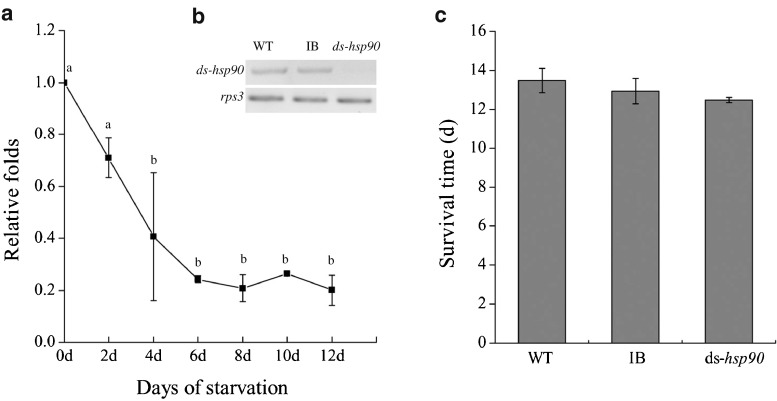
As shown in Fig. 3b, Tchsp90 expression level was significantly increased after heat stress and reached a peak after 2 h of heat treatment at 45 °C, the expression level is 3.7 ∼ 8.3-fold, higher than that without the thermal treatment, and its expression level gradually returned to normal. However, Tchsp90 did not show any response to cold stress (4 °C). After starvation, the qRT-PCR analysis indicated that Tchsp90 expression levels declined gradually and reached the lowest expression level on the sixth day, remaining at this level until the beetles died (Fig. 4a). Pupa RNAi resulted in suppression of transcript level for Tchsp90 (Fig. 4b). However, Tchsp90 knock-down insects could survive 12.5 ± 0.14 days under starvation conditions and showed comparable starvation tolerance with the wild-type and buffer-injected insects which survived 13.5 ± 0.61 and 12.9 ± 0.64 days, respectively, under starvation conditions (Fig. 4c).
Fig. 4.
The expression pattern of Tchsp90 under starvation and the survivorship after the pupa RNAi. a Time course analysis of Tchsp90 expression under starvation stress. b The Tchsp90 expression level after RNAi. c The survivorship of n WT, IB, and ds-hsp90 beetles under starvation
RNAi phenotypes of Tchsp90
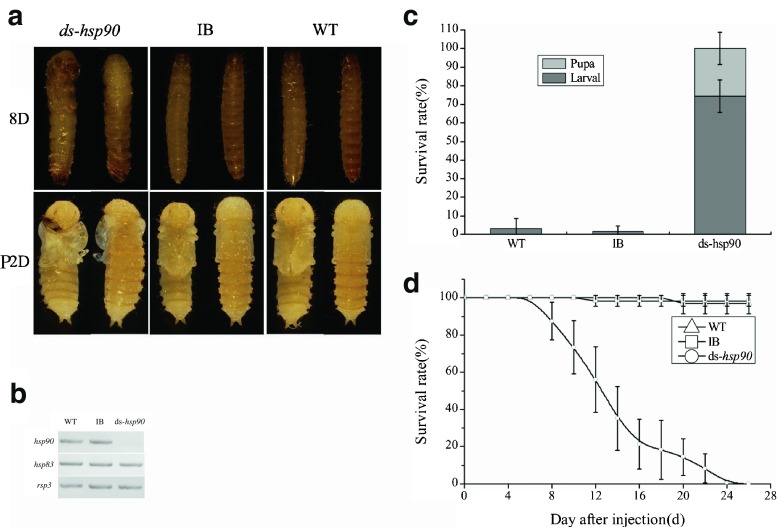
Injection of dsRNA of Tchsp90 during the late larval stage resulted in defects in larval development and suppression of transcript levels for this gene (Fig. 5). Interestingly, larval RNAi of Tchsp90 caused approximately 74 % insects to fail to molt subsequently and were unable to completely shed larval cuticles, and they finally died at larval stage. The remaining 26 % insects could molt to the pupal stage with morphological deficiency and eventually died at pupa stage (Fig. 5).
Fig. 5.
RNAi phenotype of Tchsp90. a RNA interference resulted in lethal phenotypes in both of larval/pupal stages. b Knocking down levels of Tchsp90 using larval RNAi. c Progressively increasing mortalities after late larval RNAi of ds-Tchsp90. d Cumulative adult mortality after late larval RNAi. Each point represented the mean days of three biological replications
To examine later stages of RNAi phenotypes, we injected 2-day-old pupae and assessed oviposition rate and the survival rates of the offspring. The injection of RNAi for Tchsp90 at the pupal stage caused a complete inhibition in egg production, whereas the wild-type and IB beetles laid an average of 5.4 and 5.6 eggs/day/female. Meanwhile, the inhibited oviposition rates were 40.5 ± 0.6 and 39.1 ± 2.3 were recovered by back-crossing with wild-type and IB females and could not be recovered by back-crossing with wild type males (Fig. 6a). But the egg hatch rate had no apparent differences among ds-hsp90 ♂× WT♀group, ds-hsp90 ♂× IB♀group, IB group, and WT group (Fig. 6b).
Fig. 6.
Reduced egg laying (a) and the egg hatch rate (b) as well as cumulative adult mortality (c) after injection of ds-Tchsp90 in 2-day-old pupae. Confidence intervals calculated using Student’s t test for 21 single pair mating and show standard deviations and significant differences compared with the control using single asterisk indicates p < 0.05; double asterisks indicates p < 0.001
In order to examine if Tchsp90 is involved in longevity of T. castaneum, we injected 2-day-old pupae and assessed their survival days. The pupae with injected ds-hsp90 all died during the 80 days after eclosion. However, the survival rate of wild-type and buffer-injected adults (WT and IB) remained at 97.6 ± 1.7 and 96.7 ± 3.0 percentage, respectively. Results showed that the suppression of hsp90 significantly shorten the lifespan of T. castaneum (Fig. 6c).
Discussion
In this study, we identified Tchsp90, a novel ER-based hsp90 member, based on the genome sequence of T. castaneum. Sequence analysis showed that there are 48.8 %–99.5 % amino acid identities among HSP90 groups, while there are 47.1 %–99.3 % amino acid identities among HSP83 groups. However, there are only 24.6 %–43.3 % amino acid identities between HSP83 and HSP90 groups. Moreover, phylogenetic analysis revealed that hsp90 and hsp83 were clearly divided into two groups, and they evolved separately from a common ancestor but hsp90 originated earlier than hsp83, indicating that theses two molecular chaperones may be under differential selections during their evolution.
Basically, both these two groups and other homologs of HSP90s, such as mitochondrial TRAP1 (tumor necrosis factor receptor-associated protein 1) and chloroplast HSP90, exhibit similar ATPase rates and undergo similar conformational changes. One of the key differences is that cytosolic HSP83 interacts with a large number of co-chaperones that regulate the ATPase activity of HSP83 or have other functions such as targeting clients to HSP83 (Johnson 2012). All vertebrates contain at least two isoforms of cytosolic HSP83s. Previous studies have proposed that two HSP83s evolved by duplication of a common ancestral gene more than 500 million years ago, close to the time of emergence of the vertebrates (Krone and Sass 1994; Moore et al. 1989). However, D. rerio had three hsp83 genes which are located on the same chromosome (CL20), and it seems that HSP83-1-A and HSP83-1-B were duplicated by additional recent gene duplication. It was further confirmed that these two genes possessed different functions for muscle development between HSP83-1-A (HSP90α1) and HSP83-1-B (HSP90α2) in D. rerio (Du et al. 2008). Interestingly, most invertebrates have only one HSP83 except that A. mellifera and A. pisum have a species-specific gene duplication that has resulted in two HSP83s. As the A. mellifera-specific HSP83 is caste- and age-specifically expressed in adult bees (Aamodt 2008), it was proposed that the duplicated gene might be associated with special morphological and behavioral differentiation of castes in A. mellifera (Xu et al. 2010b). However, both A. mellifera and A. pisum lived on the frequently changed humidity condition as their feed behavior to ingested plant fluids and secrete honey dew, thus the duplication might be associated with this environmental stress selection as was shown in HSP83 which has been reported to be involved in the dehydration, rehydration, and overhydration processes in Antarctic midge (Lopez-Martinez et al. 2009). However, further investigation incorporating environmental stress is required in understanding the function of these transcripts.
Induction of expression of the hsp83 gene under heat, cold, and other environmental stress conditions has been found in many species, such as D. melanogaster (Boher et al. 2012), Lucilia cuprina (Concha et al. 2012), and Spodoptera exigua (Jiang et al. 2012), and the induced expression of hsp83 under heat-shock conditions was observed in the whole body and ovary extracts of both newly hatched and mature adults of T. castaneum (Xu et al. 2010a). Currently, there are very limited studies on the expression of the HSP90 protein under environmental stress conditions, since it has been recognized that HSP90, unlike HSP83-I and II, was not induced by high temperature or other stresses that are unique to the cytosol HSP83 (Marzec et al. 2012; Subjeck and Shyy 1986). However, its upregulation is often used as a hallmark of responses to ER stress. Interestingly, in this study, a significant increase of HSP90 expression was observed under heat stress at 42 °C, and it indicated that HSP90 could also play a pivotal role in the response to heat stress as it was shown in grass carp that Cihsp90 (ER-based hsp90) was upregulated after heat shock at 34 °C or cold stress at 4 °C (Wu et al. 2012). However, Tchsp90 was only upregulated in response to heat stress but had no change under cold stress at 4 °C in T. castaneum. These results indicated that HSP90 might be involved in differential protection processes from variant stress conditions in different organisms.
Moreover, we found that Tchsp90 was significantly downregulated under starvation conditions, which is consistent with Dd-GRP94 (Dictyostelium discoideum glucose-regulated protein 94) expression that rapidly declined within 1 h in response to starvation (Morita et al. 2000). Moreover GRP94 had low expression level in energy restriction mice liver in which secretory tissues showed high expression level at normal conditions. It is possible that long-term food deprivation reduced the level of GRP94 mRNA by reducing the level of malfolded proteins in the endoplasmic reticulum of hepatic cells (Eletto et al. 2010; Stephen et al. 1990). However, one conflicting result has shown that food-deprivation (7 days) enhanced ER-based HSP90 expression in larvae of gilthead sea bream and rainbow trout. This increase of HSP90 may be associated with enhanced protein catabolism in feed-deprived fish (Cara et al. 2005). Thus, ER-based HSP90 might be involved in differential mechanisms under starvation/food-deprivation conditions for various organisms.
In addition to the response to ER-stress and environmental stress, hsp90 is also required at normal development for many organisms. Previous studies have shown that GRP94 is critical in mouse embryos as its absence provoked an embryo-lethal condition (Mao et al. 2010; Wanderling et al. 2007). Moreover, ER-based HSP90 is also required during the early developmental stage in C. elegans and fly (Marzec et al. 2012; Maynard et al. 2010). Interestingly, once knocked-down, the expression of Tchsp90 is not only arrested the larvae/pupae development, but also significantly reduced the beetle’s lifespan and their reproduction in T. castaneum besides its response to heat stress. The loss of Gp93 expression is larval-lethal in Drosophila; it seems because GRP94 expression was essential for the integrated secretory and absorptive functions of the midgut that nutrient assimilation-coupled growth control is enabled (Maynard et al. 2010).
Moreover, ds-Tchsp90 female had no offspring, and the male had low reproduction rates. This implied that Tchsp90 was a maternal effect gene similar to GRP94 (Li et al. 2010). These data suggested that Tchsp90 might be involved in the oogenesis and spermatogenesis as it was discovered that this gene is required during early embryo development of mouse, which failed to develop mesoderm, primitive streak, or proamniotic cavity once this gene was deleted (Mao et al. 2010). Meanwhile, grp94−/− ES cells grow in culture and are capable of differentiation into cells representing all the three germ layers. However, these cells do not differentiate into cardiac, smooth, or skeletal muscle (Wanderling et al. 2007). Although eggs produced by ds-Tchsp90 male have comparable hatch capability with wild-type, it is suggested that this gene seems not to be involved in the later embryo development in T. castaneum. Furthermore, we first discovered that Tchsp90 is important for beetle’s lifespan, and once this gene was knocked down, all adult beetles died within 80 days, which is significantly shorter lifespan than that of the wild-type. One possible explanation is that the decreased expression of Tchsp90 would reduce the ER stress resistance and when the misfolded proteins constant increased beyond the ER capacity would induce ER-stress-induced apoptosis (Tabas and Ron 2011). Finally, it caused a greater reduction in lifespan in T. castaneum. However, further investigation into the details of how this gene is involved in the beetle’s lifespan and its mechanism is needed to further understand the function of this gene.
Electronic supplementary material
(XLSX 14.6 kb)
(XLSX 13 kb)
(XLSX 16 kb)
(XLSX 15 kb)
(PDF 371 kb)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31172146); the Natural Science Foundation of Jiangsu Province, China (BK2011785); the Key Project of Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 10KJA180023); the PAPD of Jiangsu Higher Education Institutions; the Excellent Talent Project of Nanjing Normal University of China; and the graduate innovation research projects of Jiangsu colleges and universities.
References
- Aamodt RM. The caste- and age-specific expression signature of honeybee heat shock genes shows an alternative splicing-dependent regulation of Hsp90. Mech Ageing Dev. 2008;129:632–637. doi: 10.1016/j.mad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci U S A. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, Park Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125:984–995. doi: 10.1016/j.mod.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Begum K, Li B, Beeman RW, Park Y. Functions of ion transport peptide and ion transport peptide-like in the red flour beetle Tribolium castaneum. Insect Biochem Mol Biol. 2009;39:717–725. doi: 10.1016/j.ibmb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Boher F, Trefault N, Piulachs MD, Belles X, Godoy-Herrera R, Bozinovic F. Biogeographic origin and thermal acclimation interact to determine survival and hsp90 expression in Drosophila species submitted to thermal stress. Comp Biochem Physiol A Mol Integr Physiol. 2012;162:391–396. doi: 10.1016/j.cbpa.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Bucher G, Scholten J, Klingler M. Parental RNAi in Tribolium (Coleoptera) Curr Biol. 2002;12:R85–R86. doi: 10.1016/S0960-9822(02)00666-8. [DOI] [PubMed] [Google Scholar]
- Cara JB, Aluru N, Moyano FJ, Vijayan MM. Food-deprivation induces HSP70 and HSP90 protein expression in larval gilthead sea bream and rainbow trout. Comp Biochem Physiol B Biochem Mol Biol. 2005;142:426–431. doi: 10.1016/j.cbpb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colinet H, Lee SF, Hoffmann A. Temporal expression of heat shock genes during cold stress and recovery from chill coma in adult Drosophila melanogaster. FEBS J. 2010;277:174–185. doi: 10.1111/j.1742-4658.2009.07470.x. [DOI] [PubMed] [Google Scholar]
- Concha C, Edman RM, Belikoff EJ, Schiemann AH, Carey B, Scott MJ. Organization and expression of the Australian sheep blowfly (Lucilia cuprina) hsp23, hsp24, hsp70 and hsp83 genes. Insect Mol Biol. 2012;21:169–180. doi: 10.1111/j.1365-2583.2011.01123.x. [DOI] [PubMed] [Google Scholar]
- Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci U S A. 2008;105:554–559. doi: 10.1073/pnas.0707330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Semin Cell Dev Biol. 2010;21:479–485. doi: 10.1016/j.semcdb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliscak JP, Beeman RW. Status of malathion resistance in five genera of beetles infesting farm-stored corn, wheat and oats in the United States. J Econ Entomol. 1983;76:6. [Google Scholar]
- Haslbeck V, Kaiser CJ, Richter K. Hsp90 in non-mammalian metazoan model systems. Biochim Biophys Acta. 2012;1823:712–721. doi: 10.1016/j.bbamcr.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhai H, Wang L, Luo L, Sappington TW, Zhang L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperones. 2012;17:67–80. doi: 10.1007/s12192-011-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823:607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Kaiser E, Kroll C, Ernst K, Schwan C, Popoff M, Fischer G, Buchner J, Aktories K, Barth H. Membrane translocation of binary actin-ADP-ribosylating toxins from Clostridium difficile and Clostridium perfringens is facilitated by cyclophilin A and Hsp90. Infect Immun. 2011;79:3913–3921. doi: 10.1128/IAI.05372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katherine AB, Farrelly WF, David BF, John T, Susan L. Hsp82 is an essential protein that is required in higher concentrations for growth of cells at higher temperatures. Mol Cell Biol. 1989;9:3919–3930. doi: 10.1128/mcb.9.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim SH. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Knorr E, Vilcinskas A. Post-embryonic functions of HSP90 in Tribolium castaneum include the regulation of compound eye development. Dev Genes Evol. 2011;221:357–362. doi: 10.1007/s00427-011-0379-z. [DOI] [PubMed] [Google Scholar]
- Krone PH, Sass JB. HSP 90 alpha and HSP 90 beta genes are present in the zebrafish and are differentially regulated in developing embryos. Biochem Biophys Res Commun. 1994;204:746–752. doi: 10.1006/bbrc.1994.2522. [DOI] [PubMed] [Google Scholar]
- Krone PH, Lele Z, Sass JB. Heat shock genes and the heat shock response in zebrafish embryos. Biochem Cell Biol. 1997;75:487–497. doi: 10.1139/o97-083. [DOI] [PubMed] [Google Scholar]
- Large AT, Goldberg MD, Lund PA. Chaperones and protein folding in the archaea. Biochem Soc Trans. 2009;37:46–51. doi: 10.1042/BST0370046. [DOI] [PubMed] [Google Scholar]
- Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137:859–870. doi: 10.1242/dev.039487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Beeman RW, Park Y. Functions of duplicated genes encoding CCAP receptors in the red flour beetle, Tribolium castaneum. J Insect Physiol. 2011;57:1190–1197. doi: 10.1016/j.jinsphys.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar SP. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J Biol Chem. 2004;279:2101–2108. doi: 10.1074/jbc.M310029200. [DOI] [PubMed] [Google Scholar]
- Lopez-Martinez G, Benoit JB, Rinehart JP, Elnitsky MA, Lee RE, Jr, Denlinger DL. Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica. J Comp Physiol B. 2009;179:481–491. doi: 10.1007/s00360-008-0334-0. [DOI] [PubMed] [Google Scholar]
- Mao C, Wang M, Luo B, Wey S, Dong D, Wesselschmidt R, Rawlings S, Lee AS. Targeted mutation of the mouse Grp94 gene disrupts development and perturbs endoplasmic reticulum stress signaling. PLoS One. 2010;5:e10852. doi: 10.1371/journal.pone.0010852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Eletto D, Argon Y. GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, Newgard CB, Spana EP, Nicchitta CV. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol. 2010;339:295–306. doi: 10.1016/j.ydbio.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P, Prodromou C, Hu B, Vaughan C, Roe SM, Panaretou B, Piper PW, Pearl LH. Structural and functional analysis of the middle segment of hsp90: implications for ATP hydrolysis and client protein and cochaperone interactions. Mol Cell. 2003;11:647–658. doi: 10.1016/S1097-2765(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Moore SK, Kozak C, Robinson EA, Ullrich SJ, Appella E. Murine 86- and 84-kDa heat shock proteins, cDNA sequences, chromosome assignments, and evolutionary origins. J Biol Chem. 1989;264:5343–5351. [PubMed] [Google Scholar]
- Morita T, Saitoh K, Takagi T, Maeda Y. Involvement of the glucose-regulated protein 94 (Dd-GRP94) in starvation response of Dictyostelium discoideum cells. Biochem Biophys Res Commun. 2000;274:323–331. doi: 10.1006/bbrc.2000.3096. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–290. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- Ritossa FM, Vonborstel RC. Chromosome puffs in Drosophila induced by ribonuclease. Science. 1964;145:513–514. doi: 10.1126/science.145.3631.513. [DOI] [PubMed] [Google Scholar]
- Stephen RS, Mark DC, Patricia LM, Judith MG, Roy LW. Dietary energy restriction in mice reduces hepatic expression of glucose-regulated protein 78 (BiP) and 94 mRNA. Nutrition and Gene Expr. 1990;120:1412–1417. doi: 10.1093/jn/120.11.1412. [DOI] [PubMed] [Google Scholar]
- Street TO, Lavery LA, Agard DA. Substrate binding drives large-scale conformational changes in the Hsp90 molecular chaperone. Mol Cell. 2011;42:96–105. doi: 10.1016/j.molcel.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subjeck JR, Shyy TT. Stress protein systems of mammalian cells. Am J Physiol. 1986;250:C1–C17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoraki MA, Caplan AJ. Quality control and fate determination of Hsp90 client proteins. Biochim Biophys Acta. 2012;1823:683–688. doi: 10.1016/j.bbamcr.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu Y, Denell RE. Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Dev Genes Evol. 2004;214:575–578. doi: 10.1007/s00427-004-0434-0. [DOI] [PubMed] [Google Scholar]
- Tramentozzi E, Zamarchi R, Pagetta A, Brunati AM, Rossi E, Tibaldi E, Finotti P. Effects of glucose-regulated protein94 (Grp94) on Ig secretion from human blood mononuclear cells. Cell Stress Chaperones. 2011;16:329–338. doi: 10.1007/s12192-010-0245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CX, Zhao FY, Zhang Y, Zhu YJ, Ma MS, Mao HL, Hu CY. Overexpression of Hsp90 from grass carp (Ctenopharyngodon idella) increases thermal protection against heat stress. Fish Shellfish Immunol. 2012;33:42–47. doi: 10.1016/j.fsi.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Xu J, Shu J, Qiu X, Wang Z, Zhao F, Zhang Z, Zhang Q. Effects of heat shock on ovary development and hsp83 expression in Tribolium castaneum (Coleoptera: Tenebrionidae) Arch Insect Biochem Physiol. 2009;70:204–216. doi: 10.1002/arch.20294. [DOI] [PubMed] [Google Scholar]
- Xu J, Shu J, Zhang Q. Expression of the Tribolium castaneum (Coleoptera: Tenebrionidae) hsp83 gene and its relation to oogenesis during ovarian maturation. J Genet Genomics. 2010;37:513–522. doi: 10.1016/S1673-8527(09)60071-0. [DOI] [PubMed] [Google Scholar]
- Xu PJ, Xiao JH, Xia QY, Murphy B, Huang DW. Apis mellifera has two isoforms of cytoplasmic HSP90. Insect Mol Biol. 2010;19:593–597. doi: 10.1111/j.1365-2583.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX 14.6 kb)
(XLSX 13 kb)
(XLSX 16 kb)
(XLSX 15 kb)
(PDF 371 kb)