Abstract
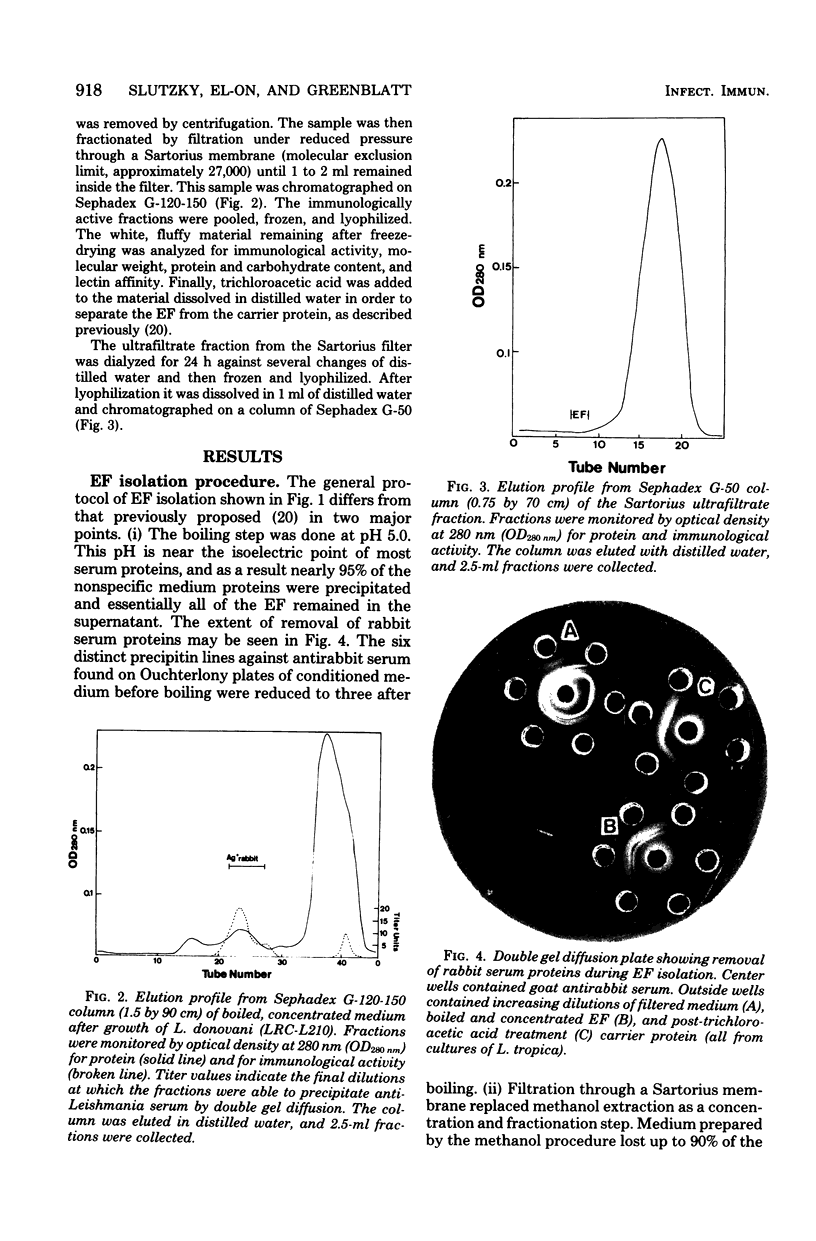
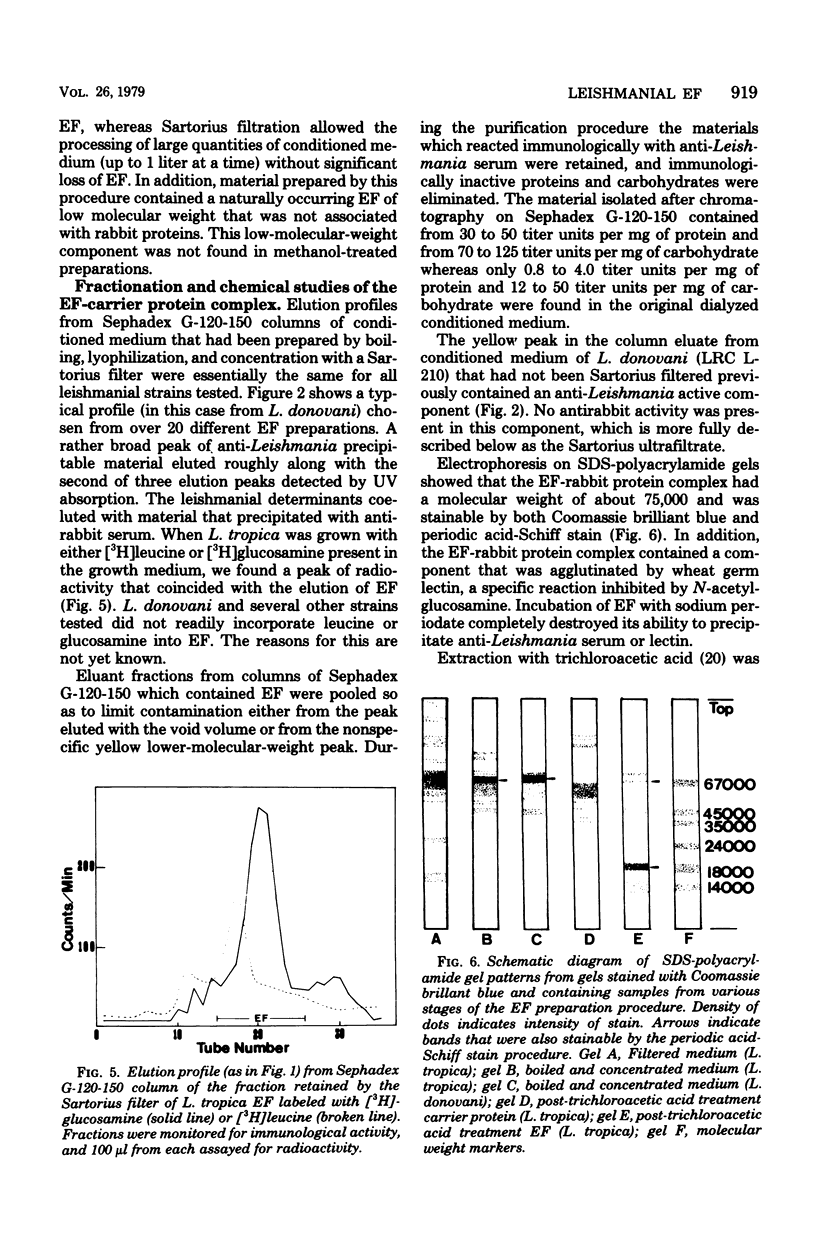
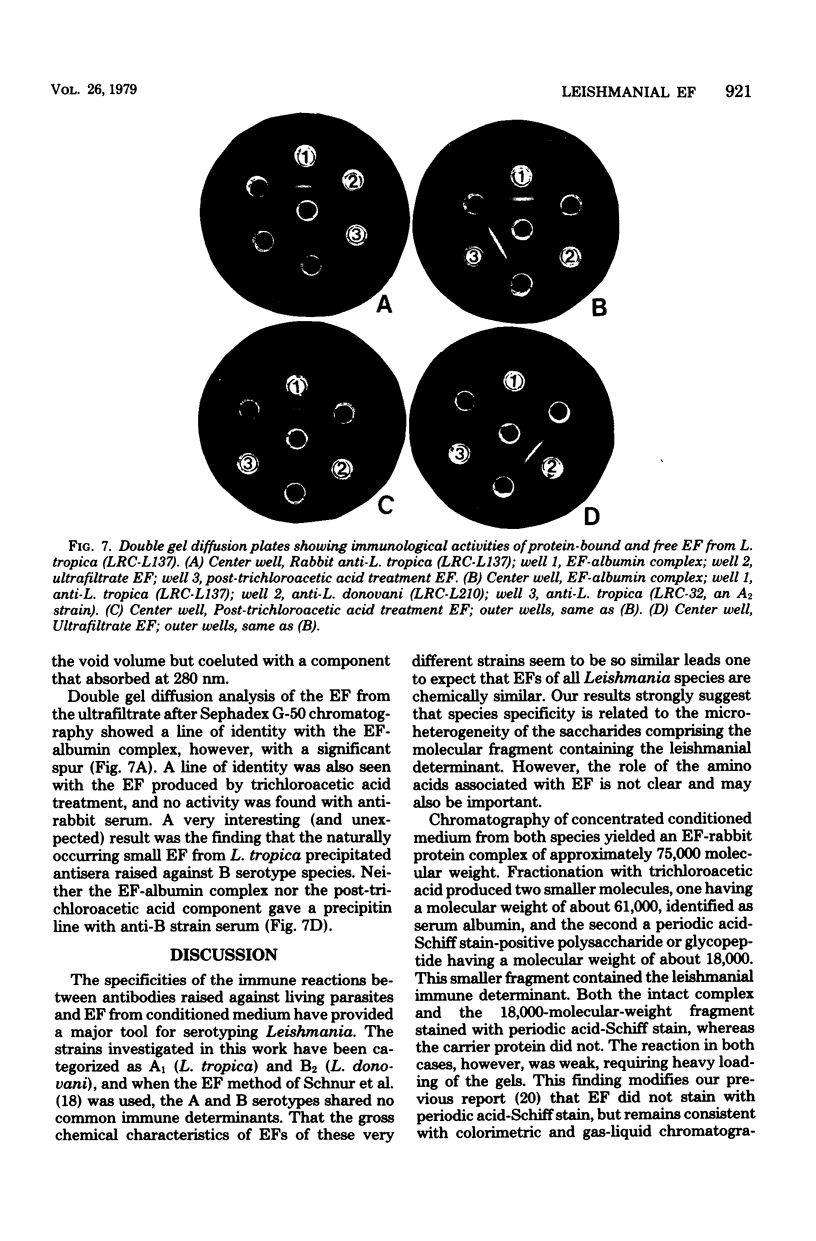
Leishmania spp. growing in culture produce an immunologically active substance called excreted factor (EF), which precipitates antibodies raised against intact cells and has been implicated as the conditioning agent for parasite infection of host macrophages. An improved method for isolation of the material is described, based on Sephadex column chromatography of growth medium which had been boiled at pH 5.0. This procedure allows the detection of differences among the EF molecules of different species, and it overcomes previous shortcomings through the monitoring of immunological activity throughout. Analysis of the products of this procedure revealed that EFs from Leishmania tropica and Leishmania donovani share a common carrier protein, identified as rabbit serum albumin, and are chemically quite similar. Growth medium from L. tropica boiled at acidic pH contains primarily an EF-albumin complex of 75,000 molecular weight. Treated growth medium from L. donovani, on the other hand, contains both the albumin complex and a smaller molecule (less than 27,000 molecular weight) that is not associated with rabbit protein. This material accounts for nearly 20% of the EF of one L. donovani strain, but constitutes only a minute fraction of L. tropica EF. Treatment of the EF-albumin complex with trichloroacetic acid separates the molecule into two major subunits, one having a molecular weight of about 61,000 (without anti-Leishmania activity) and the other having a molecular weight of about 18,000 (with no anti-rabbit activity). The protein-free EF of L. tropica differs from that released by trichloroacetic acid extraction in that it is capable of precipitating antisera of nonhomologous serotypes, whereas the albumin complex and the trichloroacetic acid-treated EF fragment are not. EFs from both species display pH-dependent affinity for certain lectins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buonassisi V., Colburn P. Interactions of sulfated mucopolysaccharides with lectins. Application to the separation of mucopolysaccharide mixtures. Arch Biochem Biophys. 1977 Oct;183(2):399–407. doi: 10.1016/0003-9861(77)90374-5. [DOI] [PubMed] [Google Scholar]
- CHARLWOOD P. A. Ultracentrifugal studies of rat, rabbit and guinea-pig serum albumins. Biochem J. 1961 Jan;78:163–172. doi: 10.1042/bj0780163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- El-On J., Schnur L. F., Greenblatt C. L. Leishmania donovani: physicochemical, immunological, and biological characterization of excreted factor from promastigotes. Exp Parasitol. 1979 Apr;47(2):254–269. doi: 10.1016/0014-4894(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L. Promotion of leishmanial infections in non-permissive host macrophages by conditioned medium. Z Parasitenkd. 1977 Sep 21;53(2):143–147. doi: 10.1007/BF00380458. [DOI] [PubMed] [Google Scholar]
- Janado M., Azuma J., Onodera K. Reversible aggregation of a human erythrocyte membrane glycoprotein. J Biochem. 1973 Nov;74(5):881–887. [PubMed] [Google Scholar]
- Kahane I., Razin S. Cholesterol-phosphatidylcholine dispersions as donors of cholesterol to Mycoplasma membranes. Biochim Biophys Acta. 1977 Nov 15;471(1):32–38. doi: 10.1016/0005-2736(77)90390-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A. Leishmanial excreted factor (EF) serotypes in Sudan, Kenya and Ethiopia. Ann Trop Med Parasitol. 1977 Sep;71(3):273–294. doi: 10.1080/00034983.1977.11687191. [DOI] [PubMed] [Google Scholar]
- Slutzky G. M., Greenblatt C. L. Analysis by SDS-polyacrylamide gel electrophoresis of an immunologically active factor of Leishmania tropica from growth media, promastigotes, and infected macrophages. Biochem Med. 1979 Feb;21(1):70–77. doi: 10.1016/0006-2944(79)90057-7. [DOI] [PubMed] [Google Scholar]
- Slutzky G. M., Greenblatt C. L. Isolation of a carbohydrate-rich immunologically active factor from cultures of Leishmania tropica. FEBS Lett. 1977 Aug 15;80(2):401–404. doi: 10.1016/0014-5793(77)80485-7. [DOI] [PubMed] [Google Scholar]