Abstract
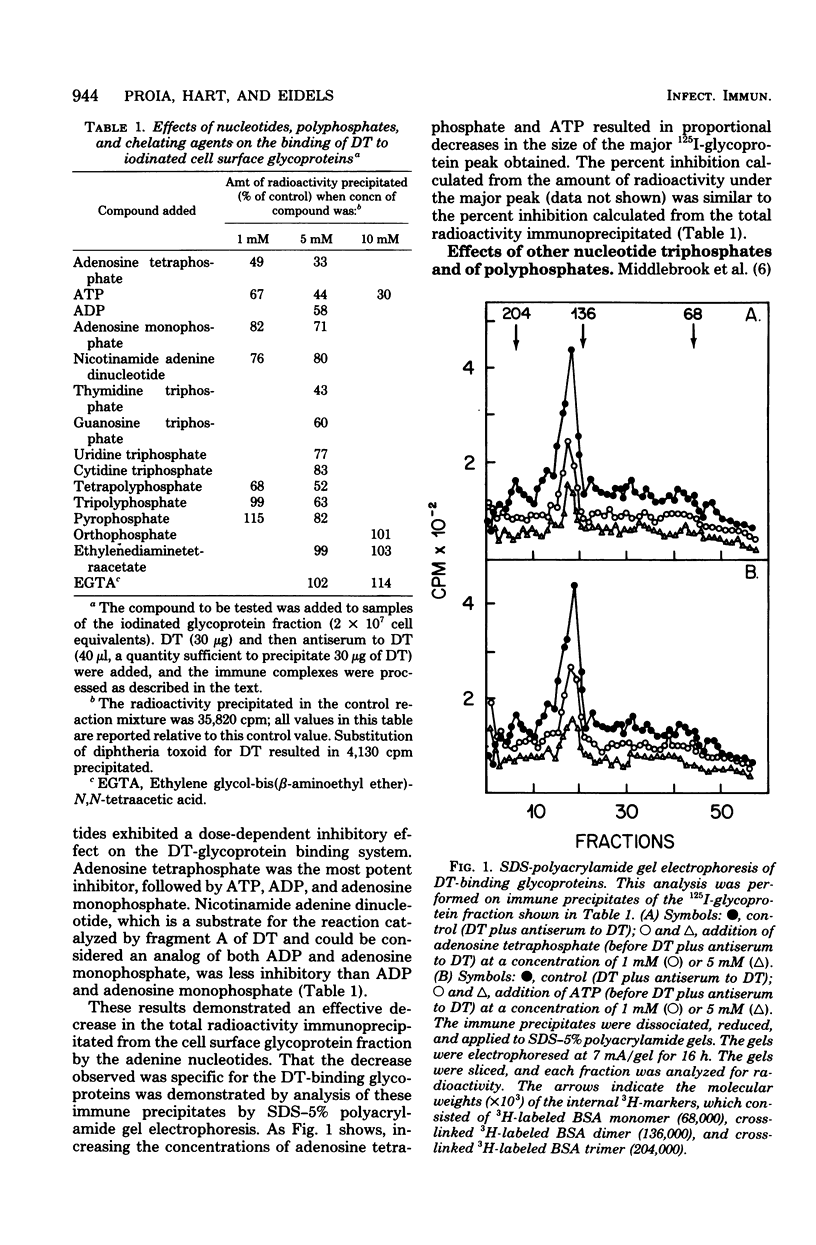
The binding of diphtheria toxin to 125I-labeled cell surface glycoproteins from hamster thymocytes was shown to be inhibited by nucleotides. The relative effectiveness of the nucleotides (at 5 mM) was found to be thymidine triphosphate greater than adenosine triphosphate greater than guanosine triphosphate greater than uridine triphosphate greater than cytidine triphosphate. When adenine-containing compounds were used, the relative effectiveness was determined to be adenosine tetraphosphate greater than adenosine triphosphate greater than adenosine diphosphate greater than adenosine monophosphate. In addition, tetrapolyphosphate, tripolyphosphate, inositol hexaphosphate (phytic acid), and the highly phosphorylated proteins casein and phosvitin were also shown to be potent inhibitors of the binding of diphtheria toxin to 125I-labeled cell surface glycoproteins. Diphtheria toxin was shown to bind directly to 125I-casein; this binding was also inhibited by the highly phosphorylated compounds and was decreased by pretreatment of the 125I-casein with alkaline phosphatase. These results suggest that diphtheria toxin binds to regions of high phosphate density and raise the possibility that the site on the cell surface glycoproteins to which diphtheria toxin binds might be polyanionic in nature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L. Corynebacterium diphtheriae and its relatives. Bacteriol Rev. 1970 Dec;34(4):378–422. doi: 10.1128/br.34.4.378-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T., Neville D. M., Jr Demonstration of diphtheria toxin receptors on surface membranes from both toxin-sensitive and toxin-resistant species. J Biol Chem. 1978 Oct 10;253(19):6866–6871. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everse J., Lappi D. A., Beglau J. M., Lee C. L., Kaplan N. O. Investigations into the relationship between structure and function of diphtheria toxin. Proc Natl Acad Sci U S A. 1977 Feb;74(2):472–476. doi: 10.1073/pnas.74.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Protection of mammalian cells from diphtheria toxin by exogenous nucleotides. Can J Microbiol. 1979 Mar;25(3):285–290. doi: 10.1139/m79-046. [DOI] [PubMed] [Google Scholar]
- Milstein C. P. An application of diagonal electrophoresis to the selective purification of serine phosphate peptides. Serine phosphate peptides from ovalbumin. Biochem J. 1968 Nov;110(1):127–134. doi: 10.1042/bj1100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Proia R. L., Eidels L., Hart D. A. Diphtheria toxin-binding glycoproteins on hamster cells: candidates for diphtheria toxin receptors. Infect Immun. 1979 Sep;25(3):786–791. doi: 10.1128/iai.25.3.786-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia R. L., Hart D. A., Holmes R. K., Holmes K. V., Eidels L. Immunoprecipitation and partial characterization of diphtheria toxin-binding glycoproteins from surface of guinea pig cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):685–689. doi: 10.1073/pnas.76.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Uchida T., Gill D. M., Pappenheimer A. M., Jr Mutation in the structural gene for diphtheria toxin carried by temperate phage . Nat New Biol. 1971 Sep 1;233(35):8–11. doi: 10.1038/newbio233008a0. [DOI] [PubMed] [Google Scholar]
- Zanen J., Muyldermans G., Beugnier N. Competitive antagonists of the action of diphtheria toxin in HeLa cells. FEBS Lett. 1976 Jul 15;66(2):261–263. doi: 10.1016/0014-5793(76)80518-2. [DOI] [PubMed] [Google Scholar]



