Abstract
In this study, we cloned a full-length cDNA of heat shock protein (HSP) gene of Apolygus lucorum (Meyer-Dür) [AlHSP90, KC109781] and investigated its expression in response to temperature and pesticide stresses. The open reading frame (ORF) of AlHSP90 is 2,169 bp in length, encoding a 722 amino acid polypeptide with a predicted molecular weight of 82.99 kDa. Transcriptional and translational expression profiles of AlHSP90 under extreme temperature or pesticide stresses were examined by fluorescent real-time quantitative PCR and Western blot. Results showed that the expression profiles of AlHSP90 protein were in high agreement with those of AlHSP90 RNA and indicated that AlHSP90 was not only an important gene for A. lucorum adults in response to extremely high temperature, but also involved in the resistance or tolerance to cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate, especially for female adults to emamectin benzoate and for male adults to cyhalothrin. Transcriptional results of AlHSP90 also confirmed that AlHSP90 was an important gene involved in the resistance or tolerance to both temperature and pesticide stresses. In addition, our study also revealed that ∼24 °C may be the suitable temperature range for A. lucorum survival, which is also confirmed by the results of the expression of AlHSP90, the nymph mortality, and the intrinsic rate of increase (rm) when A. lucorum is reared at six different temperatures. Therefore, these studies are significant in elucidating the AlHSP90 in response to temperature and pesticide stresses and would provide guidance for A. lucorum management with different pesticides or temperatures in fields.
Keywords: A. lucorum, HSP90, Pesticide, Extreme temperature, Temperature stress, Expression files
Introduction
Heat shock proteins (HSPs) belong to a protein superfamily and have been extensively studied in organisms (Feder and Hofmann 1999). HSPs are rapidly synthesized in response to a series of environmental stressors (Sonoda and Tsumuki 2007). HSPs are classified into several gene families according to their respective molecular weights, such as sHSP, HSP40, HSP60, HSP70, HSP90, and HSP100 (Feder and Hofmann 1999; Li and Srivastava 2004). Among all HSPs, HSP90 and HSP70 are the most conserved and abundant in cells and have thus been extensively studied (Taipale et al. 2010).
As an important member of the HSP protein family, the HSP90 family contains three domains: an N-terminal ATP-binding domain (25 kDa), a central connecting domain (35 kDa), and a C-terminal dimerization domain (12 kDa), which forms constitutive homodimers (Minami et al. 1994; Nemoto et al. 1995; Young et al. 2001). The HSP90 protein family comprises evolutionarily conserved abundant cytosolic proteins that have been involved in chaperone function, oncogenic transformation, cell cycle control, and antigen presentation. This family also functions in the structural folding and maintenance of the conformational integrity of various proteins, including several cellular signal transduction proteins (Csemely et al. 1998; Byrd et al. 1999; Graefe et al. 2002; Prodromou and Pearl 2003; Tsutsumi and Neckers 2007; Hao et al. 2010). HSP90 is also required for normal spermatogenesis, oogenesis, and embryogenesis in Drosophila spp. (Yue et al. 1999; Song et al. 2007; Pisa et al. 2009). Recent studies have confirmed that HSP90 has been thought to have evolutionarily conserved functions that contribute to oogenesis and compound eye development in the red flour beetle Tribolium castaneum (Xu et al. 2010; Knorr and Vilcinskas 2011). Furthermore, HSP90 messenger(m)RNA or protein have enhanced in response to heat shock (Sonoda et al. 2006; Benoit et al. 2009; Feng et al. 2010; Jiang et al. 2012; Wang et al. 2012; Li and Du 2013), heavy metal pollution (Sonoda et al. 2007; Shu et al. 2011; Wang et al. 2012), diapause (Tachibana et al. 2005), proteotoxic stresses (Jones et al. 2008), starvation stresses (Wang et al. 2012), and pesticide stresses (Feng et al. 2010; Sonoda and Tsumuki 2007).
In the past decade, transgenic Bacillus thuringiensis (Bt) cotton has been effectively used to control the cotton bollworm Helicoverpa armigera (Hübner) in China (Wu et al. 2008). However, recent frequent outbreaks of the non-target pest Apolygus lucorum (Meyer-Dür) have been reported after the wide commercialization of Bt cotton in northern China (Li et al. 2011). At present, A. lucorum is the dominant mirid species in cotton fields in China (Lu et al. 2010; Lu and Wu 2008), and calendar-based insecticide sprays were the sole management option for A. lucorum (Lu et al. 2007). We have previously studied the effects of different temperatures on A. lucorum development and reproduction. Results showed that the suitable temperature range for A. lucorum development and reproduction was ∼24 °C (Zhao et al. 2012). When A. lucorum nymph and adults were reared at 18 to 30 °C, the total survival rate of nymphs and total fecundity of female adults were significantly lower than those at other temperatures. In addition, the life span of A. lucorum adults reared at 30 °C is also significantly shorter than that at other temperatures (Zhao et al. 2012), which indicated that A. lucorum reared at 30 °C can face stronger temperature stresses than that at other temperatures within the range of 18 to 30 °C.
HSP90 is an important gene for arthropods and is used for defense against heat shock (Feng et al. 2010; Jiang et al. 2012; Wang et al. 2012; Li and Du 2013) and pesticide stresses (Feng et al. 2010; Sonoda and Tsumuki 2007). However, little is known about how HSP90 in A. lucorum responds to heat shock and pesticide stresses. Therefore, in this study we identified the full-length complementary(c)DNA of A. lucorum HSP90 (denoted as AlHSP90) and analyzed the expression pattern of AlHSP90 when A. lucorum were reared at six different temperatures. In addition, how regulated the expression of this gene of A. lucorum in response to heat shock and pesticide stresses and the transcriptional and translational expression profiles of AlHSP90 in A. lucorum under extreme temperature (4 and 40 °C) or pesticide stresses were also examined by fluorescent real-time quantitative PCR and Western blot.
Materials and methods
Collection, culture, and treatment of A. lucorum under different temperatures
A colony of A. lucorum was established from about 900 A. lucorum adults collected from the broad bean fields of Dafeng and Dongtai City, Jiangsu Province, China. The colony was reared on sautéed green beans in a light incubator at the temperatures of 25 ± 1 °C, RH 70 ± 5 % humidity, and photoperiod 12:12 (L/D).
The tested temperatures were set up at 18, 21, 24, 27, 31, and 33 °C, with 24 °C as the control. More than 400 eggs of A. lucorum were then reared at each aforementioned temperature. The collected A. lucorum samples were separated into nymph and adult stages. The nymph stage was divided into first, second, third, fourth, or fifth instars stage. Each stage had five A. lucorum nymphs with five replicates. Thus, a total of 25 nymphs were sampled for each nymph stage at each temperature.
Female and male adults were identified and reared in insect-rearing boxes after A. lucorum emergence, respectively. The adult stage of females/males were categorized based on new emergence (1 day old), pre-mating (5 days old), and post-mating (11 days old, a couple of male and female adult were placed in same insect-rearing boxes and obvious mating behaviors were observed). Each treatment sampled five female (male) A. lucorum adults with five replicates for a total of 25 female (male) adults were sampled at each stage or temperature. The total mortality rate of nymphs and the intrinsic rate of increase (rm) values of adults reared at each aforementioned temperature were also analyzed (Table 1) according to the methods reported by Zhao et al. (2012).
Table 1.
Total mortality rate of nymphs and intrinsic rate of increase (r m) in A. lucorum strains
| Temperatures | 18 °C | 21 °C | 24 °C | 27 °C | 30 °C | 33 °C |
|---|---|---|---|---|---|---|
| Total mortality rate of nymphs (%) | 28.04 | 18.71 | 17.03 | 23.14 | 41.02 | 52.00 |
| Intrinsic rate of increase (rm) | 0.0414 | 0.0645 | 0.0963 | 0.0931 | 0.0586 | 0.0486 |
Furthermore, more than 120 2-day-old female (male) A. lucorum adults were treated at extreme temperatures (4 and 40 °C) in insect-rearing boxes with sautéed green beans as host for 1 h. Same sample number of 2-day-old female (male) A. lucorum adults reared at 24 °C were set as controls. Five female (male) A. lucorum adults were collected each time after treatment, and this collection was repeated 12 times for a total of 60 female (male) adults at each temperature. These treatments were replicated two times, one was for real-time PCR, and the other was for Western blot.
Bioassay and pesticide treatment
The topical treatment technique reported by FAO (1980) and Chen et al. (2000) was adopted for the toxicity bioassay of cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate. About 95 % cyhalothrin and 98 % imidacloprid active ingredient (AI) were provided by Jiangsu Rotam Crop Sciences Ltd., about 95.5 % emamectin benzoate (AI) was provided by Hebei Veyong Bio-Chemical Co. Ltd., and 97 % chlorpyrifos was provided by Hubei Xianlong Co. Ltd. Over 30 2-day-old A. lucorum female (male) adults used as test insects were paralyzed by placing on ice just before application of each species of pesticide treatment. Serial dilutions of each insecticide in acetone were prepared, and a droplet (0.25 μL) was dispensed onto the middle-abdomen notum of the adult using a hand microapplicator (Burkard Manufacturing, Rickmansworth, UK). Sixty female or male adults were treated at each concentration, respectively. The same batches of A. lucorum adults reared at 24 °C and set as CK controls (Ct). And then, the same batches of Ct controls A. lucorum adults treated with acetone alone were set as Ac controls. A. lucorum adults that did not respond to stimulation from a writing brush were considered dead. Mortality was assessed 48 h after treatment. For bioassays, the LD50 values and slope of concentration mortality for each assay were estimated by probit analysis (Finney 1971) using POLO-PC software (LeOra Software 1987). Significant differences of LD50 values were determined by non-overlapping 95 % confidence limits.
For the expression profiles of AlHSP90 in A. lucorum induced by different pesticides with different doses, more than 30 2-day-old female (male) A. lucorum adults were treated with each aforementioned pesticide according to the log concentration probit (LCP) line. The LD50 and LD20 values of these pesticides were set as induction doses on A. lucorum adults, as shown in Table 2. Mortality was assessed 48 h after treatment. Surviving A. lucorum adults after pesticide treatments, Ct controls and Ac controls were collected and stored at −80 °C until use. These tests, in which A. lucorum adults were treated with each aforementioned pesticide (induction dose = LD50), Ct controls and Ac controls, were also performed two times, one is for real-time PCR, and the other is for Western blot.
Table 2.
Toxicity and induction dose of the four pesticides in female and male A. lucorum strains
| Pesticide | A. lucorum sexuality | LDP line | LD50 (ng/individual; 95 % confidence limit) |
Induction dose, LD50 and LD20
(ng/individual) |
|---|---|---|---|---|
| Chlorpyrifos | Female | y = 0.4608x + 4.9818 | 1.0954 (0.5747–2.0877) | LD50 = 1.0954; LD20 = 0.01633 |
| Male | y = 0.4431x + 5.1064 | 0.5753 (0.2910–1.1375) | LD50 = 0.5753; LD20 = 0.00725 | |
| Imidacloprid | Female | y = 0.5349x + 4.9540 | 1.2193 (0.6941–2.1418) | LD50 = 1.2193; LD20 = 0.03255 |
| Male | y = 0.5505x + 4.9744 | 1.1129 (0.6418–1.9299) | LD50 = 1.1129; LD20 = 0.03294 | |
| Cyhalothrin | Female | y = 0.5069x + 5.1539 | 0.4969 (0.2721–0.9074) | LD50 = 0.4969; LD20 = 0.01087 |
| Male | y = 0.4994x + 5.0805 | 0.6900 (0.3788–1.2568) | LD50 = 0.6900; LD20 = 0.01424 | |
| Emamectin benzoate |
Female | y = 0.5362x + 5.5420 | 0.0975 (0.0554–0.1718) | LD50 = 0.0975; LD20 = 0.00263 |
| Male | y = 0.5506x + 5.6171 | 0.0757 (0.0431–0.1330) | LD50 = 0.0757; LD20 = 0.00224 |
Similarly, the expression profiles of AlHSP90 in A. lucorum treated with both pesticide and temperature stresses were performed using the above methods, and except for A. lucorum that were treated with each aforementioned pesticide (induction dose = LD50), Ct controls and Ac controls were then reared at 33 °C.
Cloning and sequencing of AlHSP90
Four newly emerged A. lucorum adult samples were pulverized with a mortar and pestle. Total RNA was extracted using the TRIzol method (Invitrogen, San Diego, CA, USA) according to the manufacturer’s instructions. Poly (A+) RNA was then enriched from total RNA using a Qiagen mRNA Purification Kit (Qiagen, Valencia, CA, USA).
Double-stranded cDNA was produced from approximately 2 μg of (A+) RNA. The A. lucorum cDNA library was analyzed with cDNA from A. lucorum adults using an In-Fusion SMARTer™ direction cDNA Library Construction Kit (Clontech, Palo Alto, CA, USA) according to the manufacturer’s instructions. Subsequently, the cDNA library was ligated with a pSMART2IFD Linearized Vector (Clontech, Palo Alto, CA, USA). The constructed vector was transferred by electroporation into DH5α Escherichia coli cells (Invitrogen). Individual clones from A. lucorum newly emerged adult cDNA library were randomly collected and cultured on 96-well microtiter plates. The plasmids were used for PCR in 96-well PCR plates with forward and reverse screening primers. The primer sequences were synthesized according to the manufacturer’s instructions. Each PCR product was detected by agarose gel electrophoresis. After finishing PCR screenings, each positive plasmid longer than 500 bp was transferred to a new cuvette with LB liquid medium containing ampicillin (50 μg mL−1), incubated at 37 °C overnight and then sequenced in Sangon Biotech (Shanghai) Co., Ltd. EST sequences were used to search for similar HSP90 peptide sequences in public databases using the BlastX search algorithm. Consequently, a 1,524-bp A. lucorum HSP90 gene (AlHSP90) fragment from 1 bp to 1,524 bp of this 2,548 bp gene was obtained.
To ensure base pair accuracy and completeness of AlHSP90, two pairs of gene-specific primers (GSPs) for rapid amplification of cDNA ends (RACE) and a SMARTer™ RACE cDNA Amplification Kit (Clontech, Palo Alto, CA, USA) were used to obtain the full-length cDNA of AlHSP90. The 5′ and 3′ ends of AlHSP90 were obtained by RACE. Two 5′GSPs (5′GSP1: 5′-TGTTCAAGGGGAGATCTTCAGAGTCG-3′, 5′GSP2: 5′-GTCTTCAGCGAGTTCGTCGAAGAGTTCC-3′) and two 3′GSPs, (3′GSP1:5′-AAGACCAAGCCCATCTGGACCAGGA-3′, 3′GSP2:5′-GAGGATGTCGGTGAGGATGAGGATGAAG-3′) were designed based on the partial sequence obtained by the cDNA library using Primer 5.0 software.
Bioinformatics analysis of AlHSP90
A sequence similarity search at both nucleotide and amino acid levels was performed with the BLAST program at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The inferred amino acid sequence was analyzed with the Expert Protein Analysis System (http://www.expasy.org/). Multiple alignments of AlHSP90 were performed with GeneDoc 3.2 software. A phylogenic tree was constructed by ClustalX 2.0 and MEGA 4.0 based on the obtained AlHSP90 sequences and other known insect HSP90 amino acid sequences. Bootstrap analysis was used with 1,000 replicates to estimate the confidence of branches produced by the neighbor-joining method.
Relative quantification of AlHSP90 mRNA expression by real-time quantitative RT-PCR after A. lucorum treated by different temperatures and pesticides
Total RNA of A. lucorum samples treated with different temperatures or pesticides was extracted using the SV Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. cDNA was synthesized using MMLV Reverse Transcriptase (Promega, Madison, WI, USA), treated with Ribonuclease H (TaKaRa, Tokyo, Japan), and quantified by spectrophotometry.
The primers used for real-time quantitative reverse transcription(RT)-PCR (SYBR Green I) were set from the full-length cDNA of AlHSP90 (GenBank accession no. KC109781). The primers used for AlHSP90 were 5′-ATCGCCCAGTTGATGTCCCT-3′ and 5′-CCTTGGTCATGCCAATACCG-3′, with a 226-bp product. The A. lucorum housekeeping gene β-actin was used as an endogenous reference for data normalization (GenBank accession no. JN616391). The primers for β-actin were as follows: 5′-ACCTGTACGCCAACACCGT-3′ and 5′-TGGAGAGAGAGGCGAGGAT-3′, with a 177-bp product. All EST-specific primers were designed by Primer 5.0 software. Melting curve analysis and gel electrophoresis showed that only the target gene was synthesized. Real-time fluorescence data of reactions containing SYBR Green I using SYBR Premix Ex Taq Kit (TaKaRa, Tokyo, Japan) were carried out using the Bio-rad iCycler real-time quantitative RT-PCR detection system, and values were determined using iCycler iQ real-time detection system software (version 3.0a; Bio-Rad). Real-time reactions of each treatment were replicated five times, and non-template control reactions were performed in triplicate for each primer pair.
To estimate the real-time quantitative RT-PCR amplification efficiency of primers, a standard curve of five dilution series (1 × 102, 1 × 101, 1 × 100, 1 × 10−1, and 1 × 10−2 ng) was constructed from purified cDNA fragments obtained from previous real-time quantitative RT-PCRs using the same primer set. The efficiency of primers was calculated using the formula E = 10−1/SLOPE. The amplification efficiency values of AlHSP90 and β-actin were 2.1535 ± 0.054 and 2.115 ± 0.046, respectively.
The relative gene expression levels of AlHSP90 after A. lucorum samples treated with different temperatures and pesticides were represented by relative quantification (RQ) values calculated by the 2−ΔΔCt method (Livak and Schmittgen 2001). Significant difference in gene expression of AlHSP90 with different temperatures and pesticides were conducted by Duncan’s new multiple range method of statistical analysis (SAS software, version 8.0).
Western blot
To identify AlHSP90 expression at protein level after extreme heat, cold, or different pesticide stresses, more than 30 of the 2-day-old female or male A. lucorum adults were selected to perform Western blot for each sample. And then, total proteins were extracted using a Tissue Protein Extraction Reagent Kit (Nanjing Zoonbio Tech, Co., Ltd.) according to the manufacturer’s instructions. Total protein concentrations were determined using the bicinchoninic acid (BCA) method (Nanjing Zoonbio Tech, Co., Ltd.). Western blot analysis was performed according to studies by Song et al. (2012) with small modifications. Protein samples were electrophoresed on 10 % SDS-polyacrylamide gel and electroblotted to NC membrane (Bio-rad). Transfer was run at 100 mA for 3 h, using Tris/glycine buffer. Membranes were blocked with 5 % non-fat powdered milk in Tris-buffered saline containing 0.05 % Tween 20 (TBS-T) and incubated for 1 h at 37 °C. The primary antibody against AlHSP90, which was produced from the cDNA of AlHSP90 ORF by Nanjing Zoonbio Tech, Co., Ltd., was used at 1:1,000 dilutions and incubated for 1 h at 37 °C. The membrane was washed three times in TBS-T, 5 min each, and then incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (Nanjing Zoonbio Tech, Co., Ltd.) diluted 1:5,000 in blocking buffer for 1 h at 37 °C. The membrane was washed three times with TBS-T, 5 min each, and then developed by using an enhanced Chemiluminescence Advance Kit (GE Healthcare). For each experiment, samples containing an equal amount of total protein were run on the same gel. Densitometric analysis of the immunoblots was performed using a desktop scanner (Hanwang E60) and Image J free software (http://rsb.info.nih.gov/ij/index.html). Furthermore, the Western blot for β-actin was also conducted with the above method at the same time, and mouse anti-β-actin antibody used for Western blots was purchased from Nanjing Zoonbio Tech, Co., Ltd. The optical density of immunoblots was determined by scanning densitometry, and the result was presented in densitometric units. Translation was calculated by dividing the normalized AlHSP90 density by the normalized β-actin density (AlHSP90/β-actin ratio) (Song et al. 2012). The Western blotting was replicated three times.
Results
Sequence and phylogenetic tree analyses of AlHSP90
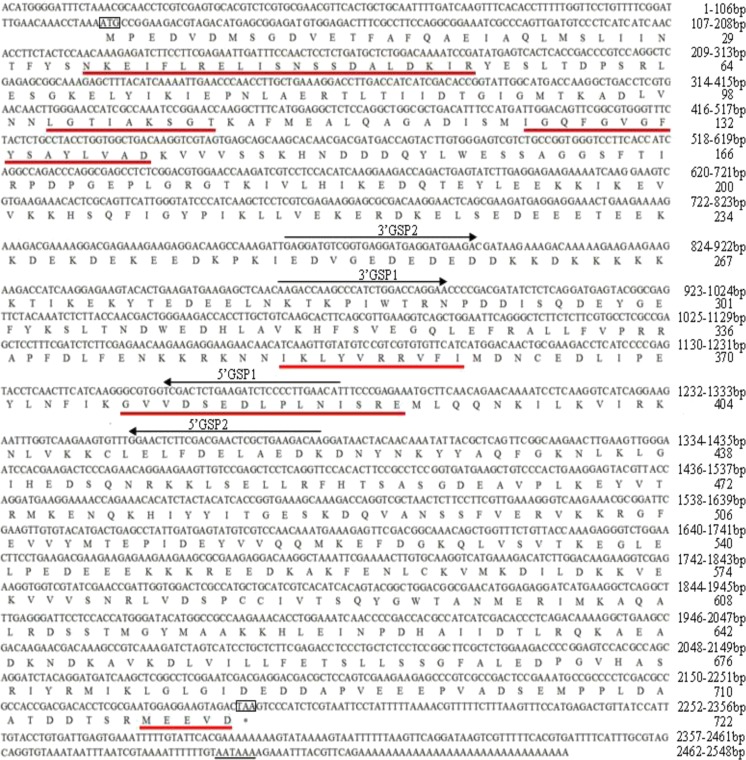
By combining the SMARTer™ cDNA library of A. lucorum with RACE approaches, two fragments corresponding to the 5′ and 3′ ends of AlHSP90 cDNA were amplified. Finally, a nucleotide sequence of 2,548 bp representing the complete cDNA sequence of AlHSP90 was obtained and deposited in GenBank (accession no. KC109781). The full-length cDNA of AlHSP90 was 2,548 bp long, including a 5′-untranslated region (UTR) of 121 bp, a 3′-UTR of 258 bp, a canonical polyadenylation signal sequence AATAAA, a poly(A) tail, and an ORF of 2,169 bp. The ORF encoded a polypeptide of 722 amino acids with a predicted molecular weight of 82.99 kDa and a theoretical isoelectric point of 4.93. The protein contained the five amino acid blocks defining the HSP90 protein family (NKEIFLRELISN[S/A]SDALDKIR, LGTIA[K/R]SGT, IGQFGVGFYSA[Y/F] LVA[E/D], IKLYVRRVFI, and GVVDS[E/D] DLPL N[I/V]SRE), as well as a consensus sequence MEEVD at the C terminus that were highly conserved in the AlHSP90 sequence (Fig. 1). Conserved domain analysis in GenBank revealed a typical histidine kinase-like ATPase domain at the amino acid positions of 35–184, which is ubiquitous in all HSP90 family members.
Fig. 1.
Nucleotide and deduced amino acid encoding region of AlHSP90 (including 5′ and 3′ UTRs). Signature sequences of HSP90 family and C-terminal five amino acids (MEEVD) are underlined in red. Start and stop codons are indicated with a black box. Tailing signals are shown in black underline. Arrows above nucleotide sequences represent the position of specific primers for RACE experiments used in PCR
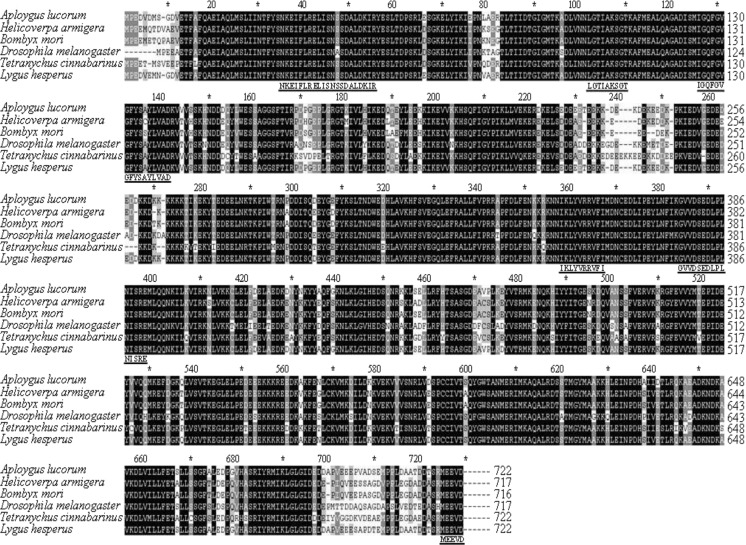
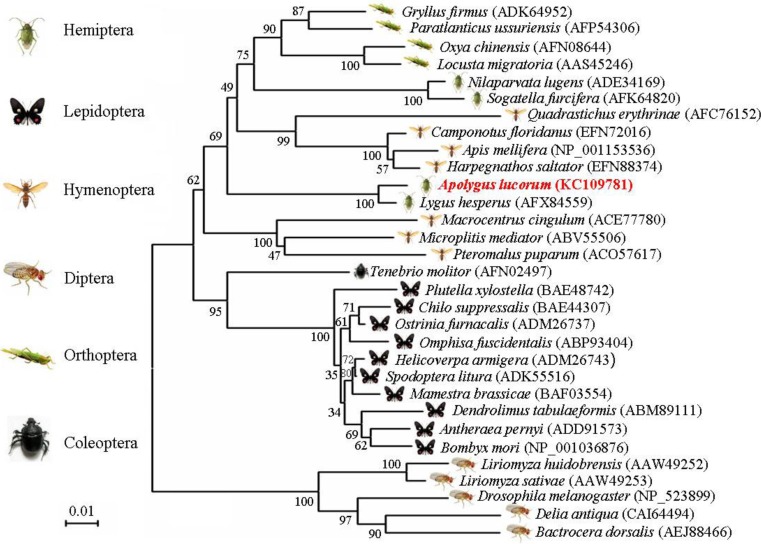
Multiple alignments of AlHSP90 with those of the other five arthropod species showed high conservation (Fig. 2). Results indicated that in compared canonical signature sequences, fewer substitutions were observed in the signature sequences of these arthropods HSP90. Among the HSP90 proteins compared, the highest percentage identity of AlHSP90 was with HSP90 from Lygus hesperus (98 % identity). AlHSP90 and TcHSP90 also had a high sequence similarity of 80 %. The lowest percentage identity and similarity was those of Drosophila melanogaster HSP90 with TcHSP90 from Tetranychus cinnabarinus (77 % identity). The identity of HSP90 was high among arthropods, especially in the signature regions of the HSP90 family, which indicated that these HSP90 may have similar roles in these organisms. BLAST results from GenBank indicated a high percentage identity of AlHSP90 compared with other insect HSP90s, and the lowest percentage identity and similarity was that of AlHSP90s with HSP90 from D. melanogaster (81 % identity). A phylogenetic tree was constructed with ClustalX1.83 and MEGA 4 based on 31 HSP90s from six different insect orders (Fig. 3). Results of the phylogenetic tree showed that 15 HSP90s belonged to Hemiptera, Hymenoptera, and Orthoptera insects located in a close branch, and the sequence similarity among these HSP90s was at least 85 %. Furthermore, the HSP90 from Spodoptera litura also shared high similarities with HSP90 from H. armigera (98 % identity). In addition, AlHSP90 and HSP90 from S. litura (H. armigera) also had a high sequence similarity of 86 %. HSP90s belonging to Diptera insects is located in the branch far from that of the other five insect orders. However, the sequence similarity among these 31 HSP90s was at least 80 %, which indicated that these HSP90 genes may be derived from the same ancestral gene.
Fig. 2.
Comparison of the amino acid sequences of HSP90 protein family. The GenBank accession numbers and species names are as follows: Apolygus lucorum, KC109781; Helicoverpa armigera, ADM26743; Bombyx mori, BAB41209; Drosophila melanogaster, NP_523899; Tetranychus cinnabarinus, ACF75907; and Lygus hesperus, AFX84559
Fig. 3.
Phylogenetic tree of AlHSP90 from A. lucorum and HSP90 from other insects constructed by the neighbor-joining method based on amino acid sequences. Numbers at each branch indicate the percentage of times a node is supported in 1,000 bootstraps pseudo-replication by neighbor joining
Quantitative analysis of AlHSP90 of A. lucorum reared at different temperatures
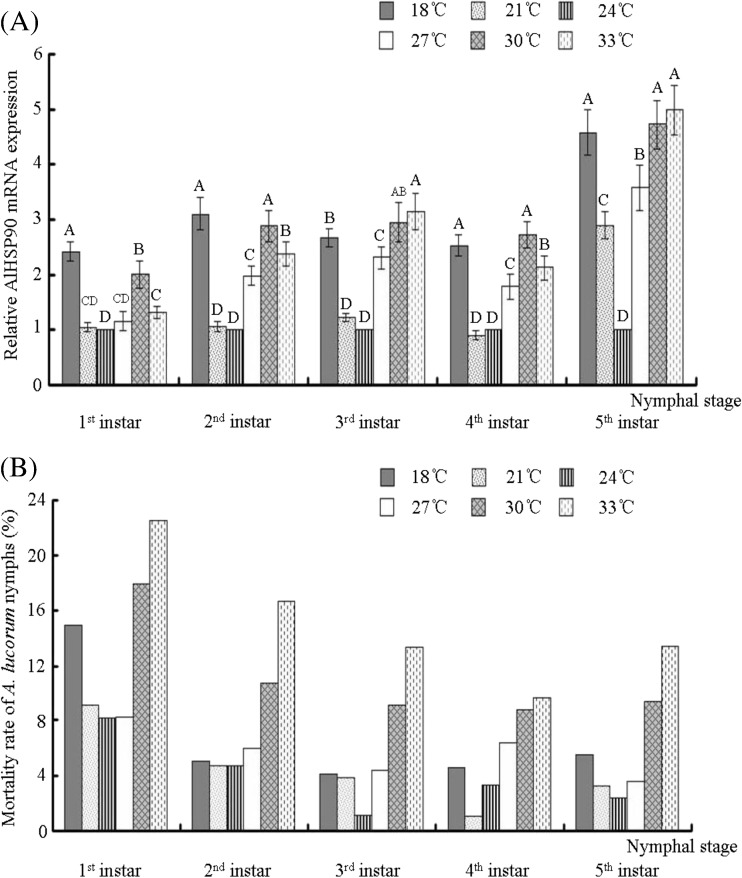
After A. lucorum nymphs and adults were reared at 18, 21, 24, 27, 30, and 33 °C, the intrinsic rate of increase (rm) of A. lucorum reared at 24 °C was the highest among these six temperatures. The total nymph mortality of A. lucorum reared at 24 °C was the lowest among these six temperatures (Table 1). Except for A. lucorum nymphs at the fourth instar, the nymph mortality at the four other different stages (first, second, third, and fifth instars) reared at 24 °C was also the lowest among the six temperatures (Fig. 4). The nymph mortality at the fourth instar reared at 21 °C was the lowest among six different temperatures (Fig. 4). Furthermore, the total of A. lucorum nymph mortality reared at 33 °C was the lowest among six different temperatures (Table 1), and the total nymph mortality reared at 33 °C reached to 52 %. In addition, the intrinsic rate of increase (rm) of A. lucorum reared at 33 °C was the lowest among six different temperatures (Fig. 4). These results indicated that ∼24 °C was the suitable temperature range for A. lucorum survival and development, while 33 °C was the unsuitable temperature for A. lucorum survival and development.
Fig. 4.
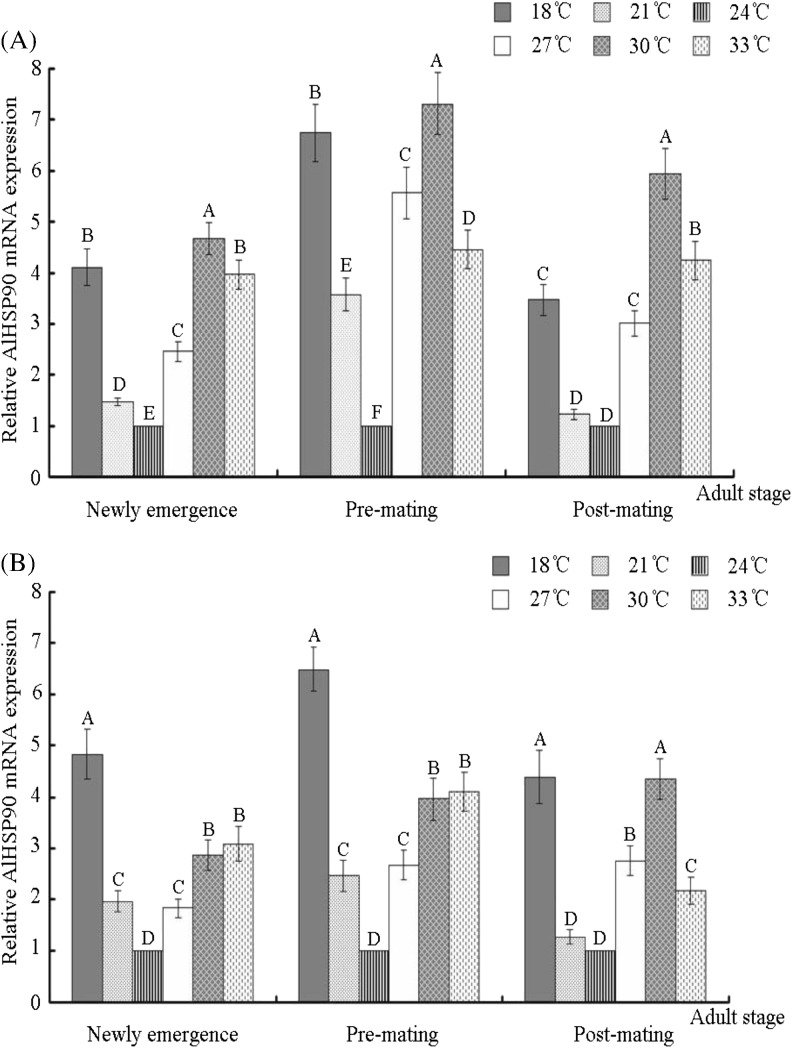
Comparative quantitative RT-PCR analysis of the relative expression of AlHSP90 and analysis of nymph’s mortality after culturing A. lucorum nymphs at different temperatures. a Relative AlHSP90 mRNA expression of A. lucorum nymphs (first to fifth instars) reared at six different temperatures. b The mortality rate of A. lucorum nymphs (first to fifth instars) reared at six different temperatures. A. lucorum insects reared at 24 °C were set up as controls. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
Fluorescent real-time quantitative PCR was used to measure the mRNA expression level of AlHSP90 in A. lucorum when its nymphs and adults were reared at six different temperatures. Results indicated that the expression of AlHSP90 in the nymphs of first and fifth instars of A. lucorum reared at 24 °C was significantly lower than that at other five different temperatures, and the expression of AlHSP90 in the nymphs of second, third, or fourth instars A. lucorum reared at 21 and 24 °C was significantly lower than that at the other four different temperatures (p < 0.01) (Fig. 4). Furthermore, the expression of AlHSP90 can be also upregulated by both cold shock (18 °C) and heat shock (30 and 33 °C). However, the expression of AlHSP90 in the nymphs of first, second, and fourth instars nymphs reared at 33 °C was significantly lower than that at 30 °C (p < 0.01) (Fig. 4). In addition, results also indicated that the expression of AlHSP90 in female or male adults reared at 24 °C was lower than at the other five different temperatures when these adults were at the newly emerged and pre-mating stage (p < 0.01) (Fig. 5). And then, the expression of AlHSP90 in female (or male) adults reared at 21 and 24 °C was lower than that at the other four different temperatures when these adults were at post-mating stage (p < 0.01) (Fig. 5). Similarity, the expression of AlHSP90 in adults can be also upregulated by both cold shock (18 °C) and heat shock (30 and 33 °C) (Fig. 5). However, the expression of AlHSP90 in female and male adults (post-mating stage) reared at 33 °C was significantly lower than that at 30 °C (p < 0.01) (Fig. 4).
Fig. 5.
Comparative quantitative RT-PCR analysis of the relative expression of AlHSP90 after A. lucorum female (male) adults cultured at different temperatures. a Relative AlHSP90 mRNA expression of female A. lucorum adults (new emergence, pre-mating, and post-mating stages) reared at six different temperatures. b Relative AlHSP90 mRNA expression of male A. lucorum adults (new emergence, pre-mating, and post-mating stages) reared at six different temperatures; A. lucorum insects reared at 24 °C were set up as controls. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
These results also indicated that a lower stress from temperature was found in A. lucorum nymphs and adults that survived at ∼24 °C deduced from the expression of the AlHSP90. In addition, the intrinsic rate of increase (rm) of A. lucorum reared at 24 °C was higher than that at other temperatures while the nymph mortality of A. lucorum reared at 24 °C was lower than that at other temperatures (Table 1), indicating that AlHSP90 was also an important gene for A. lucorum defense against temperature stresses.
Quantitative analysis of AlHSP90 expression when A. lucorum was subjected to extreme temperatures (4 and 40 °C)
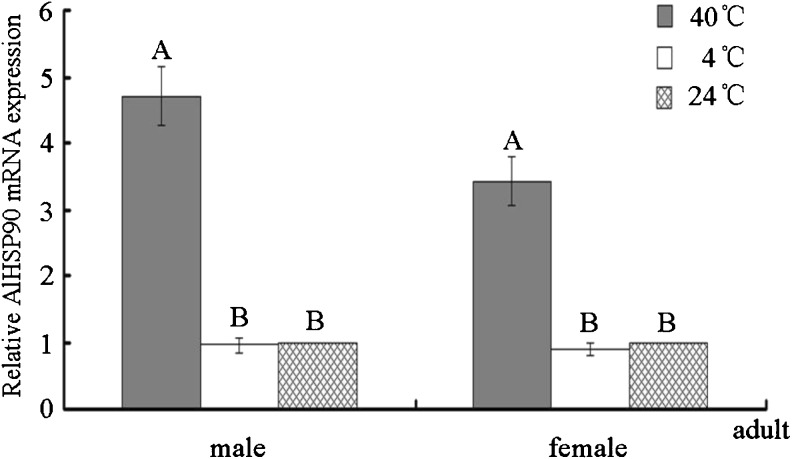
When subjected to extreme temperatures (40 and 4 °C), the AlHSP90 mRNA level in 2-day-old A. lucorum adults increased only after being induced at 40 °C for 1 h compared with the corresponding controls reared at 24 °C. The mRNA levels in females and males were 3.43- and 4.71-fold higher than those of the controls, respectively, and all differences were significant (p < 0.01). After treatment at 4 °C for 1 h, the mRNA levels in female and male adults were 0.89- and 0.96-fold lower than those of the controls, respectively, but all differences were not significant (p > 0.05) (Fig. 6). Therefore, AlHSP90 was an important gene for A. lucorum adult in response to extremely high temperatures.
Fig. 6.
Comparative quantitative RT-PCR analysis of the relative expression of AlHSP90 after subjecting 2-day-old female and male A. lucorum adults to extreme temperatures (4 and 40 °C). The same batches of 2-day-old female (male) A. lucorum adults reared at 24 °C were set up as controls. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
Bioassay and pesticide treatment
The LD50 (LD20) of emamectin benzoate and chlorpyrifos was much higher in female than that in male adults, and the LD50 values of emamectin benzoate and chlorpyrifos in female adults were 0.0975 and 1.0954 (in nanograms/individual), respectively, whereas those in male adults were 0.0757 and 0.5753 (in nanograms/individual), respectively. However, the LD50 (LD20) of cyhalothrin in male adults was much higher than that in female adults, and the LD50 of cyhalothrin in male adults was 0.6900 (in nanograms/individual). By contrast, the LD50 of cyhalothrin in female adults was 0.4969 (in nanograms/individual). We also observed that the LD50 (LD20) of imidacloprid in female adults was similar to that in male adults (Table 2).
Quantitative analysis of AlHSP90 expression after A. lucorum treated with four different pesticides at 24 °C
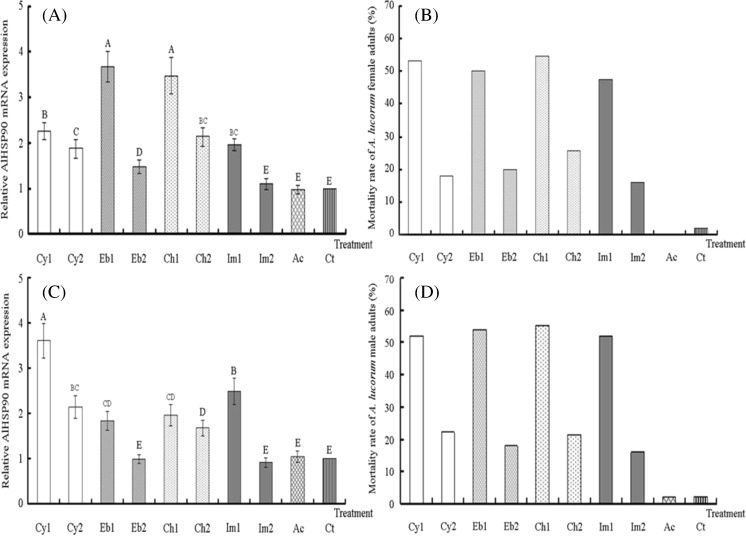
After treatment with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction doses = LD50 and LD20), the mortality rate of female and male adults reached 50 and 20 %, respectively (Fig. 7b, d). In addition, the mortality rate in Ac controls was similar to that in Ct controls. After four different pesticides treatments at 24 °C, results from the AlHSP90 mRNA levels of the surviving adults measured by real-time PCR indicated that the expression of AlHSP90 in female and male adults treated with four different pesticides (induction dose = LD50) was significantly higher than that in the Ac controls, Ct controls, and adults treated with the corresponding pesticides (induction dose = LD20; p < 0.01) (Fig. 7a, c). Furthermore, significant differences were not found between Ac controls and Ct controls (p > 0.05) (Fig. 7a, c). After treating female adults with emamectin benzoate and chlorpyrifos (induction dose = LD50), AlHSP90 expression was significantly higher than that with the other pesticide treatments, Ac controls and Ct controls (p < 0.01). However, after treating female adults with imidacloprid (induction dose = LD20), AlHSP90 expression was significantly lower than when other pesticide treatments were used (p < 0.01), and significant differences were not found among imidacloprid treatment, Ac controls and Ct controls (p > 0.05) (Fig. 7a).
Fig. 7.
Comparative quantitative RT-PCR analysis of the relative expression of AlHSP90 after treating 2-day-old A. lucorum adults with four different pesticides. Cy1, Eb1, Ch1, and Im1 are 2-day-old A. lucorum adults treated with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction dose = LD50). Cy2, Eb2, Ch2, and Im2 are 2-day-old A. lucorum adults treated with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction dose = LD20). a Relative AlHSP90 mRNA expression of female A. lucorum adults treated with four different pesticides. b Total mortality of female A. lucorum adults treated with four different pesticides (induction dose = LD50, LD20). c Relative AlHSP90 mRNA expression of male A. lucorum adults treated with four different pesticides. d Total mortality of male A. lucorum adults treated with four different pesticides (induction dose = LD50, LD20). Ct are the same batches of A. lucorum reared at 24 °C, set up as controls; Ac are the Ct control A. lucorum adults treated with acetone. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
The expression trends of AlHSP90 in male adults treated with pesticides differed from those of female adults. After treating male adults with cyhalothrin (induction dose = LD50), AlHSP90 expression was significantly higher than that with the other pesticide treatments, Ac controls and Ct controls (p < 0.01). However, after treating male adults with emamectin benzoate and imidacloprid (induction dose = LD20), AlHSP90 expression was significantly lower than that with the other pesticide treatments (p < 0.01), and significant differences were not found among the imidacloprid treatments, emamectin benzoate treatments, Ac control and Ct control (p > 0.05) (Fig. 7c).
Similar results showed that AlHSP90 was an important gene for A. lucorum to protect itself from harmful pesticides, and AlHSP90 expression increased with an increase of mounds of pesticide AI. Also, in coping with the same pesticide stress, the difference of the expression trends of AlHSP90 revealed that AlHSP90 may play different roles in female and male adults.
Quantitative analysis of AlHSP90 expression after treating A. lucorum both with 4 pesticides and heat stresses (33 °C)
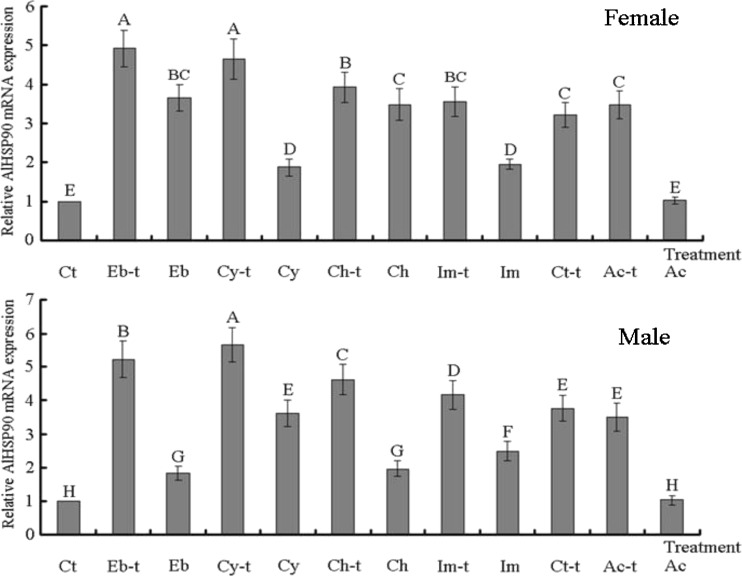
After treatment with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction doses = LD50) at 33 °C, the AlHSP90 mRNA levels in the surviving adults were also measured by fluorescent real-time quantitative PCR. Results indicated that AlHSP90 expressions in female and male adults treated with these four pesticides (induction dose = LD50) at 33 °C were all significantly higher than that at 24 °C and those of the four species controls (Ac, Ac-t, Ct, and Ct-t) (p < 0.01) (Fig. 8). Furthermore, after treatment with acetone alone at 33 °C (Ac-t) and 24 °C (Ac), the AlHSP90 mRNA levels in female and male adults at 33 °C (Ac-t) were all significantly higher than those at 24 °C (Ac) and Ct control A. lucorum adults (p < 0.01) (Fig. 8). In addition, the AlHSP90 mRNA levels in female and male adult controls at 33 °C (Ct-t) were all significantly higher than those at 24 °C (Ct) in A. lucorum adults (p < 0.01) (Fig. 8). However, significant differences were not found between Ac and Ct controls (p > 0.05). In addition, significant differences were also not found between Ac-t and Ct-t controls (p > 0.05) (Fig. 8). These results indicated that AlHSP90 mRNA in A. lucorum adults subjected to both temperature (33 °C) and the aforementioned four pesticide stresses increased significantly than in those treated with a single stress from heat shock or pesticide treatments (p < 0.01). These results also confirmed that AlHSP90 is an important gene for A. lucorum defense against both temperature and pesticide stresses.
Fig. 8.
Comparative quantitative RT-PCR analysis of the relative expression of AlHSP90 after treating 2-day-old female (male) A. lucorum adults with four different pesticides and heat shock (33 °C). Eb, Cy, Ch, and Im are 2-day-old A. lucorum adults treated with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction dose = LD50); Eb-t, Cy-t, Ch-t, and Im-t are the 2-day-old A. lucorum adults treated with four different pesticides (cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction dose = LD50)) and heat shock (33 °C) at the same time. Ct are the same batches of A. lucorum adults reared at 24 °C, set up as the controls; Ct-t are the Ct control A. lucorum adults treated with heat shock (33 °C). Ac are the Ct control A. lucorum adults treated with acetone; Ac-t are the Ct control A. lucorum adults treated with both acetone and heat shock (33 °C) at the same time. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
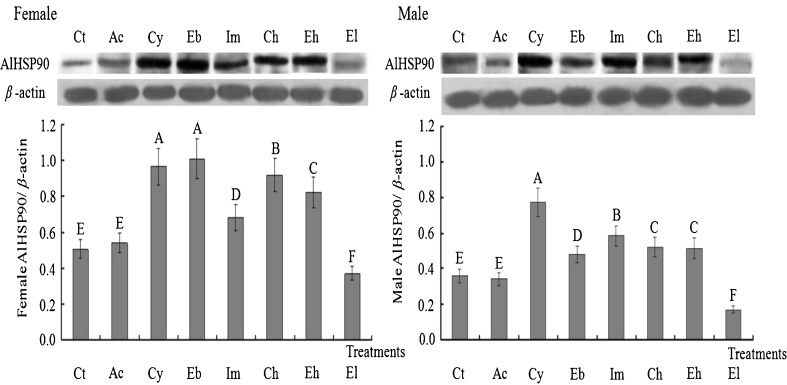
Western blot analysis of AlHSP90 protein expression after A. lucorum treated with four different pesticides or extreme temperatures (4 and 40 °C)
Western blot results showed that the expression profiles of AlHSP90 protein were in high agreement with those of AlHSP90 RNA after 2-day-old A. lucorum adults treated with four different pesticides or extreme temperatures (4 and 40 °C). Results indicated that the expression profiles of AlHSP90 protein in A. lucorum adults treated with extreme high temperature (40 °C) were significantly higher than those in Ct controls (p < 0.01). However, the expression profiles of AlHSP90 protein treated by extreme low temperature (4 °C) were significantly lower than those in Ct controls (p < 0.01) (Fig. 9). Treated by cyhalothrin, imidacloprid, chlorpyrifos, or emamectin benzoate, the expression of AlHSP90 protein was significantly upregulated when compared with Ct and Ac controls (p < 0.01) (Fig. 9), indicating AlHSP90 was very important for A. lucorum defense against aforementioned four pesticides. The expression profiles of AlHSP90 protein in female adults treated with cyhalothrin, chlorpyrifos, and emamectin benzoate (induction dose = LD50) and in male adults treated with cyhalothrin (induction dose = LD50) were significantly higher than those with other treatments (p < 0.01) (Fig. 9). Furthermore, the expression profiles of AlHSP90 protein in female/male adults treated with each of four different pesticides were all significantly higher than those in the Ct control (p < 0.01) (Fig. 9).
Fig. 9.
Western blot showing expression profiles of AlHSP90 protein induced by extreme temperatures (40 and 4 °C) and four different pesticides in 2-day-old female (male) adults of A. lucorum. Cy, Eb, Ch, and Im are 2-day-old A. lucorum adults treated with cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate (induction dose = LD50); Ac, Eh, and El are 2-day-old A. lucorum adults treated with acetone, extreme high temperature (40 °C), and extreme low temperature (4 °C); Ct are the same batches of 2-day-old A. lucorum adults reared at 24 °C, set up as control. Different letters above each bar indicate statistical difference with a statistical analysis system (SAS) followed by Duncan’s multiple comparison test (p < 0.01)
Discussion
The HSP90 family plays an important role in the environmental adaptation of various organisms (Sonoda et al. 2007; Sonoda and Tsumuki 2007; Jiang et al. 2012). To explore the response of HSP90 to thermal and pesticide stresses in A. lucorum, full-length cDNA AlHSP90 genes were cloned by cDNA library and RACE. Conserved sequences and characteristic motifs such as HSP90 family signatures and histidine kinase (from 35 to 184 amino acid residues) (Gupta 1995) were found, as well as the major structural and functional domains typical in HSP90 (Buchner 1999; Caplan 1999) in the inferred AlHSP90 protein sequence. Several special motifs were also found, including the EEVD tetrapeptide located at the C termini of AlHSP90, which may form a structure involved in mediating cofactor binding (Fuertesa et al. 2004). Furthermore, the presence of motif MEEVD at the C terminus is a characteristic shared by all eukaryotic HSP90 proteins (Feng et al. 2010).
As members of the HSP gene family, the deduced amino acid sequences of insect HSP90 genes were highly conserved compared with other insects (84.8–96.0) (Jiang et al. 2012), which is also demonstrated in our study based on the results of sequence alignment, phylogenetic analysis, and sequence similarity analysis. Our study showed that the deduced amino acid sequence of AlHSP90 shared high similarities with other known HSP90s in insects and T. cinnabarinus, especially with HSP90 from L. hesperus Knight (98 % identity). Previous studies and our results suggested that these arthropod HSP90 genes may be derived from the same ancestral gene, and then diverged through different natural selection pressures.
Similar to A. lucorum, the tarnished plant bug L. hesperus, which also belongs to the family Miridae, is a multiple-host pest that destroys vegetables, cotton, and alfalfa, thereby causing considerable economic losses (Wright et al. 2006). With the exception of related species, given the similar variety of hosts, the selection pressures of A. lucorum may be similar to that of L. hesperus which resulted in the high similarity of these two HSP90s. In addition, AlHSP90 also shared high similarities with HSP90 from H. armigera and S. litura (Lepidoptera: Noctuidae) (86 % identity). However, these two insects show a different reaction to Bt cottons. A recent study shows that Bt toxin was not effective against later instar larvae of S. litura (Govindan et al. 2012) while H. armigera is sensitive to Bt cottons (Wu et al. 2008). In addition, HSP90 from H. armigera also shared high similarities with HSP90 from S. litura (98 % identity). Therefore, these results indicated that the high or low of HSP90 identity among these cotton pests mainly depends on the related species, but not on insensitivity or sensitivity to Bt cottons.
A. lucorum is the dominant mirid species in various crops in China (Lu and Wu 2008), and temperature is the key limiting factor affecting A. lucorum growth development and population increase (Men et al. 2008; Zhao et al. 2012). When A. lucorum was reared at 17 to 29 °C, an average of ∼23 °C was the most favorable temperature for an increase of A. lucorum population; nevertheless, a temperature of 17 to 29 °C was not favorable for an increase in the A. lucorum population (Men et al. 2008). Therefore, previous studies by Men et al. (2008) and Zhao et al. (2012) have indicated that ∼24 °C was the best temperature for A. lucorum development, and that A. lucorum may suffer the lowest temperature stress (Men et al. 2008; Zhao et al. 2012). In this study, our nymph mortality and rm results also confirmed that 24 °C may be the most suitable temperature for A. lucorum development, and that <18 or >30 °C were unsuitable temperature ranges for A. lucorum survival. Furthermore, the expression pattern of AlHSP90 when A. lucorum was reared at different temperatures from 18 to 33 °C indicated that A. lucorum reared at 24 °C suffered the lowest temperature stress, whereas those reared at <18 or >30 °C suffered a higher temperature stress than at any other temperature. Therefore, AlHSP90 may be an important gene for the adaptation of A. lucorum to unsuitable temperatures.
When A. lucorum nymphs were reared at 33 °C continuously, the total nymph mortality was more than that at five other different temperatures, and the mortality rate was up to 52 %. Furthermore, the rm of A. lucorum was also much lower than that at four other different temperatures (21, 24, 27, and 30 °C) and was only up to 0.0486. These results indicated that a continuous temperature of 33 °C was highly unsuitable for A. lucorum survival, and they suffered more temperature stresses than that at 30 °C. Therefore, consistent high temperature stress at 33 °C could make female and male adults much less healthy than those living at 30 °C. So, the expression of some genes in A. lucorum reared at 33 °C seem to be confused, such as AlHSP90, AlSP3, and AlSP4 (AlSP3 and AlSP4 were important serine proteases for A. lucorum, and these results will be published in another journal). Consequently, the expression of AlHSP90 in A. lucorum adults (female at all adult stages, male at post-mating stage) reared at 33 °C is much lower than that at 30 °C.
Although heat shock was the first stress to induce the synthesis of HSP90, extreme heat and cold shock also regulated the expression of these proteins (Feng et al. 2010). In this study, after A. lucorum was subjected to extreme heat (40 °C) for 1 h, AlHSP90 expression in A. lucorum significantly increased compared with that of the 24 °C control (p < 0.01). In addition, the translational expression profiles of AlHSP90 in A. lucorum at extreme high temperature (40 °C) were also in high agreement with those of AlHSP90 transcriptional expression profiles. These results were similar to that of TcHSP90 in T. cinnabarinus treated at 40 °C for 1 h (Feng et al. 2010), indicating that AlHSP90 was an important gene for A. lucorum in response to extreme heat shock. Previous studies on insects have shown that the expression of HSP90, HSP70, HSP40, and some sHSPs gene can be upregulated by cold shock and even induced by cold shock for 1 h (Goto et al. 1998; Huang and Kang 2007; Huang et al. 2009). However, after A. lucorum was subjected to an extremely low temperature (4 °C) for 1 h, AlHSP90 gene and protein expressions insignificantly increased compared with that of the 24 °C controls. This result was similar to that of HSP90 gene in Liriomyza huidobrensis and Liriomyza sativae treated at 4 °C for 1 h (Huang and Kang 2007). However, TcHSP90 expression in T. cinnabarinus treated at 4 °C for 1 h was significantly enhanced compared with the control (Feng et al. 2010). The expression of HSP90 in L. huidobrensis and L. sativae also significantly increased compared with the controls after treatment at −12.5 and −10 °C, respectively (Huang and Kang 2007). After exposing Drosophila trapezifrons to 0 °C for 12 h and 6 °C for 24 h, about 40 % of the individuals died; the former treatment induced HSP70 mRNA expression but the latter one did not (Goto et al. 1998). Consequently, the induction temperatures and times of cold shock were also important factors affecting the expression of HSPs in insects. Therefore, the relationship of AlHSP90 expression with cold shock as affected by different temperatures and treatment durations needs to be studied in detail.
After female (male) A. lucorum adults were treated with each of four different pesticides (induction dose = LD50), the translational profiles of AlHSP90 indicated that the expression of AlHSP90 protein in female adults treated with cyhalothrin and emamectin benzoate (induction dose = LD50) and in male adults treated with cyhalothrin (induction dose = LD50) were significantly higher than that with other treatments (p < 0.01). In addition, transcriptional expression profile results also indicated that the expression of AlHSP90 in female adults treated with chlorpyrifos and emamectin benzoate (induction dose = LD50) and in male adults treated with cyhalothrin (induction dose = LD50) were higher than that with other treatments. These results indicated that AlHSP90 may be involved in the resistance or tolerance to cyhalothrin, imidacloprid, chlorpyrifos, and emamectin benzoate for A. lucorum adults, especially for female adults to emamectin benzoate and for male adults to cyhalothrin.
Previous studies have suggested that organisms can survive under the cross-protection of different stresses: one type of resistance can be induced by another type (Feng et al. 2010), such as the sublethal concentration of propoxur that induces the resistance of Anopheles stephensi and Aedes aegypti to a higher temperature, whereas several hours of heat shock can protect these two insects from pesticides (Patil et al. 1996). Feng et al. (2010) also reported that TcHSP90 may be involved in the cross-protection of temperature and emamectin benzoate stresses in T. cinnabarinus (Feng et al. 2010). A. lucorum is an important pest of Bt cottons, and calendar-based insecticide sprays are presently the sole management option for A. lucorum (Lu et al. 2007). In this study, transcriptional and translational results of AlHSP90 indicated that AlHSP90 is an important gene for A. lucorum defense against both temperature and pesticide stresses. Therefore, there seem to be some relationship between temperature and pesticides in A. lucorum by AlHSP90, which requires further study in the future. Overall, our results not only confirmed that AlHSP90 is an important gene for A. lucorum to defend against both temperature and pesticide stresses, but also confirmed that ∼24 °C was the most favorable temperature for A. lucorum development and the temperature at which it suffered the lowest temperature stress, which could provide guidance for A. lucorum management using different pesticides or temperatures in crops.
Acknowledgments
This work was supported by the Open Fund in State Key Laboratory for Biology of Plant Diseases and Insect Pests (SKL2012OP03), the Natural Science Fund of Jiangsu Province (BK20130717), the Special Fund for Agro-scientific Research in the Public Interest of China (201103012-04), and the National Key Technology Research and Development Program of China (2012BAD19B05-003). We thank Professor G.U. Zhong Yan for providing the pesticide AI for this study.
Footnotes
Yang Sun and Yang Sheng equally contributed to this paper.
References
- Benoit JB, Lopez-Martinez G, Teets NM, Phillips SA, Denlinger DL. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418–425. doi: 10.1111/j.1365-2915.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- Buchner J. Hsp90 & Co.—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/S0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Bornmann W, Erdjument-Bromage H, Tempst P, Pavletich N, Rosen N, Nathan CF, Ding A. Heat shock protein 90 mediates macrophage activation by Taxol and bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1999;96:5645–5650. doi: 10.1073/pnas.96.10.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ. Hsp90’s secrets unfold: new insights from structural and functional studies. Trends Cell Biol. 1999;9:262–268. doi: 10.1016/S0962-8924(99)01580-9. [DOI] [PubMed] [Google Scholar]
- Chen CK, Li XF, Han ZJ. Method for monitoring insecticide resistance in rice stem borer Chilo suppressalis Walker and relative susceptible baseline data. J Nanjing Agric Univ. 2000;23:25–28. [Google Scholar]
- Csemely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical application. Compr Rev Pharmacol Ther. 1998;79:129–168. doi: 10.1016/S0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- FAO (1980) Method for larvae of the rice stem borer (Chilo suppressalis Walker), FAO Method No. 3 [A], in: Pest resistance to pesticides and crop loss assessment-2[2]. FAO, Rome, pp. 25–28
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Feng H, Wang L, Liu Y, He L, Li M, Lu W, Xue C. Molecular characterization and expression of a heat shock protein gene (HSP90) from the carmine spider mite, Tetranychus cinnabarinus (Boisduval) J Insect Sci. 2010;10:112. doi: 10.1673/031.010.11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. 3. Cambridge: Cambridge University Press; 1971. [Google Scholar]
- Fuertesa MA, Peŕezb JM, Sotoa M, Menéndezc M, Alonsoa C. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. Biochim Biophys Acta. 2004;1699:45–56. doi: 10.1016/j.bbapap.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Goto SG, Yoshida KM, Kimura MT. Accumulation of Hsp70 mRNA under environmental stresses in diapausing and nondiapausing adults of Drosophila triauraria. J Insect Physiol. 1998;44:1009–1015. doi: 10.1016/S0022-1910(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Govindan K, Gunasekaran K, Kuttalam S. Evaluation of Indian transgenic Bt cotton and non Bt cotton against Spodoptera litura Fab. (Noctuidae: Lepidoptera) fourth and fifth instar larvae. J Biol Pest. 2012;5:171–177. [Google Scholar]
- Graefe SE, Wiesgigl M, Gaworski I, Macdonald A, Clos J. Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation. Eukaryotic Cell. 2002;1:936–943. doi: 10.1128/EC.1.6.936-943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RS. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol. 1995;12:1063–1073. doi: 10.1093/oxfordjournals.molbev.a040281. [DOI] [PubMed] [Google Scholar]
- Hao H, Naomoto Y, Bao X, Watanabe N, Sakurama K, Noma K, Motoki T, Tomono Y, Fukazawa T, Shirakawa Y, Yamatsuji T, Matsuoka J, Takaoka M. HSP90 and its inhibitors. Oncol Rep. 2010;23:1483–1492. doi: 10.3892/or_00000787. [DOI] [PubMed] [Google Scholar]
- Huang LH, Kang L. Cloning and inter-specific altered expression of heat shock protein genes in two leaf miner species in response to thermal stress. Insect Mol Biol. 2007;16:491–500. doi: 10.1111/j.1365-2583.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Huang LH, Wang CZ, Kang L. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leaf miner, Liriomyza sativa. J Insect Physiol. 2009;55:279–285. doi: 10.1016/j.jinsphys.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Jiang XF, Zhai HF, Wang L, Luo LZ, Sappington TW, Zhang L. Cloning of the heat shock protein 90 and 70 genes from the beet armyworm, Spodoptera exigua, and expression characteristics in relation to thermal stress and development. Cell Stress Chaperones. 2012;17:67–80. doi: 10.1007/s12192-011-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Anderson S, Singha UK, Chaudhuri M. Protein phosphatase 5 is required for Hsp90 function during proteotoxic stresses in Trypanosoma brucei. Parasitol Res. 2008;102:835–844. doi: 10.1007/s00436-007-0817-z. [DOI] [PubMed] [Google Scholar]
- Knorr E, Vilcinskas A. Post-embryonic functions of HSP90 in Tribolium castaneum include the regulation of compound eye development. Dev Genes Evol. 2011;221:357–362. doi: 10.1007/s00427-011-0379-z. [DOI] [PubMed] [Google Scholar]
- POLO-PC. A user’s guide to probit analysis or logit analysis. Berkeley: LeOra Software; 1987. [Google Scholar]
- Li HB, Du YZ (2013) Molecular cloning and characterization of an Hsp90/70 organizing protein gene from Frankliniella occidentalis (Insecta: Thysanoptera, Thripidae). Gene published online [DOI] [PubMed]
- Li Z, Srivastava P (2004) Heat-shock proteins. Curr Protoc Immunol Appendix 1: Appendix 1 T [DOI] [PubMed]
- Li GP, Feng HQ, McNeil JN, Liu B, Chen PY, Qiu F. Impacts of transgenic Bt cotton on a non-target pest, Apolygus lucorum (Meyer-Dür) (Hemiptera: Miridae), in northern China. Crop Prot. 2011;30:1573–1578. doi: 10.1016/j.cropro.2011.08.015. [DOI] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu YH, Wu KM. Biology and control of the mirids. Beijing: Golden Shield; 2008. [Google Scholar]
- Lu YH, Liang GM, Wu KM. Advances in integrated management of cotton mirids. Plant Prot. 2007;33:10–15. [Google Scholar]
- Lu YH, Wu KM, Jiang YY, Xia B, Li P, Feng HQ, Wyckhuys KAG, Guo YY. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science. 2010;328:1151–1154. doi: 10.1126/science.1187881. [DOI] [PubMed] [Google Scholar]
- Men XY, Yu Y, Zhang AS, Li LL, Zhang JT, Ge F. Life table of the laboratory population of Lygus lucorum Meyer-Dür (Hemiptera: Miridae) at different temperatures. Acta Entomol Sin. 2008;51:1216–1219. [Google Scholar]
- Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol Cell Biol. 1994;14:1459–1464. doi: 10.1128/mcb.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto T, Ohara-Nemoto Y, Ota M, Takagi T, Yokoyama K. Mechanism of dimer formation of the 90-kDa heat-shock protein. Eur J Biochem. 1995;233:1–8. doi: 10.1111/j.1432-1033.1995.001_1.x. [DOI] [PubMed] [Google Scholar]
- Patil NS, Lole KS, Deobagkar DN. Adaptive larval thermo-tolerance and induced cross-tolerance to propoxur insecticide in mosquitoes Anopheles stephensi and Aedes aefypti. Med Vet Entomol. 1996;10:277–282. doi: 10.1111/j.1365-2915.1996.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Pisa V, Cozzolino M, Gargiulo S, Ottone C, Piccioni F, Monti M, Gigliotti S, Talamo F, Graziani F, Pucci P, Verrotti AC. The molecular chaperone Hsp90 is a component of the capbinding complex and interacts with the translational repressor Cup during Drosophila oogenesis. Gene. 2009;432:67–74. doi: 10.1016/j.gene.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3:301–323. doi: 10.2174/1568009033481877. [DOI] [PubMed] [Google Scholar]
- Shu YH, Du Y, Wang JW. Molecular characterization and expression patterns of Spodoptera litura heat shock protein 70/90, and their response to zinc stress. Comp Biochem Physiol. 2011;158A:102–110. doi: 10.1016/j.cbpa.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Song Y, Fee L, Lee TH, Wharton RP. The molecular chaperone Hsp90 is required for mRNA localization in Drosophila melanogaster embryos. Genetics. 2007;176:2213–2222. doi: 10.1534/genetics.107.071472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song NN, Ding WH, Chu SY, Zhao J, Dong X, Di BB, Tang CS. Urotensin II stimulates vascular endothelial growth actor secretion from adventitial fibroblasts in synergy with angiotensin II. Vasc Med. 2012;76:1267–1273. doi: 10.1253/circj.cj-11-0870. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tsumuki H. Induction of heat shock protein genes by chlorfenapyr in cultured cells of the cabbage armyworm, Mamestra brassicae. Pestic Biochem Physiol. 2007;89:185–189. doi: 10.1016/j.pestbp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. Cloning and nucleotide sequencing of three heat shock protein genes (hsp90, hsc70, and hsp19.5) from the diamondback moth, Plutella xylostella (L.) and their expression in relation to developmental stage and temperature. Arch Insect Biochem Physiol. 2006;62:80–90. doi: 10.1002/arch.20124. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Ashfaq M, Tsumuki H. A comparison of heat shock protein genes from cultured cells of the cabbage armyworm, Mamestra brassicae, in response to heavy metals. Arch Insect Biochem Physiol. 2007;65:210–222. doi: 10.1002/arch.20178. [DOI] [PubMed] [Google Scholar]
- Tachibana SI, Numata H, Goto SG. Gene expression of heat-shock proteins (Hsp23, Hsp70 and Hsp90) during and after larval diapause in the blow fly Lucilia sericata. J Insect Physiol. 2005;51:641–647. doi: 10.1016/j.jinsphys.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. Hsp90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Neckers L. Extracellular heat shock protein 90: a role for a molecular chaperone in cell motility and cancer metastasis. Cancer Sci. 2007;98:1536–1539. doi: 10.1111/j.1349-7006.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li K, Zhu JY, Fang Q, Ye GY. Cloning and expression pattern of heat shock protein genes from the endoparasitoid wasp, Pteromalus puparum in response to environmental stresses. Arch Insect Biochem Physiol. 2012;79:247–263. doi: 10.1002/arch.21013. [DOI] [PubMed] [Google Scholar]
- Wright MK, Brandt SL, Coudron TA, Wagner RM, Habibi J, Backus EA, Huesinge JE. Characterization of digestive proteolytic activity in Lygus hesperus Knight (Hemiptera: Miridae) J Insect Physiol. 2006;52:717–728. doi: 10.1016/j.jinsphys.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wu KM, Lu YH, Feng HQ, Jiang YY, Zhao JZ. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin containing cotton. Science. 2008;5896:1676–1678. doi: 10.1126/science.1160550. [DOI] [PubMed] [Google Scholar]
- Xu J, Shu J, Zhang Q. Expression of the Tribolium castaneum (Coleoptera: Tenebrionidae) hsp83 gene and its relation to oogenesis during ovarian maturation. J Genet Genom. 2010;37:513–522. doi: 10.1016/S1673-8527(09)60071-0. [DOI] [PubMed] [Google Scholar]
- Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics. 1999;151:1065–1079. doi: 10.1093/genetics/151.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HX, Tan YA, Xiao LB, Wu GQ, Bai LX. Effects of different temperatures on the development and reproduction of Apolygus lucorum. Chin J Appl Entomol. 2012;49:585–590. [Google Scholar]