Abstract
Accumulation of excess hepatic lipids contributes to insulin resistance and liver disease associated with endoplasmic reticulum (ER) stress. Exendin-4 is an agonist of the glucagon-like peptide 1 receptor and plays a role in improving insulin resistance and liver disease by increasing silent mating type information regulation 2 homolog (SIRT) 1. However, the effects and mechanism of action of exendin-4 on responses to palmitic acid (PA)-induced ER stress in hepatocytes have not been clearly defined. We investigated whether exendin-4 attenuates PA-induced ER stress via SIRT1 in HepG2 cells. PA treatment induced increased expression of PRKR-like endoplasmic reticulum kinase, inositol-requiring kinase 1α (IRE1α), activating transcription factor 6 (ATF6), and C/EBP homologous protein (CHOP) mRNA. Exendin-4 decreased the expression of P-IRE1α, ATF6, X-box binding protein-1 and CHOP, and increased the expression of SERCA2b. A significant decrease in the hepatic expression of PUMA, BAX, cytochrome c, and cleaved caspase-3 were observed in hepatocytes treated with exendin-4. The TUNEL assay consistently showed that exendin-4 reversed hepatocyte apoptosis induced by treatment with PA. Inhibition of SIRT1 by nicotinamide and siRNA significantly increased the expression of ER stress marker genes in cells treated with both PA and exendin-4. In conclusion, increased SIRT1 by exendin-4 attenuates PA-induced ER stress and mitochondrial dysfunction in hepatocytes.
Keywords: Exendin-4, SIRT1, ER stress, Mitochondrial dysfunction, Apoptosis, Hepatocyte
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of chronic diseases ranging from fatty liver (hepatic steatosis) to fibrosis or cirrhosis and is linked to obesity and type 2 diabetes (Clark and Diehl 2002; Festi et al. 2004). Hepatic steatosis is typically associated with an overload of free fatty acids (FFAs). Unlike unsaturated fatty acids (e.g., oleate and linoleate), saturated fatty acids (e.g., palmitate and stearate) induce insulin resistance through mitochondrial dysfunction and impaired glucose metabolism (Hirabara et al. 2010) and cause cell apoptosis (Ricchi et al. 2009).
Excessive lipid accumulation in hepatocytes is involved with lipid metabolism and inflammation. Endoplasmic reticulum (ER) stress is involved in lipogenesis, insulin resistance, and apoptosis in fatty acid-induced hepatic steatosis (Liu et al. 2010; Flamment et al. 2010; Eizirik et al. 2008). ER stress is induced by the accumulation and aggregation of unfolded proteins due to stresses that disturb the cellular energy levels, the redox state, or Ca2+ concentration, leading to the unfolded protein response (UPR) pathway (Szegezdi et al. 2006).
The UPR initially restores normal ER function by increasing the production of chaperone glucose-regulated protein 78 kDa (GRP78), also known as Bip, which activates the UPR signaling molecules inositol-requiring 1α (IRE1α), pancreatic ER kinase (PERK), and activating transcription factor 6 (ATF6). If cellular damage is too severe, IRE1α, PERK, and ATF6 lead to apoptosis by activating downstream apoptosis genes, including C/EBP homologous protein (CHOP), JNK, caspase, and the Bcl2 family (Ron and Walter 2007; Akazawa et al. 2010). Recent studies have shown that palmitic acid increases the activity of UPR-associated genes CHOP, GRP78, and GRP94 and caspase-3 in primary rat hepatocytes (Zhang et al. 2011). Enhanced ER stress has been observed in the liver and adipose tissue of patients with NAFLD (Gentile et al. 2011).
We recently reported that exendin-4, a GLP-1 receptor agonist, improved steatohepatitis by increasing SIRT1 in mice fed with a high-fat diet (Lee et al. 2012). Overexpression of SIRT1 in the liver protects against ER stress and insulin resistance in obese LDLR−/− mice (Li et al. 2011). In this study, we investigated whether the protective effects of exendin-4 on ER stress are mediated via SIRT1 in hepatocytes.
Materials and methods
Cell culture and treatment
The HepG2 and Huh7 human hepatoma cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and Korean Cell Line Bank (KCLB, Seoul, Korea) and cultured in 6-well plates in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) containing 10 % (v/v) fetal bovine serum and 1 % (v/v) penicillin/streptomycin. Palmitic acid (PA) (Sigma-Aldrich, St. Louis, MO, USA), oleic acid (OA) (Sigma-Aldrich, St. Louis, MO, USA), and tunicamycin (Tuni) (Sigma-Aldrich, St. Louis, MO, USA) were used to induce ER stress in cultured hepatocytes. To examine the effects of exendin-4 on ER stress, cells were supplemented with media alone or media containing exendin-4 (100 nM) (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Nicotinamide (Fluka AG, Buchs SG, Switzerland) and exendin fragment 9-39 (Sigma-Aldrich, St. Louis, MO, USA) were used as a SIRT1 inhibitor and a GLP-1 receptor antagonist, respectively.
Transfection
For the gene knockdown approach, the specific siRNAs of SIRT1 (Bioneer, Daejeon, Korea) and negative control (Invitrogen, Carlsbad, CA, USA) were purchased. HepG2 cells were cultured in a 6-well plate at 2.5 × 105 cells/well with DMEM supplemented with 10 % (v/v) FBS without antibiotics. Transfection of siRNA into HepG2 cells was carried out using Lipofectamine RNAiMAX reagent, according to manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and incubated at 37 °C for 24 h.
Apoptosis
Cell death was quantified using the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) assay (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Chemicon, Temecula, CA, USA) according to the manufacturer’s instructions. Apoptotic hepatocytes were examined by light microscopy (magnification, ×400) and stained dark brown. The apoptotic index was calculated as the percentage of TUNEL-positive cells by the following formula: apoptotic cells (%)= (number of TUNEL positive cells/total number of cells) × 100.
Total RNA isolation and real-time RT-PCR
Total RNA was isolated from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and complementary DNA was prepared and amplified by reverse transcribing 2 μg of total RNA with moloney murine leukemia virus reverse transcriptase (MMLV-RT) and oligo (dT) 12-18 primer (Invitrogen, Carlsbad, CA, USA) as described previously (Lee et al. 2012). Synthesized cDNA was amplified by real-time PCR (Light-Cycler 480; Roche, Lewis, UK) using SYBR green (Invitrogen, Carlsbad, CA, USA) and specific primers (Bioneer Co., Daejeon, Korea) according to the manufacturer’s instructions. The cycle conditions were as follows: denaturation at 94 °C for 15 s, annealing at 55 °C for 10 s, and extension at 72 °C for 20 s. To analyze the expression of the target genes, the comparative Ct method (2-delta delta Ct) was used, with β-actin (Actb) as an internal control.
Western blot analysis
For Western blot analysis, hepatocytes were homogenized at 4 °C in RIPA buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) supplemented with a cocktail of protease and phosphatase inhibitors. Protein was obtained by centrifugation at 13,000 rpm for 20 min at 4 °C, and the protein concentration was quantified using the Bradford protein assay (Bio-Rad Protein Assay, BioRad, Hercules, CA) with bovine serum albumin standard (Thermo Scientific, Rockford, IL, USA). Extraction of nuclear and cytoplasmic proteins was performed using a nuclear extraction kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer’s instructions. Equal amounts of protein were separated on 4–12 % (w/v) bis-Tris Nupage gels (Invitrogen, Carlsbad, CA, USA) and transferred to polyvinylidene difluoride membranes (GE Healthcare, Chalfont St. Giles, UK). After transferring, the membranes were incubated overnight at 4 °C with antibodies against PERK ([H-300], sc-13073; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), P-PERK ([Thr 981], sc-32577; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), IRE1α (#3294; Cell Signaling Technology, Danvers, MA, USA), P-IRE1α (ab104157; Abcam, Cambridge, MA, USA), ATF6 ([H-280], sc-22799; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), XBP1 ([M-186], sc-7160; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CHOP (#2895; Cell Signaling Technology, Danvers, MA, USA), SERCA2 (#4388; Cell Signaling Technology, Danvers, MA, USA), PUMA (#4976; Cell Signaling Technology, Danvers, MA, USA), BAX (#5023; Cell Signaling Technology, Danvers, MA, USA), cytochrome C (#11940; Cell Signaling Technology, Danvers, MA, USA), Cleaved caspase-3 (#9664; Cell Signaling Technology, Danvers, MA, USA), caspase-3 (#9665; Cell Signaling Technology, Danvers, MA, USA), GLP-1R ([H-55], sc-66911; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), SIRT1 ([H-300], sc-15404; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and β-actin (#4967; Cell Signaling Technology, Danvers, MA, USA) followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Antibody–antigen complexes were visualized with enhanced chemiluminescence Western blotting detection reagents (Invitrogen, Carlsbad, CA, USA).
Statistical analysis
All statistical analyses were performed using SPSS 12.0 software (SPSS Inc., Chicago, IL, USA). Results are expressed as the mean ± SEM. For evaluation of the significant differences between the mean values in the different experimental groups, the one-way analysis of variance test or two-tailed Student’s t test was used. p < 0.05 was considered significant.
Results
Effects of exendin-4 on the expression of genes associated with unfolded protein response and calcium homeostasis
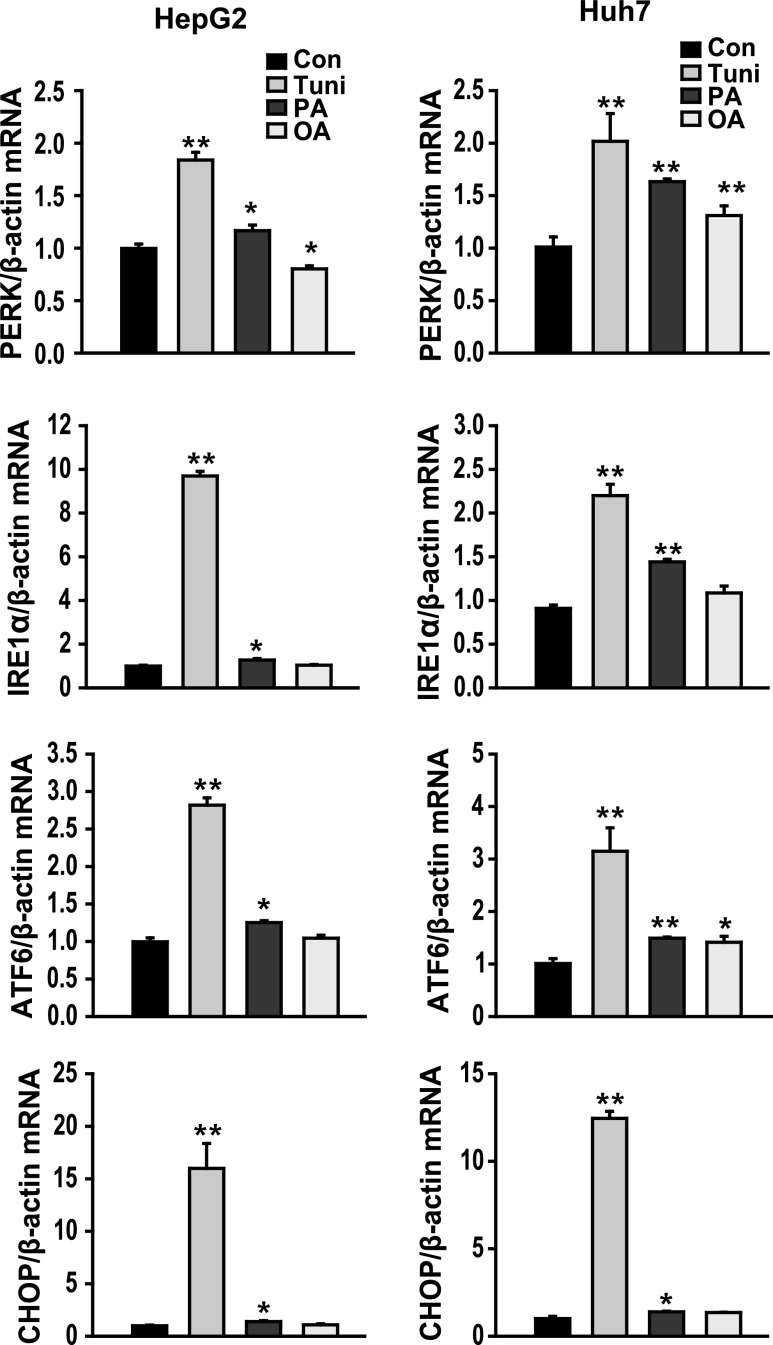
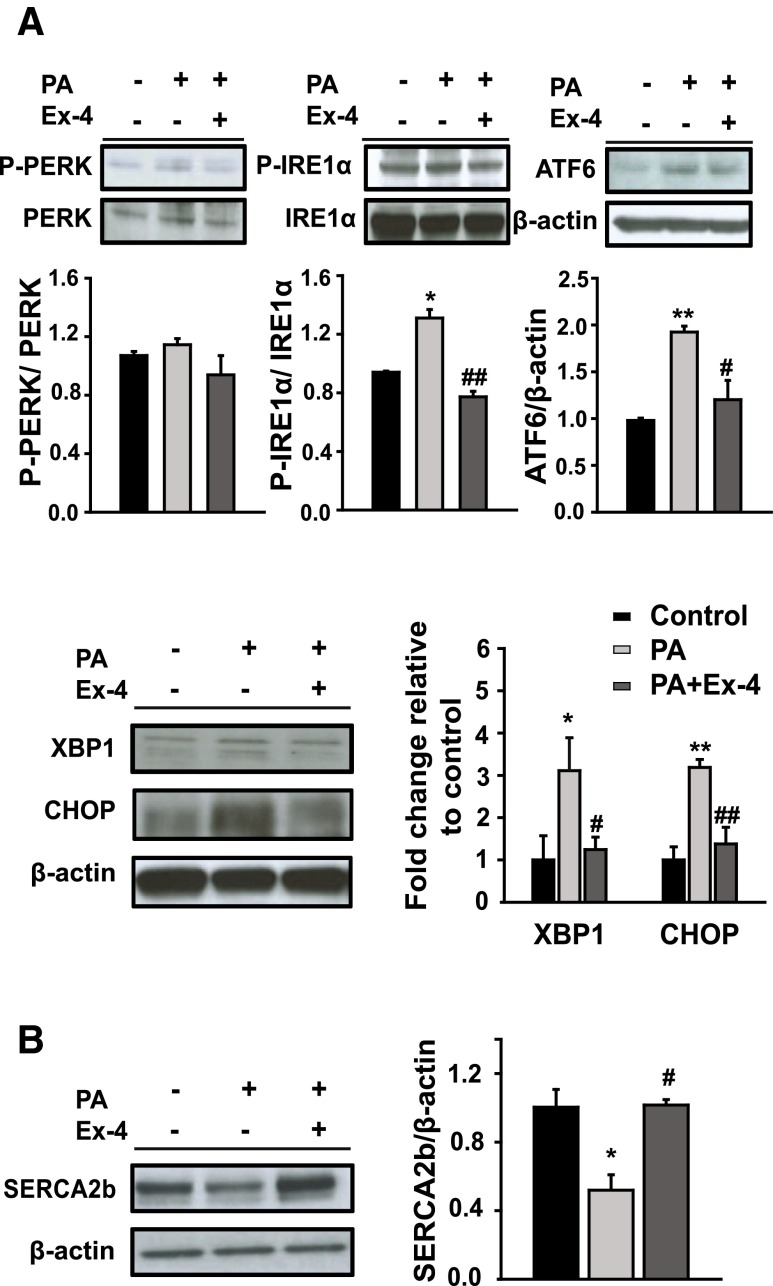
Characterization of FFA-induced hepatic steatosis is associated with increased liver injury, markers of ER stress, and cell death (Wang et al. 2006). As shown in Fig. 1, PA, but less than a positive control (tunicamycin), increased the expression of PRKR-like endoplasmic reticulum kinase (PERK), IRE1α, ATF6, and CHOP mRNA in both HepG2 and huh7 cell lines. No change on the expression of ER stress marker genes was observed between the cells treated with oleic acid (an unsaturated FFA) and untreated HepG2 cells (control). To investigate whether exendin-4 (Ex-4) regulates UPR signaling under conditions of ER stress induced by increased saturated fatty acids, HepG2 cells were treated with PA in the absence or presence of Ex-4. No change in the expression of P-PERK was observed between in the cells treated with PA and PA + Ex-4. The expression of P-IRE1α, ATF6, X-box binding protein 1 (XBP1), and CHOP proteins was increased in the cells treated with PA alone compared to the untreated controls and significantly decreased in the cells treated with Ex-4 compared to the cells treated with PA (Fig. 2a). In contrast, the expression of SERCA2, which is associated with calcium uptake from the cytosol to the endoplasmic reticulum, was increased in the cells treated with Ex-4 compared to the cells treated with PA (Fig. 2b). These results suggest that palmitic acid, a saturated FFA, induces ER stress in hepatocytes and that exendin-4 can reduce the negative effects of ER stress.
Fig. 1.
PA induces ER stress in cultured hepatocytes. HepG2 and Huh7 cells were treated with 400 μM palmitic acid (PA), 400 μM oleic acid (OA), or 2 μg/ml tunicamycin (Tuni) for 24 h. The expression of PERK, IRE1α, ATF6, and CHOP mRNA were analyzed using quantitative real-time RT-PCR. Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01 compared with the control
Fig. 2.
Exendin-4 ameliorates ER stress and calcium homeostasis in HepG2 cells treated with PA. HepG2 cells were exposed to PA (400 μM) and treated with or without Ex-4 (100 nM) for 24 h. The protein expression of a ER stress pathway, P-PERK, P-IRE1α, ATF6, XBP1, and CHOP and b sarco(endo)plasmic reticulum calcium ATPase SERCA2b were analyzed using Western blot and normalized based on the β-actin control. Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01 compared with the control and # p < 0.05, ## p < 0.01 compared with PA
Effects of exendin-4 on mitochondrial dysfunction and apoptosis
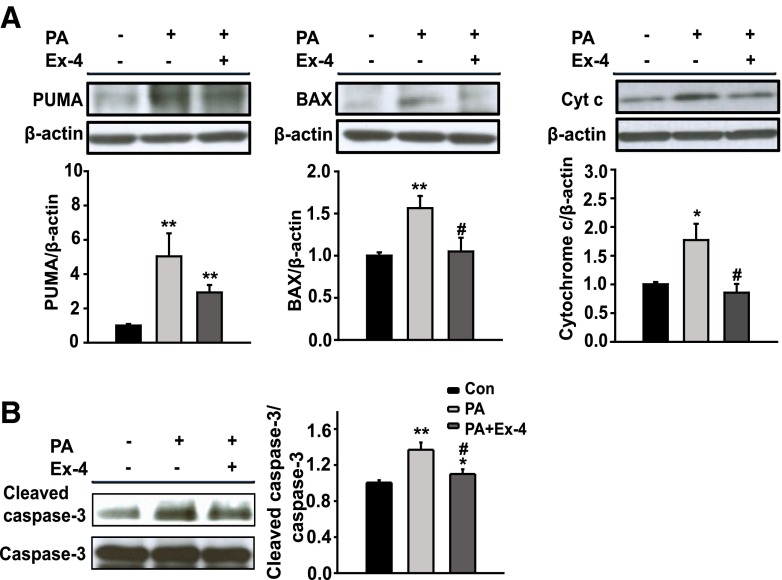
To investigate the effects of exendin-4 on mitochondrial dysfunction and apoptosis associated with ER stress, the expression of members of the proapoptotic Bcl2 family, cytochrome C, and caspase-3 protein were measured. As shown in Fig. 3a, the p53-upregulated modulator of apoptosis (PUMA), Bcl2-associated X protein (BAX), and cytochrome C were increased in the cells treated with PA compared to the untreated controls, whereas BAX and cytochrome C were decreased in the cells treated with Ex-4 compared to the cells treated with PA. No significant change in the expression level of PUMA was observed between the cells treated with PA and PA + Ex-4. In addition, lower expression levels of cleaved caspase-3 were observed in the cells treated with Ex-4 (Fig. 3b), and a similar trend was observed using TUNEL assay analysis, suggesting that exendin-4 inhibits apoptosis (Fig. 4). These results suggest that exendin-4 can reverse the PA-induced mitochondrial dysfunction and apoptosis.
Fig. 3.
Exendin-4 decreases mitochondrial dysfunction and apoptosis-related gene expression. HepG2 cells were exposed to PA (400 μM) and treated with or without Ex-4 (100 nM) for 24 h. a, b Protein expression of PUMA, BAX, cytochrome c, and cleaved caspase-3 were analyzed by Western blot. PUMA, BAX, and cytochrome c were normalized based on the β-actin control, and cleaved caspase-3 was normalized to the total caspase-3 of each sample. Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01 compared with the control and # p < 0.05, ## p < 0.01 compared with PA
Fig. 4.
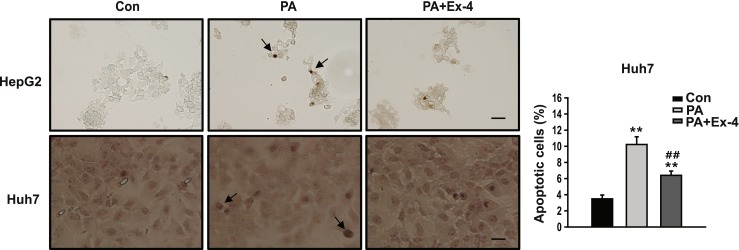
Exendin-4 improves PA-induced apoptosis. DNA fragments were detected using the TUNEL assay in HepG2 and Huh7 cell lines. TUNEL-positive cells were stained dark brown (×400 magnification, scale bars = 50 μm) and apoptotic cell death was measured as mean percentage of TUNEL-positive cells
The effects of exendin-4 on ER stress are mediated by SIRT1
To investigate the protective effect of exendin-4 against ER stress is mediated by SIRT1, HepG2 cells were treated with Ex-4, a GLP-1receptor agonist, in the absence or presence of exendin fragment 9-39 [Ex(9-39)], a GLP-1 receptor antagonist. As shown in Fig. 5a, the expression of SIRT1 was increased in the cells treated with Ex-4 compared to the untreated controls and was decreased in the cells treated with both Ex-4 and Ex(9-39) compared to the cells treated with Ex-4. In the absence of Ex-4, increased mRNA expression of IRE1α, PERK, and ATF6 and their target genes XBP1, GRP78/BiP, ER degradation-enhancing alpha-mannosidase-like 1 (EDEM1), growth arrest and DNA damage (GADD34), and CHOP were observed in the cells treated with PA compared to the untreated controls (Fig. 5b). These were significantly decreased in the cells treated with Ex-4. However, the expression levels of the ER stress marker genes were similar to the levels observed in the cells treated with PA after addition of nicotinamide (NAM), a known inhibitor of SIRT1, to cultured cells with both PA and Ex-4 (Fig. 5b). In addition, SIRT1 deficiency by siRNA in the cells treated with PA and PA + Ex-4 also dramatically increased the protein expression of ATF6, XBP1, and CHOP and the expression of P-PERK and P-IRE1α increased in SIRT1 siRNA-transfected cell treated both with PA and Ex-4 compared to untreated controls (Fig. 5c). These results suggest that the protective effect of exendin-4 against PA-induced ER stress is mediated by SIRT1.
Fig. 5.
Exendin-4 attenuates ER stress via SIRT1. a HepG2 cells were pretreated with 50 nM exendin fragment 9-39 [Ex (9-39)], a GLP-1receptor antagonist, for 6 h and were then treated with exendin-4, a GLP-1 receptor agonist, for 24 h. The expression of GLP-1R and SIRT1 protein was measured by Western blot. b HepG2 cells were exposed to PA (400 μM) and treated with or without Ex-4 (100 nM) for 24 h. Nicotinamide (NAM) was added to the cells treated with both PA and Ex-4. Inositol-requiring 1α (IRE1α), activating transcription factor 6 (ATF6), pancreatic ER kinase (PERK), X-box binding protein 1 (XBP1), ER degradation-enhancing alpha-mannosidase-like 1 (EDEM1), glucose-regulated protein 78 kDa (GRP78/BiP), C/EBP homologous protein (CHOP), and growth arrest and DNA damage (GADD34) were measured by quantitative real-time RT-PCR and normalized based on the β-actin control. Data are expressed as the mean ± SEM. *p < 0.05, **p < 0.01 compared with the control, + p < 0.05, ++ p < 0.01 compared with PA, and # p < 0.05, ## p < 0.01 compared with PA + Ex-4. c Cells were transfected with 10 nM siRNA against SIRT1 for 24 h and were then exposed to PA (400 μM) and treated with or without Ex-4 (100 nM) for 24 h. The expression of P-PERK, P-IRE1α, ATF6, XBP1, and CHOP protein was measured by Western blot
Discussion
In this study, exendin-4 treatment resulted in reduced ER stress, mitochondrial dysfunction, and apoptosis-associated gene expression in hepatocytes treated with palmitic acid. We demonstrate for the first time that the actions of exendin-4 are mediated via SIRT1, which is associated with insulin sensitivity and improvement of hepatic steatosis.
Disrupted lipid metabolism can cause ER stress in the liver, which has been implicated in the development and progression of metabolic diseases (Fu et al. 2011; Ozcan and Tabas 2012). We proposed that unlike oleic acid, an unsaturated fatty acid, palmitic acid, a saturated fatty acid, induces ER stress in hepatocytes, and it was confirmed through a comparison of the mRNA expression level of PERK, IRE1α, ATF6, and CHOP, which is involved in endoplasmic reticulum stress-induced apoptosis. Exendin-4, a GLP1 receptor agonist, significantly affects glucose and insulin sensitivity and control of lipid metabolism in peripheral tissue (Lee et al. 2007) and has protective effects against ER stress in the pancreatic β-cells (Tsunekawa et al. 2007; Xiang et al. 2012). In this study, we showed that the expressions of P-IRE1α, ATF6, XBP1, and CHOP were decreased by exendin-4 treatment in hepatocytes treated with palmitic acid, suggesting that GLP-1 might act on ER stress-associated cell death in hepatocytes.
Alterations in ER calcium homeostasis are associated with ER stress and apoptosis. The ER enzymes and chaperones require high calcium levels for protein folding activity (Ashby and Tepikin 2001; Araki and Nagata 2011) and release of calcium from the ER induce mitochondrial-mediated apoptosis (Jeong and Seol 2008). Sarco(endo)plasmic reticulum Ca2+−ATPase (SERCA) is an enzyme that function in translocation of Ca2+ from the cytosol into the ER lumen and has three isoforms (SERCA1-3). SERCA2b in the liver is the main isoform of SERCA2, and the decrease of SERCA2b was shown in the liver of high-fat diet-fed obese mice and ob/ob mice (Park et al. 2010; Vangheluwe et al. 2005). In the present study, we showed that SERCA2b expression was decreased by palmitic acid treatment and reversed by exendin-4 treatment. This result suggests that one of diverse effects of exendin-4 is to maintain an ER homeostasis in hepatocytes.
ER stress plays a role in mitochondrial dysfunction-induced apoptosis via regulation of the apoptosis-associated Bcl-2 family of proteins that localize on the mitochondrial membrane (Lee et al. 2010; Leem and Koh 2012). There are reports that B cell lymphoma-2 (Bcl-2) proteins act as proapoptotic (e.g., BAX, BAD, BAK, and BIK) or antiapoptotic (e.g., BCL-2 proper, BCL-XL, and BCL-w) regulators to influence mitochondrial function (Wang et al. 1996; Certo et al. 2006). The upregulation of PUMA and Bim was observed in hepatocytes with palmitate-induced lipoapoptosis (Akazawa et al. 2010). Similarly, the current study found that exendin-4 inhibits the expression of PUMA and BAX, leading to inhibition of cytochrome C and caspase-3. These data suggest that the protective effects of exendin-4 on ER stress-induced cell death might be linked to the recovery of mitochondrial function.
SIRT1 is a NAD+-dependent deacetylase and is an important regulator of energy homeostasis in response to nutrient deprivation (Cohen et al. 2004; Nemoto et al. 2004). In a previous study, we observed that increased SIRT1 by treatment with exendin-4 regulated hepatic lipid metabolism and led to improvement of steatohepatitis in diet-induced obese mice (Lee et al. 2012). The protective effects of SIRT1 on ER stress have already been demonstrated. Jung et al. reported that SIRT1 attenuates palmitate-induced ER stress and insulin resistance by increasing the levels of ORP150, an ER stress protein, via FOXO1 in hepatocytes (Jung et al. 2012a). In this study, we showed that inhibition of SIRT1 by nicotinamide, an inhibitor of SIRT1, and by siRNA increased the expression of ER stress marker genes that were reduced by treatment with exendin-4, indicating that inhibition of SIRT1 induces ER stress. In addition, increased expression of ER stress marker genes was also observed in cells transfected with SIRT1 siRNA alone. However, we showed a decrease of SIRT1expression by exendin fragment 9-39 (GLP-1 receptor antagonist) treatment to certify that sirt1 is regulated by GLP-1 (Fig. 5). These results suggest the potential role of SIRT1 in mediating the effects of exendin-4.
Although it is unclear how increased SIRT1 by exendin-4 reduces the expression of ER stress marker genes, the activation of AMP-activated protein kinase (AMPK), a key sensor of cellular energy status, appears to be important (Hou et al. 2008). SIRT1 is an upstream factor of AMPK in hepatocytes, but not in skeletal muscle (Lee et al. 2012; Hou et al. 2008; Canto et al. 2009). Metformin, an antidiabetic drug that stimulates phosphorylation of AMPK, has been shown to reduce ER stress-induced apoptosis in both hepatocytes and pancreatic beta cells (Kim et al. 2010; Jung et al. 2012b). AMPK inhibits palmitate-induced cell apoptosis by suppressing the generation of reactive oxygen species associated with mitochondrial dysfunction and ER stress (Kim et al. 2008; Hayashi et al. 2005; Maria et al. 2012). However, these findings will require additional studies to draw any firm conclusions.
In conclusion, this study suggests that treatment with exendin-4 may attenuate palmitic acid-induced ER stress and mitochondrial dysfunction through a SIRT1-dependent mechanism and may explain, at least in part, the pharmacological effects of exendin-4 in improving the outcome of patients with liver disease, including steatohepatitis.
Acknowledgments
This study was supported by a grant from Daewoong Pharmaceutical (# MRI-110907-004) and the Korea Science and Engineering Foundation by the Ministry of Education, Science and Technology # S-2010-1115-000 (http://www.mest.go.kr). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52(4):586–593. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Nagata K. Protein folding and quality control in the ER. Cold Spring Harb Perspect Biol. 2011;3(11):a007526. doi: 10.1101/cshperspect.a007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol. 2001;12(1):11–17. doi: 10.1006/scdb.2000.0212. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo MV, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diabetes Rep. 2002;2(3):210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29(1):42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev: Off J Int Assoc Study Obes. 2004;5(1):27–42. doi: 10.1111/j.1467-789X.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- Flamment M, Kammoun HL, Hainault I, Ferre P, Foufelle F. Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr Opin Lipidol. 2010;21(3):239–246. doi: 10.1097/MOL.0b013e3283395e5c. [DOI] [PubMed] [Google Scholar]
- Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile CL, Frye MA, Pagliassotti MJ. Fatty acids and the endoplasmic reticulum in nonalcoholic fatty liver disease. Biofactors. 2011;37(1):8–16. doi: 10.1002/biof.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metabol. 2005;25(1):41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- Hirabara SM, Curi R, Maechler P. Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol. 2010;222(1):187–194. doi: 10.1002/jcp.21936. [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283(29):20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41(1):11–22. doi: 10.5483/BMBRep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem Biophys Res Commun. 2012;422(2):229–232. doi: 10.1016/j.bbrc.2012.04.129. [DOI] [PubMed] [Google Scholar]
- Jung TW, Lee MW, Lee YJ, Kim SM. Metformin prevents endoplasmic reticulum stress-induced apoptosis through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun. 2012;417(1):147–152. doi: 10.1016/j.bbrc.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Kim JE, Kim YW, Lee IK, Kim JY, Kang YJ, Park SY. AMP-activated protein kinase activation by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) inhibits palmitate-induced endothelial cell apoptosis through reactive oxygen species suppression. J Pharmacol Sci. 2008;106(3):394–403. doi: 10.1254/jphs.FP0071857. [DOI] [PubMed] [Google Scholar]
- Kim DS, Jeong SK, Kim HR, Kim DS, Chae SW, Chae HJ. Metformin regulates palmitate-induced apoptosis and ER stress response in HepG2 liver cells. Immunopharmacol Immunotoxicol. 2010;32(2):251–257. doi: 10.3109/08923970903252220. [DOI] [PubMed] [Google Scholar]
- Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, Yoon JW, Jun HS. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56(6):1671–1679. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim WH, Yeo J, Jung MH. ER stress is implicated in mitochondrial dysfunction-induced apoptosis of pancreatic beta cells. Mol Cells. 2010;30(6):545–549. doi: 10.1007/s10059-010-0161-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Hong SW, Chae SW, Kim DH, Choi JH, Bae JC, Park SE, Rhee EJ, Park CY, Oh KW. Exendin-4 improves steatohepatitis by increasing Sirt1 expression in high-fat diet-induced obese C57BL/6J mice. PloS one. 2012;7(2):e31394. doi: 10.1371/journal.pone.0031394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem J, Koh EH. Interaction between mitochondria and the endoplasmic reticulum: implications for the pathogenesis of type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:242984. doi: 10.1155/2012/242984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, Donmez G, Li J, Luo Z, Walsh K, Guarente L, Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J: Off Publ Fed Am Soc Exp Biol. 2011;25(5):1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jin X, Yu CH, Chen SH, Li WP, Li YM. Endoplasmic reticulum stress involved in the course of lipogenesis in fatty acids-induced hepatic steatosis. J Gastroenterol Hepatol. 2010;25(3):613–618. doi: 10.1111/j.1440-1746.2009.06086.x. [DOI] [PubMed] [Google Scholar]
- Maria DA, de Souza JG, Morais KL, Berra CM, Zampolli HD, Demasi M, Simons SM, de Freitas SR, Chammas R, Chudzinski-Tavassi AM. A novel proteasome inhibitor acting in mitochondrial dysfunction, ER stress and ROS production. Investig New Drugs. 2012 doi: 10.1007/s10637-012-9871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306(5704):2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012;63:317–328. doi: 10.1146/annurev-med-043010-144749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+−ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107(45):19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricchi M, Odoardi MR, Carulli L, Anzivino C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni S, Lonardo A, Carulli N, Loria P. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunekawa S, Yamamoto N, Tsukamoto K, Itoh Y, Kaneko Y, Kimura T, Ariyoshi Y, Miura Y, Oiso Y, Niki I. Protection of pancreatic beta-cells by exendin-4 may involve the reduction of endoplasmic reticulum stress; in vivo and in vitro studies. J Endocrinol. 2007;193(1):65–74. doi: 10.1677/JOE-06-0148. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium. 2005;38(3–4):291–302. doi: 10.1016/j.ceca.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- Xiang JN, Chen DL, Yang LY. Effect of PANDER in betaTC6-cell lipoapoptosis and the protective role of exendin-4. Biochem Biophys Res Commun. 2012;421(4):701–706. doi: 10.1016/j.bbrc.2012.04.065. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang X, Shi H, Dong L, Bai J. Effect of alpha-linolenic acid on endoplasmic reticulum stress-mediated apoptosis of palmitic acid lipotoxicity in primary rat hepatocytes. Lipids Health Dis. 2011;10:122. doi: 10.1186/1476-511X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]