Abstract
The increasing availability of novel oral anticoagulants (NOAC) for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF) offers alternatives for patients currently prescribed warfarin. This article provides a brief overview on the mechanism and clinical use of these drugs as well as a review of the pivotal clinical trials providing the basis for each agent’s safety and efficacy. While these agents are currently Food and Drug Administration (FDA) approved for anticoagulation of patients with nonvalvular AF, additional studies continually emerge offering further insight into the application of these agents in other areas.
Keywords: Dabigatran, warfarin, apixaban, rivaroxaban, nonvalvular atrial fibrillation (AF), anticoagulation
Background
Atrial fibrillation (AF) is the most common cardiac arrhythmia and increases the risk of stroke and systemic embolism (1). The prevalence of AF increases with age and is more common in the male population (2). In patients with AF, the risk of stroke increases with increasing age, rising from 1.5% for those aged 50-59 years to 23.5% for those aged 80-89 years (3). Oral anticoagulation therapy is the standard of care for outpatient prevention of stroke and systemic embolism. A systematic review of the literature follows describing the role of novel oral anticoagulants (NOAC) in patients with nonvalvular AF.
Introduction
Warfarin, first approved in 1954 for use as a blood thinner in humans, has long remained the most widely prescribed oral anticoagulant in the United States (4). Warfarin works as a vitamin K antagonist, inhibiting the function of vitamin K epoxide reductase and reducing the production of vitamin K dependent proteins in the coagulation cascade (5,6). However, clinical management of patients taking warfarin can be complex due to multiple drug-drug interactions, essential dietary counseling, and routine monitoring in an outpatient setting (5,7).
In nonvalvular AF, the goal international normalized ratio (INR) for patients on warfarin is 2-3 with an objective of maintaining patients in this therapeutic window. A measured marker of the efficacy of this drug is percent time in therapeutic range (TTR), which tends to range from 50-70% depending on select patient cohorts (8,9). Further, in the initial phase, when initiating warfarin in sub-therapeutic patients, there is a period of hypercoagulability associated with an increased incidence of embolic events. Therefore some patients are bridged with unfractionated or low molecular weight heparin (such as enoxaparin) while starting warfarin until they reach the target INR. Thus, the emergence of a new generation of oral anticoagulants offers the potential for improved patient management and superior outcomes.
In the last five years, three new oral anticoagulants—dabigatran, rivaroxaban, and apixaban—have been approved by the Food and Drug Administration (FDA) for use in patients with nonvalvular AF for the prevention of embolic stroke. These NOACs do not require routine laboratory monitoring and have been extensively studied with regards to safety and efficacy.
Dabigatran
The first of the NOACs to be studied and FDA approved was dabigatran—a direct thrombin inhibitor with a serum half-life of 12-17 hours (Table 1) (10). Thrombin enables the conversion of fibrinogen to fibrin during the coagulation cascade and thus inhibition by dabigatran prevents the development of a thrombus (Figure 1). Dabigatran has 6% bioavailability and 80% renal elimination (10). Peak plasma levels are achieved approximately two hours after administration. Unlike warfarin, dabigatran does not require any routine monitoring and is approved for the treatment of nonvalvular AF (11). Current recommended dosing is 150 mg twice daily for patients with preserved renal function, i.e., a creatinine clearance (CrCl) greater than 30 mL/min (11). For patients with CrCl 15-30 mL/min, the recommended dosing is 75 mg twice daily (11).
Table 1. Pharmacological properties of new oral anticoagulants.
| Drug | Dabigatran (pradaxa™) | Rivaroxaban (xarelto™) | Apixaban (eliquis™) |
|---|---|---|---|
| Mechanism of action | Direct thrombin inhibitor | Direct factor Xa inhibitor | Direct factor Xa inhibitor |
| Approved indications | Stroke prevention in nonvalvular AF; VTE treatment; VTE prevention | Stroke prevention in nonvalvular AF; VTE treatment; recurrent VTE prevention; prophylaxis of VTE following hip/knee replacement | Stroke prevention in nonvalvular AF; prophylaxis of VTE following hip/knee replacement |
| Dosing in AF | 150 mg twice daily (CrCl >30 mL/min) | 20 mg once daily (CrCl >50 mL/min) | 5 mg twice daily |
| 75 mg twice daily (CrCl 15-30 mL/min) | 15 mg once daily (CrCl 15-50 mL/min) | 2.5 mg twice daily (age >80, body weight <60 kg, serum creat >1.5 mg/dL) | |
| Bioavailability (%) | 3-7 | 80-100 | 50 |
| Half-life (h) | 12-17 | 5-9 | 8-15 |
| Renal elimination (%) | >80 | 66 | 25-27 |
| Routine monitoring | No | No | No |
| Adverse reactions | Major bleeding; dyspepsia; nausea; upper abdominal pain; diarrhea; gastritis; hypersensitivity reaction | Major bleeding; abdominal pain; dyspepsia; toothache; fatigue; back pain; hypersensitivity; angioedema; Stevens-Johnson syndrome; cholestasis/jaundice | Major bleeding; drug hypersensitivity (<1%), nausea, transaminitis; epistaxis; hematuria; ocular hemorrhage; gingival bleeding |
| Drug interactions | Increased activity with P-gp inhibitors dronaderone, ketoconazole Decreased activity with P-gp inducer rifampin No effect of P-gp inhibitors amiodarone, verapamil, quinidine, clarithromycin |
Increased activity with CYP3A4/5, CYP2J2 inhibitors—ketoconazole, itraconzaole, ritonavir, clarithromycin Decreased activity with inducers of CYP3A4-rifampin, carbamazepine, phenytoin, St. John’s Wort |
Increased activity with CYP3A4 inhibitors—ketoconazole, itraconzaole, ritonavir, clarithromycin Decreased activity with inducers of CYP3A4-rifampin, carbamazepine, phenytoin, St. John’s Wort |
| Effect on coagulation tests | ↑: TCT, ECT, aPTT ↑Or no change: PT |
↑Anti-factor Xa ↑Or no change: PT, aPTT No change: TCT, ECT |
↑Anti-factor Xa ↑Or no change: PT, aPTT No change: TCT, ECT |
| Reversal in emergency bleeding | Oral charcoal Hemodialysis PCC Desmopressin Antifibrinolytic agents |
PCC Desmopressin Antifibrinolytic agents |
PCC Desmopressin Antifibrinolytic agents |
AF, atrial fibrillation; VTE, venous thromboembolism; CrCl, creatinine clearance; CYP3A4, cytochrome P450 3A4; CYP2J2, cytochrome P450 2J2; PT, prothrombin time; aPTT, activated partial thromboplastin time; TCT, thrombin clotting time; ECT, ecarin clotting time; PCC, prothrombin complex concentrate. Data obtained from Pradaxa (dabigatran etexilate) US Prescribing Information, Xarelto (rivaroxaban) US Prescribing Information, Eliquis (Apixaban) US Prescribing Information.
Figure 1.
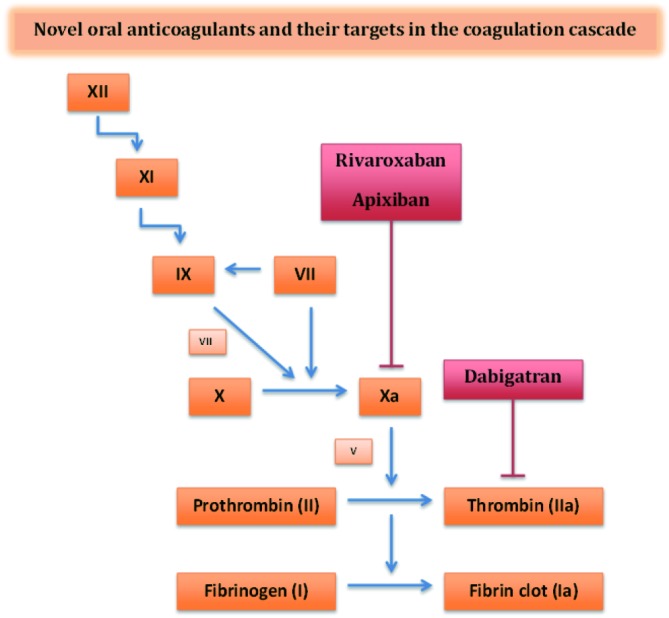
Site of action of novel oral anticoagulants. Coagulation factors are noted in orange squares. Oral anticoagulants are displayed in red boxes. Rivaroxaban and apixaban are direct factor Xa inhibitors. Dabigatran is a direct thrombin inhibitor (Adapted from Bhatt et al.).
Although no monitoring is required, dabigatran does prolong activated partial thromboplastin time (aPTT). INR is relatively unchanged by dabigatran and cannot be used for monitoring or assessing anticoagulant effect. In patients with preserved renal function, the anticoagulant effect as measured by aPTT seems to completely diminish by 48 hours. In those with a CrCl less than 30 mL/min, that effect can last more than four days. Of note, the ecarin clotting time (ECT) is another, less commonly used coagulation marker which can also be used to measure the drug effect of dabigatran (11).
The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial was a noninferiority study that compared the long-term efficacy and safety of dabigatran and warfarin in patients with AF (Table 2) (12). The trial randomly assigned 18,113 patients to one of three cohorts—adjustable dose warfarin to goal INR 2-3, dabigatran 150 mg twice daily and dabigatran 110 mg twice daily. Study investigators concluded that 150 mg twice daily dosing was superior to warfarin with respect to primary efficacy endpoints of stroke or systemic embolism without a statistically significant increased risk of major bleeding. Major bleeding was defined as a reduction in hemoglobin level of at least 2 g/dL, bleeding requiring a transfusion of at least two units of packed red blood cells, or symptomatic bleeding in a critical area or organ. Life-threatening bleeding, a subset of major bleeding, was defined as fatal bleeding, symptomatic intracranial bleeding, bleeding with a decrease of hemoglobin level of at least 5 g/dL, or bleeding requiring a transfusion of at least four units of packed red blood cells, inotropic agent support, or surgery. Dabigatran 110 mg twice daily compared to warfarin reduced rates of major bleeding while remaining noninferior in terms of stroke prevention, though both doses increased risk of gastrointestinal bleed. Importantly, both doses of dabigatran were associated with significantly lower incidence of hemorrhagic stroke in comparison to warfarin. The most frequent adverse reactions to dabigatran in the RE-LY trial, other than bleeding, were gastrointestinal events such as dyspepsia, nausea, diarrhea and abdominal pain.
Table 2. Pivotal trials for new oral anticoagulants.
| Drug | Dabigatran (pradaxa™) |
Rivaroxaban (xarelto™) |
Apixaban (eliquis™) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6,076, NOAC† (%/yr) | 6,022, Warf (%/yr) | RR (95% CI) | P value | 7,081, NOAC†,‡ | 7,090, Warf‡ | HR (95% CI) | P value | 9,120, NOAC† (%/yr) | 9,081, Warf (%/yr) | HR (95% CI) | P value | |||
| Drug trial | RE-LY | ROCKET-AF | ARISTOTLE | |||||||||||
| Study design and analysis | Multicenter, open-label, intent-to-treat | Multicenter, double-blind, per protocol | Multicenter, double-blind, intent-to-treat | |||||||||||
| No. in sample | 18,153 | 14,264 | 18,201 | |||||||||||
| Efficacy | ||||||||||||||
| SSE | 1.11 | 1.69 | 0.66 (0.74-1.11) | <0.001* | 2.1 | 2.4 | 0.88 (0.75-1.03) | <0.001* | 1.27 | 1.6 | 0.79 (0.66-0.95) | 0.01 | ||
| Stroke (all) | 1.01 | 1.57 | 0.64 (0.51-0.81) | <0.001 | 1.65 | 1.96 | 0.85 (0.70-1.03) | 0.092 | 1.19 | 1.51 | 0.79 (0.65-0.95) | 0.01 | ||
| Hemorrhagic | 0.1 | 0.38 | 0.26 (0.14-0.49) | <0.001 | 0.26 | 0.44 | 0.59 (0.37-0.93) | 0.024 | 0.24 | 0.47 | 0.51 (0.35-0.75) | <0.001 | ||
| Ischemic or NOS | 0.92 | 1.2 | 0.76 (0.60-0.98) | 0.03 | 1.34 | 1.42 | 0.94 (0.75-1.17) | 0.581 | 0.97 | 1.05 | 0.92 (0.74-1.13) | 0.42 | ||
| Disabling | 0.66 | 1 | 0.66 (0.50-0.88) | 0.005 | 0.39 | 0.5 | 0.77 (0.52-1.14) | 0.188 | ||||||
| Non-disabling | 0.37 | 0.58 | 0.62 (0.43-0.91) | 0.01 | 0.79 | 0.77 | 1.03 (0.76-1.38) | 0.863 | ||||||
| MI | 0.74 | 0.53 | 1.38 (1.00-1.91) | 0.048 | 0.91 | 1.12 | 0.81 (0.63-1.06) | 0.121 | 0.53 | 0.61 | 0.88 (0.66-1.17) | 0.37 | ||
| PE | 0.15 | 0.09 | 1.61 (0.76-3.42) | 0.21 | ||||||||||
| All-cause mortality | 3.64 | 4.13 | 0.88 (0.77-1.00) | 0.051 | 1.87 | 2.21 | 0.85 (0.70-1.02) | 0.073 | 3.52 | 3.94 | 0.89 (0.80-0.998) | 0.047 | ||
| Safety | ||||||||||||||
| Major bleed | 3.11 | 3.36 | 0.93 (0.81-1.07) | 0.31 | 3.6 | 3.4 | 1.04 (0.90-1.20) | 0.58 | 2.13 | 3.09 | 0.69 (0.60-0.80) | <0.001 | ||
| Fatal bleed | 1.45 | 1.8 | 0.81 (0.66-0.99) | 0.04 | 0.2 | 0.5 | 0.50 (0.31-0.79) | 0.003 | ||||||
| ICH hemorrhage | 0.3 | 0.74 | 0.40 (0.27-0.60) | <0.001 | 0.5 | 0.7 | 0.67 (0.47-0.93) | 0.02 | 0.33 | 0.8 | 0.42 (0.30-0.58) | <0.001 | ||
| Any bleed | 16.42 | 18.15 | 0.91 (0.86-0.97) | 0.002 | 14.9 | 14.5 | 1.03 (0.96-1.11) | 0.44 | 18.1 | 25.8 | 0.71 (0.68-0.75) | <0.001 | ||
NOAC, new oral anticoagulant; Warf, warfarin; RR, relative risk; HR, hazard ratio; SSE, stroke or systemic embolism; MI, myocardial infarction; PE, pulmonary embolism; ICH, intracranial hemorrhage. *, indicates analysis for noninferiority; †, analysis shown only for high doses formulations of anticoagulants; ‡, event/100 patient-yr; §, bleeding was defined as: RE-LY, reduction of hemoglobin level of at least 20 g/L, transfusion of at least 2 units of packed red blood cells, symptomatic bleeding in a critical area or organ; ROCKET-AF, reduction of hemoglobin of at least 2 g/dL, transfusion of at least 2 units of packed red blood cells, overt bleeding involving a critical anatomical site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, intramuscular with compartment syndrome) permanent disability, fatal outcome; ARISTOTLE, acute or sub-acute clinically overt bleeding accompanied by at least one of the following: decrease in hemoglobin level of at least 2 g/dL, bleed requiring transfusion of at least 2 units of packed red blood cells, or bleeding that was fatal or occurred in a critical site (intracranial, spinal, ocular, pericardial, articular, intramuscular with compartment syndrome, retroperitoneal).
A long-term observational study of patients in the RE-LY cohort were followed for a median of 2.3 years and reported in a follow-up study (RELY-ABLE) (13). The rate of stroke was 1.46%/year for the 150 mg twice daily dosing regimen of dabigatran whereas the rate of major hemorrhage was 3.74%/year. In comparison, the 110 mg twice daily dosing revealed rate of stroke at 1.60%/year and major bleeding rate of 2.99%/year, which was a statistically significant reduction in bleeding risk without a significant increase in development of stroke. Similarly, lower dose dabigatran had a death rate of 3.10% vs. 3.02% in the 150 mg twice daily cohort. Since RE-LY, numerous subgroup analyses have examined safety in patient cohorts isolating certain risk factors including renal dysfunction, heart failure, older age, and a higher CHADS2 score, and all have demonstrated similar safety to warfarin (14-22).
A major limitation of the use of dabigatran is the lack of an FDA approved antidote for use in medical emergencies. Though no routine laboratory monitoring is required for general outpatient follow-up, some laboratory assays (ECT, aPTT) are available for emergency situations in which patients are suffering from life-threatening bleeding. In 2011, the EudraVigilance dataset reported 256 cases of serious bleeding resulting in death associated with the use of dabigatran (23). These findings prompted an in depth FDA investigation which concluded that there was no evidence that bleeding rates were higher with dabigatran compared to other oral agents (24). Emergent hemodialysis has been proposed and studied as a technique for drug removal. In a study of seven patients with end stage renal disease, a four-hour session of hemodialysis was shown to rapidly reduce the dabigatran blood concentration and reduce the concomitant anticoagulant effect (25). Interestingly, an analysis of genetic variants in the RE-LY study cohorts suggested that the carriage of a specific allele (CES1 rs2244613) was associated with a lower risk of bleeding without an increased ischemic event risk (26).
Rivaroxaban
Rivaroxaban is an oral factor Xa inhibitor that is administered as a fixed oral dose and does not require any routine monitoring. It is FDA approved for treatment of nonvalvular AF (27). Current recommended dosing is 20 mg daily for patients with CrCl >50 mL/min and 15 mg daily for patients with CrCl between 15 and 50 mL/min (27).
Activated factor Xa mediates the conversion of prothrombin to thrombin ultimately leading to fibrin clot formation (Figure 1). Rivaroxaban selectively and competitively inhibits free and prothrombinase/clot-associated factor Xa through reversible interactions thereby inhibiting thrombin formation and decreasing fibrin clot formation (28,29). It achieves peak plasma levels 2-4 hours after oral administration and has a half-life of 9-13 hours with 50% renal clearance (30). Approximately two-thirds of an administered dose of rivaroxaban is metabolized via cytochrome P450 enzymes (CYP3A4 and CYP2J2) (31). As such, an increased risk of bleeding has been observed when rivaroxaban is combined with cytochrome P450 isoenzyme and P-glycoprotein inhibitors such as itraconazole and voriconazole (32).
The gold standard for monitoring rivaroxaban is high-performance liquid chromatography coupled with tandem mass spectrometry; however this is not practical in most clinical laboratory settings (33). Rivaroxaban does prolong the PT and aPTT but the extent is dependent on the reagent used (34). Several different types of PT reagents have been studied to assess the pharmacodynamic effects, but the difference between these reagents could not be standardized with INR calibration (35). For this reason, routine clinical assessment of coagulation parameters is not feasible.
The “Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation” (ROCKET AF) compared the efficacy of oral rivaroxaban in preventing stroke and systemic embolism in patients with nonvalvular AF with that of warfarin (36). ROCKET AF was a large, multi-center, randomized, double-blinded, event-driven trial (37). This trial demonstrated that rivaroxaban was not inferior to warfarin when it came to the combined incidence of stroke and systemic embolism (event rate per 100 patient-years 1.7 vs. 2.2: HR 0.79; 95% CI, 0.66-0.96) (36). Rivaroxaban, however, was found to have a significantly higher incidence of stroke or systemic embolism when transitioning to warfarin therapy from rivaroxaban (event rate per 100 patient-years 6.42 vs. 1.73; HR 3.72; 95% CI, 1.51-9.16). To prevent these events, rivaroxaban should be continued with warfarin until a therapeutic INR is achieved (38).
No statistically significant difference was discovered when comparing rivaroxaban and warfarin in regards to major or non-major clinically relevant bleeding (HR 1.03; 95% CI, 96-1.11). Major bleeding was defined as overt bleeding involving a critical anatomical site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, intramuscular with compartment syndrome) or associated with transfusion of at least two units of packed red blood cells, a drop in hemoglobin of greater than 2 g/dL, permanent disability or death. Nonmajor bleeding was defined as bleeding associated with pain or impairment of activities of daily living, requiring medical intervention, unscheduled physician contact or temporary discontinuation of the study drug (36). Similar to dabigatran in the RE-LY trial, there was a higher incidence of major gastrointestinal bleeding when compared to patients in the warfarin cohort (36).
Apixaban
Apixaban is also a direct factor Xa inhibitor (Figure 1) (39). It has 66% bioavailability with a plasma half-life of 8-15 hours and 25% renal clearance (39). It is FDA approved for prevention of stroke and systemic embolism in patients with nonvalvular AF. Current recommended dosing is 5 mg twice daily for patients with preserved renal function and 2.5 mg twice daily for patients with two of the following characteristics: age >80 years, body weight <60 kg, serum creatinine >1.5 mg/dL (39).
The Apixaban for Reduction In STroke and Other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial was a double-blinded randomized controlled trial of 18,201 patients randomized to either warfarin or apixaban (40). A primary endpoint of stroke or systemic embolism occurred in 1.27%/yr in the apixaban cohort vs. 1.60%/yr in those taking warfarin. This was a statistically significant difference indicating superiority of apixaban. Major bleeding, defined according to the International Society on Thrombosis and Haemostasis (ISTH) criteria, occurred in 2.13%/yr in the apixaban group compared to 3.09%/yr in the warfarin group, a statistically significant reduction in bleeding risk (40,41). Major bleeding was defined as acute or sub-acute clinically overt bleeding accompanied by at least one of the following: decrease in hemoglobin level of at least 2 g/dL, bleed requiring transfusion of at least two units of packed red blood cells, or bleeding that was fatal or occurred in a critical site (intracranial, spinal, ocular, pericardial, articular, intramuscular with compartment syndrome, retroperitoneal). Additionally, all-cause mortality was found to be significantly lower in the apixaban cohort—3.52%/yr vs. 3.94%/yr in the warfarin cohort (hazard ratio: 0.89; 95% CI, 0.80-0.99; P=0.047). Thus, the ARISTOTLE trial demonstrated that apixaban is not only more effective than warfarin at preventing stroke, but also safer in terms of bleeding risk and risk of death.
In the AVERROES trial (apixaban versus acetylsalicyclic acid to prevent stroke in AF patients who have failed or are unsuitable for vitamin K antagonist treatment), apixaban was compared to aspirin alone for stroke prevention (42). The trial was terminated early because of a clear, overwhelming benefit of anticoagulation in the apixaban cohort. The rate of major bleeding in the apixaban group was similar to that seen in the ARISTOTLE trial.
Discussion
A meta-analysis performed by Gómez-Outes et al. analyzed safety and efficacy of all three oral anticoagulants (43). In all, more than 50,000 patients were included in the analysis. All three drugs demonstrated similar efficacy in prevention of non-hemorrhagic stroke when compared to warfarin in nonvalvular AF. Each of the respective pivotal trials indicated an overall statistically significant reduction in all strokes, both hemorrhagic and ischemic compared to warfarin. Two other meta-analyses have supported these conclusions (44,45).
A common disadvantage among all three oral agents is the lack of an antidote in cases when patients suffer major bleeding complications. The most worrisome complication of anticoagulation is the risk of intracranial hemorrhage. In this regard, the NOACs appear to be superior in that they confer a lower risk of intracranial bleeding compared to warfarin. However, the effects of warfarin can be reversed with administration of vitamin K or recombinant factor X. Though no antidote currently is available for NOACs, their short half-lives allow relatively quick elimination in patients with preserved renal function. Some studies have suggested that activated charcoal can be used to decrease the absorption of dabigatran (46). Prothrombin complex concentrates have been shown to reverse the anticoagulant effect of rivaroxaban completely in healthy subjects (47). In addition to these agents, general measures for treating acute bleeding should also be undertaken including mechanical compression, volume resuscitation, close hemodynamic monitoring, and packed red blood cell transfusions as indicated. For patients with severe or life threatening bleeding, transfer to an intensive care setting where additional hemodynamic supportive care with vasopressors and emergency dialysis can be delivered should be strongly considered.
Importantly, a recent randomized, double-blind, placebo controlled trial of 145 healthy volunteers demonstrated that idarucizumab, a humanized antibody that binds dabigatran with high affinity, could result in immediate and sustained reversal of the anticoagulant effect (48,49). Idarucizumab appears to be well tolerated without significant side effects and completely reverses prolonged clotting time within five minutes of administration. This is the first report of a specific antidote for one of the NOACs, but further studies are required before it can be made available for widespread clinical use.
The use of oral anticoagulants in numerous other clinical scenarios remains uninvestigated. Currently, concomitant use of clopidogrel and aspirin is approved only alongside warfarin. The PETRO trial showed that aspirin in conjunction with dabigatran increased the risk of bleeding, but only in patients receiving doses higher than currently recommended (300 mg twice daily); there was no increased risk of bleeding when combined with only 150 mg (50). However, the PETRO trial was limited by a small sample size and further studies are required. To date, there is a paucity of data evaluating the safety of combined ticagrelor or prasugrel with any of the factor Xa inhibitors or dabigatran (51-55).
Another scenario requiring further study is perioperative anticoagulation for patients on NOACs. The periprocedural management of patients on NOACs is a common clinical problem that is very complex and patient specific. The short half-lives and predictable pharmacokinetics of the NOACs allows for potentially improved perioperative management. A careful assessment of patient and procedure risk of thrombosis and bleeding must be completed prior to developing an anticoagulation strategy. Table 3 summarizes current recommendations on perioperative management for each of the NOACs depending on risk of surgery and renal function; however, these recommendations are based primarily on expert opinion (56). Further investigation is needed to evaluate the safety of NOAC in the perioperative window for patients needing both elective and emergent surgery. Of note, the European Heart Rhythm Association (EHRA) recommends these medications be held 18-24 hours prior to a planned procedure and restarted six hours post-procedure for those with minimal bleeding risk, i.e., ophthalmological and dermatological procedures (57). The EHRA recommends holding these agents >48 hours prior to elective procedures with high bleeding risk, e.g., abdominal and cardiothoracic surgeries, vascular procedures, liver and kidney biopsies.
Table 3. Perioperative use of novel oral anticoagulants*.
| Risk of surgery | Dabigatran (pradaxa™) |
Rivaroxaban (xarelto™) |
Apixaban (eliquis™) |
|||||
|---|---|---|---|---|---|---|---|---|
| CrCl >50 mL/min | CrCl 30-50 mL/min* | CrCl >50 mL/min | CrCl 30-50 mL/min* | CrCl >50 mL/min | CrCl 30-50 mL/min* | |||
| Low | ||||||||
| Hold | 2 days (2 doses) | 2 days (4 doses) | 2 days (1 dose) | 2 days (1 dose) | 2 days (2 doses) | 2 days (2 doses) | ||
| Resume | 1 day after | 1 day after | 1 day after | 1 day after | 1 day after | 1 day after | ||
| High | ||||||||
| Hold | 3 days (4 doses) | 5 days (8 doses) | 3 days (2 doses) | 3 days (2 doses) | 3 days (4 doses) | 3 day (4 doses) | ||
| Resume | 2-3 days after | 2-3 days after | 2-3 days after | 2-3 days after | 2-3 days after | 2-3 days after | ||
NOAC, new oral anticoagulant; CrCl, creatinine clearance. *, Doses for reduced renal function as described in Table 1. Adapted from Spyropoulos et al.
Lastly, little is known about the relative cost-effectiveness of each NOAC compared to warfarin. Some data are available based on studies conducted in the United Kingdom (58,59). Recently, Desai et al. published a study that evaluated the utilization practices and relative cost of each NOAC versus warfarin by exploring total drug expenditures for patients and insurers in the United States (60). A total of 6,893 patients taking warfarin or a NOAC were enrolled over a 33-month period. Patient out-of-pocket spending (copayments, coinsurance, deductible) and insurer spending (plan-paid amount) for each drug were plotted over a six-month period. Patient out-of-pocket spending was five-fold higher in NOACs compared to warfarin and 15-fold higher for insurance spending—an absolute total cost difference of approximately $900 per month. However, patients taking NOACs had fewer office visits, hospital days and hospitalizations within 30 days of medication initiation as compared to patients prescribed warfarin. Of note, patients on NOACs were younger with lower CHADS2 scores. Importantly, patients on a NOAC in RE-LY, ROCKET-AF, and ARISTOTLE had relatively higher CHADS2 scores when compared to current prescribing practices.
Recommendations to prescribers
In prescribing NOACs to patients for stroke prevention in nonvalvular AF, a thorough discussion regarding risks and benefits based on an in depth understanding of each patient’s comorbidities and personal preferences should occur. For patients currently on warfarin and desiring conversion to one of the newer agents, NOACs would be recommended only for patients who have proven excellent medication compliance in the past. Given the shorter half-life of these medications, even a few consecutively missed doses could result in a higher risk of stroke or systemic embolism. Additionally, patients who have suffered gastrointestinal hemorrhage should be warned of the higher risk for this complication. As such, adherence to medical therapy and close self-monitoring for any bleeding complications needs to be emphasized when first prescribing any of these drugs. Although routine monitoring is not required for these medications, the lack of a clear marker of anticoagulant activity makes verifying compliance very difficult. For patients already on these agents, close clinical follow-up for adverse events must be pursued. In the near future, more data regarding safety and efficacy of these drugs will become available and will further inform our clinical practice.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Atrial fibrillation: current understandings and research imperatives. The National Heart, Lung, and Blood Institute Working Group on Atrial Fibrillation. J Am Coll Cardiol 1993;22:1830-4 [PubMed] [Google Scholar]
- 2.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53 [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8 [DOI] [PubMed] [Google Scholar]
- 4.Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095-106 [DOI] [PubMed] [Google Scholar]
- 5.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:160S-98S. [DOI] [PubMed] [Google Scholar]
- 6.Freedman MD. Oral anticoagulants: pharmacodynamics, clinical indications and adverse effects. J Clin Pharmacol 1992;32:196-209 [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Outes A, Suarez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol 2012;9:83-104 [DOI] [PubMed] [Google Scholar]
- 8.Rose AJ, Ozonoff A, Henault LE, et al. Warfarin for atrial fibrillation in community-based practise. J Thromb Haemost 2008;6:1647-54 [DOI] [PubMed] [Google Scholar]
- 9.Ryan F, Byrne S, O’Shea S.Randomized controlled trial of supervised patient self-testing of warfarin therapy using an internet-based expert system. J Thromb Haemost 2009;7:1284-90 [DOI] [PubMed] [Google Scholar]
- 10.Stangier J.Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet 2008;47:285-95 [DOI] [PubMed] [Google Scholar]
- 11.Boehringer Ingelheim I. Pradaxa (dabigaran etexilate): US prescribing information. Available online: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf Accessed on 14 August 2014.
- 12.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51 [DOI] [PubMed] [Google Scholar]
- 13.Connolly SJ, Wallentin L, Ezekowitz MD, et al. The Long-Term Multicenter Observational Study of Dabigatran Treatment in Patients With Atrial Fibrillation (RELY-ABLE) Study. Circulation 2013;128:237-43 [DOI] [PubMed] [Google Scholar]
- 14.Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321-8 [DOI] [PubMed] [Google Scholar]
- 15.Ferreira J, Ezekowitz MD, Connolly SJ, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE-LY trial. Eur J Heart Fail 2013;15:1053-61 [DOI] [PubMed] [Google Scholar]
- 16.Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014;129:961-70 [DOI] [PubMed] [Google Scholar]
- 17.Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation 2012;125:669-76 [DOI] [PubMed] [Google Scholar]
- 18.Oldgren J, Alings M, Darius H, et al. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med 2011;155:660-7, W204. [DOI] [PubMed]
- 19.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363-72 [DOI] [PubMed] [Google Scholar]
- 20.Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010;9:1157-63 [DOI] [PubMed] [Google Scholar]
- 21.Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83 [DOI] [PubMed] [Google Scholar]
- 22.Hori M, Connolly SJ, Ezekowitz MD, et al. Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation--sub-analysis in Japanese population in RE-LY trial. Circ J 2011;75:800-5 [DOI] [PubMed] [Google Scholar]
- 23.European Medicines Agency. European Medicine Agency Updates on Safety of Pradaxa. N.p. 2011. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/11/WC500117818.pdf Accessed on 14 August 2014.
- 24.Administration USFAD. FDA Drug and Safety Communication: Safety review of post-market reports of serious bleeding events with the anticoagulant Pradaxa (dabigatran etexilate mesylate).
- 25.Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596-605 [DOI] [PubMed] [Google Scholar]
- 26.Paré G, Eriksson N, Lehr T, et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation 2013;127:1404-12 [DOI] [PubMed] [Google Scholar]
- 27.Janssen Pharmaceuticals I. Xarelto (rivaroxaban): US prescribing information. Available online: http://www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100 Accessed on 14 August 2014.
- 28.Laux V, Perzborn E, Kubitza D, et al. Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor. Semin Thromb Hemost 2007;33:515-23 [DOI] [PubMed] [Google Scholar]
- 29.Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost 2005;3:514-21 [DOI] [PubMed] [Google Scholar]
- 30.Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005;61:873-80 [DOI] [PubMed] [Google Scholar]
- 31.Duggan ST, Scott LJ, Plosker GL. Rivaroxaban: a review of its use for the prevention of venous thromboembolism after total hip or knee replacement surgery. Drugs 2009;69:1829-51 [DOI] [PubMed] [Google Scholar]
- 32.Mueck W, Kubitza D, Becka M.Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol 2013;76:455-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohde G.Determination of rivaroxaban--a novel, oral, direct Factor Xa inhibitor--in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2008;872:43-50 [DOI] [PubMed] [Google Scholar]
- 34.Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost 2011;9:133-9 [DOI] [PubMed] [Google Scholar]
- 35.Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban--an oral, direct factor Xa inhibitor. Thromb Haemost 2010;103:815-25 [DOI] [PubMed] [Google Scholar]
- 36.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91 [DOI] [PubMed] [Google Scholar]
- 37.ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 2010;159:340-7.e1. [DOI] [PubMed]
- 38.Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013;61:651-8 [DOI] [PubMed] [Google Scholar]
- 39.Squib BM. Eliquis (Apixaban): US prescribing information. 2012. Available online: http://packageinserts.bms.com/pi/pi_eliquis.pdf Accessed on 14 August 2014.
- 40.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92 [DOI] [PubMed] [Google Scholar]
- 41.Schulman S, Kearon C.Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4 [DOI] [PubMed] [Google Scholar]
- 42.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17 [DOI] [PubMed] [Google Scholar]
- 43.Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, et al. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis 2013;2013:640723. [DOI] [PMC free article] [PubMed]
- 44.Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 2012;126:2381-91 [DOI] [PubMed] [Google Scholar]
- 45.Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012;110:453-60 [DOI] [PubMed] [Google Scholar]
- 46.Siegal DM, Crowther MA. Acute management of bleeding in patients on novel oral anticoagulants. Eur Heart J 2013;34:489-498b [DOI] [PubMed] [Google Scholar]
- 47.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573-9 [DOI] [PubMed] [Google Scholar]
- 48.Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554-62 [DOI] [PubMed] [Google Scholar]
- 49.Glund S, Stangier J, Schmohl M, et al. A Specific Antidote for Dabigatran: Immediate, Complete and Sustained Reversal of Dabigatran Induced Antiocoagulation in Healthy Male Volunteers. Circulation 2013;128:A17765 [Google Scholar]
- 50.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419-26 [DOI] [PubMed] [Google Scholar]
- 51.Vranckx P, Verheugt FW, de Maat MP, et al. A randomised study of dabigatran in elective percutaneous coronary intervention in stable coronary artery disease patients. EuroIntervention 2013;8:1052-60 [DOI] [PubMed] [Google Scholar]
- 52.Oldgren J, Budaj A, Granger CB, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J 2011;32:2781-9 [DOI] [PubMed] [Google Scholar]
- 53.Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 2013;127:634-40 [DOI] [PubMed] [Google Scholar]
- 54.Mega JL, Braunwald E, Wiviott SD, et al. Comparison of the efficacy and safety of two rivaroxaban doses in acute coronary syndrome (from ATLAS ACS 2-TIMI 51). Am J Cardiol 2013;112:472-8 [DOI] [PubMed] [Google Scholar]
- 55.Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011;365:699-708 [DOI] [PubMed] [Google Scholar]
- 56.Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012;120:2954-62 [DOI] [PubMed] [Google Scholar]
- 57.Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2013;34:2094-106 [DOI] [PubMed] [Google Scholar]
- 58.Kansal AR, Sorensen SV, Gani R, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012;98:573-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lip GY, Kongnakorn T, Phatak H, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210.e20. [DOI] [PubMed]
- 60.Desai NR, Krumme AA, Schneeweiss S, et al. Patterns of Initiation of Oral Anticoagulants in Patients with Atrial Fibrillation - Quality and Cost Implications. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.05.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


