Abstract
Studies that compare tumor genotype with phenotype have provided the basis of a new histological/molecular classification of hepatocellular adenomas. Based on two molecular criteria (presence of a TCF1/HNF1α or β-catenin mutation), and an additional histological criterion (presence or absence of an inflammatory infiltrate), subgroups of hepatocellular adenoma can be defined and distinguished from focal nodular hyperplasia. Analysis of 96 hepatocellular adenomas performed by a French collaborative network showed that they can be divided into four broad subgroups: the first one is defined by the presence of mutations in TCF1 gene inactivating the hepatocyte nuclear factor 1 (HNF1α); the second by the presence of β-catenin activating mutations; the category without mutations of HNF1α or β-catenin is further divided into 2 subgroups depending on the presence or absence of inflammation. Therefore, the approach to the diagnosis of problematic benign hepatocytic nodules may be entering a new era directed by new molecular information. It is hoped that immunohistological tools will improve significantly diagnosis of liver biopsy in our ability to distinguish hepatocellular adenoma from focal nodular hyperplasia (FNH), and to delineate clinically meaningful entities within each group to define the best clinical management. The optimal care of patients with a liver nodule will benefit from the recent knowledge coming from molecular biology and the combined expertise of hepatologists, pathologists, radiologists, and surgeons.
Keywords: Hepatocellular adenoma, HNF1α mutation, β-catenin mutation, Inflammatory adenoma, Telangiectatic adenoma, Maturity-onset diabetes of the young, Hepatocyte nuclear factor 1, CTNNB1, Focal nodular hyperplasia
INTRODUCTION
Hepatocellular adenomas (HCA) are benign liver tumors which, in occidental countries, usually occur in young women when they have been taking oral contraceptives for more than two years[1]. HCA are rare tumors, and their estimated incidence is one case per 100 000 women. The classification and nomenclature of benign hepatocytic nodules is largely based on a document written by the International Working Party in 1995[2] and at present, HCA cannot be identified conclusively by any currently available imaging technique. Without a histopathological examination, HCA, which represent a heterogeneous group of tumors, can only be at best strongly suspected.
The diagnosis of HCA is made under different clinical conditions: emergency (bleeding inside the nodule, the liver, the peritoneum), abdominal pain to mild discomfort (usually related to minor form of hemorrhage or necrosis), abnormal liver function tests, liver mass or frequently by chance (usually an ultrasound performed for a different purpose). Ultrasound identifies one or several nodules whose nature will be confirmed by CT scan or MRI. Imaging techniques cannot prove the diagnosis of adenoma but depending on the aspect of the nodule and the clinical background make a diagnosis of probability: very likely to cannot be excluded. The final diagnosis is made by the pathologist on the resected specimen. It is beyond the scope of this review to give clinical, radiological and pathological criteria for the identification of HCA. It is important to keep in mind that the diagnosis may be straightforward in many cases and on the other hand extremely difficult just because HCA can undergo changes (mainly bleeding, necrosis), therefore leading to wrong diagnosis.
The main aim of this review is to give a brief overview of the recent progress made in this field[3,4].
CLASSIFICATION AND DIAGNOSIS
Recent identification of genes recurrently mutated in hepatocellular adenomas enabled us to perform studies to search for correlation between genotype and phenotype of HCA[5,6]. These results provided the basis of a new histological/molecular classification of HCA[3]. Based on 2 molecular criteria (HNF1α mutations and β-catenin mutations), and an additional histological criterion (the presence/absence of inflammation), subgroups of HCA[3] can be defined. Analysis of 96 HCA performed by our group with a French collaborative network showed that HCA can be divided into 4 broad subgroups: the first one is defined by the presence of mutations in gene coding for HNF1α; the second by the presence of mutations in gene coding for β-catenin; the category without mutations of HNF1α or β-catenin gene is further divided into 2 subgroups depending on the presence or absence of inflammation.
Group 1- Hepatocellular adenomas with mutations of HNF1α gene
HNF1α mutations are observed in approximately 30%-50% of adenomas[3,5]. Biallelic inactivating HNF1α mutations have been identified in tumor tissue: in most of the cases, both mutations are of somatic origin, whereas in 15% of the cases, one mutation is germline and the other is somatic[3,5]. Correlations with pathological and clinical data have shown that HNF1α mutations are mainly observed in a histologically homogeneous group of tumors, characterized by marked steatosis, no cytological abnormalities and no inflammatory infiltrate (Figure 1). HNF1α mutations have been reported in association with hepatocellular carcinoma (HCC), but are uncommon (less than 5% of the HCC cases)[5]. Patients with germline HNF1α mutations were younger than those with somatic mutations, with or without clinical diabetes (see below) and they frequently had a family history of liver adenomatosis[7].
Figure 1.
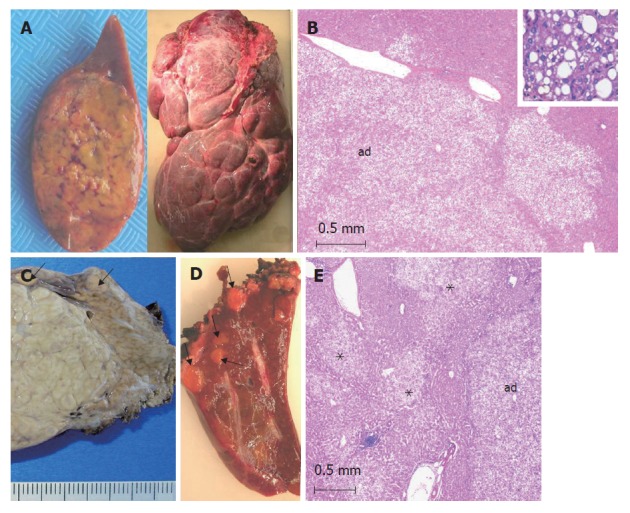
HNF1α-mutated adenomas (from different cases). A: left: single, yellowish HCA; right: a massive form involving the whole right lobe made of several numerous adjacent nodules of different sizes; B: typical aspect of a steatotic HCA (ad) with a lobulated contour. Inset: HCA at a higher magnification showing macro and microvesicular steatosis (HE); C: left: part of a large focal nodular hyperplasia (indication of surgery), nearby small adenomas (arrows) discovered by chance on the surface of the liver (corresponding to a somatic HNF1α-mutated adenomatosis). D: several yellow microadenomas in a somatic HNF1α-mutated adenomatosis; E: multiple not well limited steatotic microadenomas (asterix) nearby a larger adenoma (ad) in a patient with a constitutional HNF1α-mutated adenomatosis.
Group 2- Hepatocellular adenomas with mutations of β-catenin gene
In our series, 10% to 15% of adenomas demonstrate an activating β-catenin gene mutation including exon 3 deletion or amino acid substitution altering a site of phosphorylation[3]. Interestingly, in Taiwan, HCA are developed mainly in men and β-catenin mutations seem to be more frequently found[6,8]. In β-catenin-mutated tumors, two β-catenin target genes, glutamine synthetase (GS) and GPR49, are greatly over-expressed when compared to non-tumor tissues. Correlations with the corresponding pathological and clinical data have shown that β-catenin-mutated tumors occur more frequently in males, are usually characterized by the occurrence of cytological abnormalities and acinar pattern whereas they are less frequently steatotic (Figure 2). They are also more frequently interpreted as borderline lesions between HCA and HCC and are more frequently associated with the development of unequivocal HCC than the other HCA subtypes[3].
Figure 2.
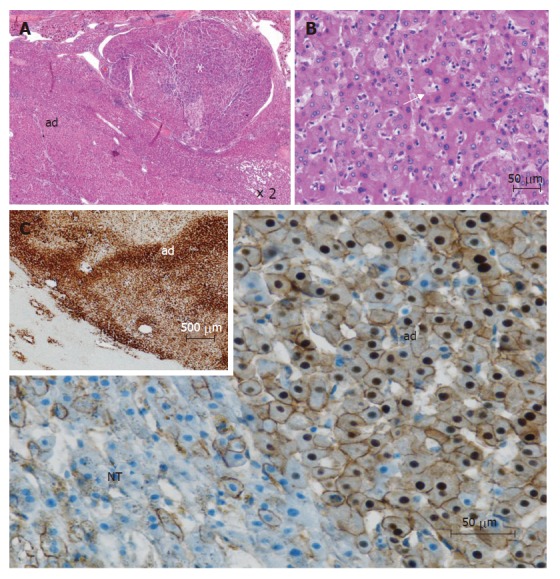
β-catenin mutated adenomas (from different cases). A: This tumor is composed of 2 parts: an adenoma (ad) and a hepatocellular carcinoma (white asterix) (HE); B: In this adenoma, some irregular nuclei and acinar arrangements are seen (white arrow) (HE); C: immunohistochemistry: cytoplasmic and nuclear overexpression of β-catenin in adenoma (ad), contrasting with normal membranous staining in adjacent non tumoral liver (NT); Inset (upper left): homogeneous immunostaining with glutamine synthetase in adenoma (ad), contrasting with normal staining of pericentral hepatocytes (lower left).
Group 3- Hepatocellular adenomas without mutations of HNF1α or β-catenin genes and with inflammatory features
This group (approximately 35%) of HCA is defined by the presence of inflammatory infiltrates and often telangiectasia[3]; it corresponds, at least partly, to the newly characterized entity (previously called telangiectatic FNH), which is a variant of HCA, often designated as inflammatory adenoma and/or telangiectatic adenoma[9,10] depending upon the importance of inflammatory infiltrates and/or sinusoidal dilatation (Figure 3). We believe this subset of tumors is HCA rather than FNH for two reasons[9]. First, the lesions display macroscopic appearances of HCA that include typical gross features of soft large tumors with a significant risk of hemorrhage instead of the nodular and fibrous appearance of typical FNH. Secondly, these lesions also have molecular characteristics of HCA, which include monoclonality and Ang1/Ang2 mRNA ratio in the range found in HCA of the usual type[9,10], and different from FNH[11]. Recently we identified a typical case of inflammatory adenomas with clinical manifestation of an inflammatory syndrome and expressing serum amyloid A (SAA) in the tumoral tissue[12]. After surgery the inflammatory syndrome disappeared. More data are needed regarding inflammatory/telangiectatic cases, which are also β-catenin mutated. In our experience, these patients are also at risk of HCC.
Figure 3.
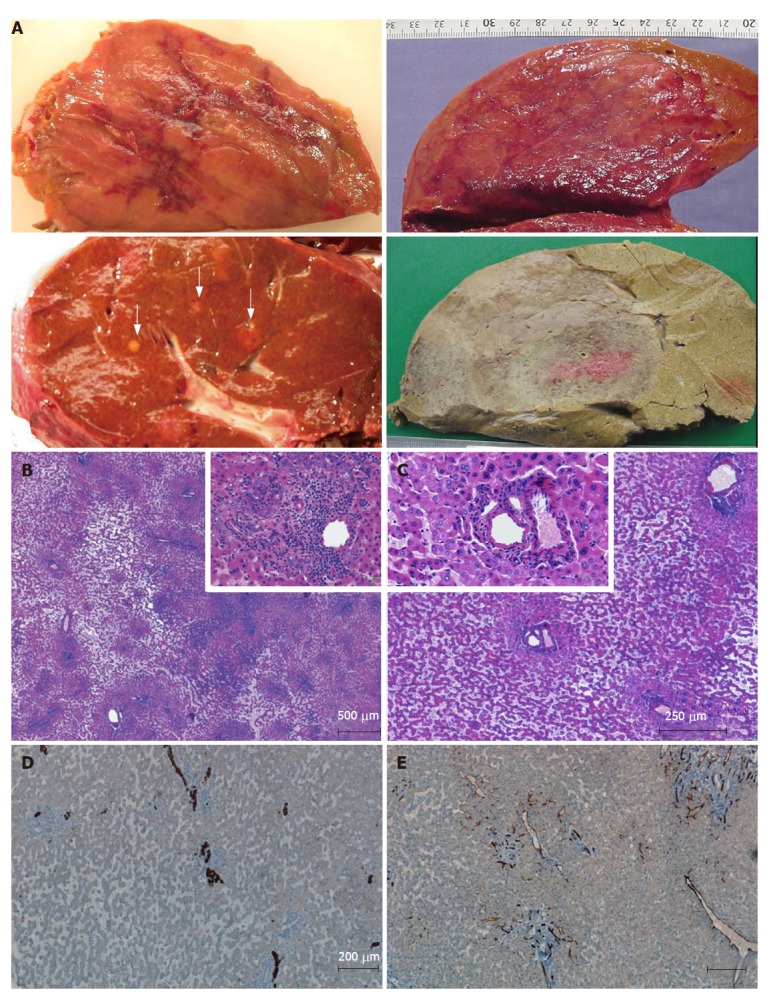
Inflammatory/telangiectatic adenomas (from different cases). A: several sections of adenomas showing different aspects. Upper left: hemorrhagic areas in the center of the tumor; lower left: several micronodules (white arrows) in a patient with a large inflammatory/telangiectatic adenoma (not shown); upper right: this massive adenoma is difficult to identify on fresh tissue; the same is more easily identified after fixation (lower right); B, C and insets: typical aspects with obvious telangiectasia and inflammatory infiltrates (B) around thick arteries (C) (HE); D: some ductular reaction is visible on cytokeratin 7 immunostaining; E: CD34 immunostaining highlights capillarized sinusoids around arteries.
Group 4- Hepatocellular adenomas without mutations of HNF1α or β-catenin genes nor specific features
This group represents less than 5%-10% of cases.
POTENTIAL CLINICAL IMPLICATIONS
This genotype/phenotype classification will help to solve some of the problems listed below. (1) The complex etiology of adenomas. Occurring essentially in women using oral contraceptives it is not, however, exceptional in women without oral contraceptives and in men[4]. There are, in addition, rare clinical conditions such as glycogenosis, familial polyposis coli, liver with vascular abnormalities, drugs related (particularly male hormones), etc[4]. It will be interesting to study to which phenotype/genotype groups they belong. Recently, several cases of HCA developed in patients with adenomatous polyposis coli were identified with an activated β-catenin pathway[13,14]; however, interestingly, we have also found a case mutated for HNF1α[15]. It will be also of interest to study the number of HCA (single, multiple, adenomatosis), the association with other hepatic (i.e. FNH, hemangioma) or extrahepatic abnormalities (i.e. meningioma)[4], the identification of small nodules in adenomatosis according to our patho-molecular classification[16]. The search for genetic predisposition to development of HCA is of particular interest particularly in association with oral contraceptive use. This field of research enabled us to recently identify germline mutations of the CYP1B1 gene in 15% of the women who developed a HNF1α-mutated HCA[17]. These results showed that inactivating mutation of CYP1B1 predisposes to develop HNF1α-mutated HCA associated with oral contraceptive use or in a familial context. (2) The radiological, clinical identification of adenomas. Magnetic resonance imaging could be an interesting tool to identify the 2 main types of adenomas. HNF1α-mutated adenomas being frequently steatotic, a signal dropout with fat suppression sequences on magnetic resonance imaging might be expected, whereas inflammatory adenomas may show strong arterial enhancement and persistent enhancement at the portal and delayed phase. It will be important to know if inflammatory adenomas are at a greater risk of bleeding and can be identified clinically. Preliminary results suggest that overweight patients are more frequently observed in the inflammatory group than in the others[18]. (3) The identification of the risk of hepatocellular carcinoma. This remains a major issue because it requires ablation of the adenoma or a stringent follow-up. Presently liver biopsy coupled to immunostaining (β-catenin and/or glutamine synthetase) is the only method available to identify patients at risk of developing hepatocellular carcinoma, this risk being greater in patients with HCA bigger than 4-5 cm and in men[18]. Patients with glycogenosis and patients taking androgens seem also at a high risk[19-21]. (4) The diagnosis of HCA variants. This remains problematic because its typical pathological features may be absent, even in the resected specimen and its pathological appearance is heterogeneous and overlaps with that of FNH[4], an entity which deserves a conservative approach. For instance, until now, the presence of ductules (CK7 positive and usually CK19 negative) in hepatocytic nodules has prevented the diagnosis of HCA for a lesion otherwise closely similar to classical HCA. One important lesson learned from molecular studies is that the presence of ductules does not preclude the diagnosis of HCA particularly when associated with an obvious sinusoidal dilatation (the so-called telangiectasia in telangiectatic FNH). These ductules can be dystrophic and differ from those observed in FNH. (5) Specific investigations. A young patient with HCA and maturity-onset diabetes of the young, type 3 (MODY 3) should be investigated for HNF1α constitutional mutation[7]. If the blood test is positive, a familial investigation for both diabetes and liver nodules is mandatory[7,22,23]. It has been shown recently that inflammatory/telangiectatic adenomas could be identified by immunohistochemistry[12] as well as HNF1α-mutated adenomas[18] on resected specimens. Work is in progress to know if these could also apply to liver biopsy material.
CONCLUSION
Molecular analysis of HCA could offer new tools for the differential diagnosis between benign lesions and suggests a revision of histological criteria currently used for their diagnosis. Genetic analysis allows us to understand the peculiar histological features and biological behavior of lesions formerly defined as telangiectatic FNH, now reclassified as adenomas. It is very difficult to offer generalized practical, evidence-based management guidelines. The details of each case have to be considered, such as patient preference, age, gender, lesional size, location, number, aetiology (glycogenosis, factors associated with HCA such as oral contraceptives, androgens, diabetes...), mode of presentation (hemorrhage, mass lesion and associated symptoms), availability of specialist techniques and expertise (hepatology, pathology, radiology, and surgery). However, the approach to the diagnosis of problematic benign hepatocytic nodules may be entering a new era directed by new molecular information. It is hoped that the available immunohistological tools will permit significant improvements in liver biopsy interpretation and in our ability to distinguish HCA from FNH, and to delineate clinically meaningful entities within each group of HCA[18]. The optimal care of patients with a liver nodule requires the combined expertise of hepatologists, pathologists, radiologists, and surgeons. Progress will also need the expertise of molecular biologists. Finally, in view of the fact that there have been and continue to be considerable difficulties in correctly identifying some cases of HCA and FNH, the literature concerning this group of tumors should be read critically and interpreted with care.
Footnotes
S- Editor Zhu LH L- Editor Zhu LH E- Editor Liu Y
References
- 1.Edmondson HA, Henderson B, Benton B. Liver-cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470–472. doi: 10.1056/NEJM197602262940904. [DOI] [PubMed] [Google Scholar]
- 2.Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 3.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Balabaud C, Bedossa P, Scoazec JY, Chiche L, Dhillon AP, Ferrell L, Paradis V, Roskams T, Vilgrain V, et al. Pathological diagnosis of liver cell adenoma and focal nodular hyperplasia: Bordeaux update. J Hepatol. 2007;46:521–527. doi: 10.1016/j.jhep.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–315. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 6.Chen YW, Jeng YM, Yeh SH, Chen PJ. P53 gene and Wnt signaling in benign neoplasms: beta-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology. 2002;36:927–935. doi: 10.1053/jhep.2002.36126. [DOI] [PubMed] [Google Scholar]
- 7.Bacq Y, Jacquemin E, Balabaud C, Jeannot E, Scotto B, Branchereau S, Laurent C, Bourlier P, Pariente D, de Muret A, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor 1alpha inactivation. Gastroenterology. 2003;125:1470–1475. doi: 10.1016/j.gastro.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Zucman-Rossi J. Genetic alterations in hepatocellular adenomas: recent findings and new challenges. J Hepatol. 2004;40:1036–1039. doi: 10.1016/j.jhep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Bioulac-Sage P, Rebouissou S, Sa Cunha A, Jeannot E, Lepreux S, Blanc JF, Blanché H, Le Bail B, Saric J, Laurent-Puig P, et al. Clinical, morphologic, and molecular features defining so-called telangiectatic focal nodular hyperplasias of the liver. Gastroenterology. 2005;128:1211–1218. doi: 10.1053/j.gastro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Paradis V, Benzekri A, Dargère D, Bièche I, Laurendeau I, Vilgrain V, Belghiti J, Vidaud M, Degott C, Bedossa P. Telangiectatic focal nodular hyperplasia: a variant of hepatocellular adenoma. Gastroenterology. 2004;126:1323–1329. doi: 10.1053/j.gastro.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Paradis V, Laurent A, Flejou JF, Vidaud M, Bedossa P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997;26:891–895. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- 12.Sa Cunha A, Blanc JF, Lazaro E, Mellottee L, Le Bail B, Zucman-Rossi J, Balabaud C, Bioulac-Sage P. Inflammatory syndrome with liver adenomatosis: the beneficial effects of surgical management. Gut. 2007;56:307–309. doi: 10.1136/gut.2006.0106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bala S, Wünsch PH, Ballhausen WG. Childhood hepatocellular adenoma in familial adenomatous polyposis: mutations in adenomatous polyposis coli gene and p53. Gastroenterology. 1997;112:919–922. doi: 10.1053/gast.1997.v112.pm9041254. [DOI] [PubMed] [Google Scholar]
- 14.Bläker H, Sutter C, Kadmon M, Otto HF, Von Knebel-Doeberitz M, Gebert J, Helmke BM. Analysis of somatic APC mutations in rare extracolonic tumors of patients with familial adenomatous polyposis coli. Genes Chromosomes Cancer. 2004;41:93–98. doi: 10.1002/gcc.20071. [DOI] [PubMed] [Google Scholar]
- 15.Jeannot E, Wendum D, Paye F, Mourra N, de Toma C, Fléjou JF, Zucman-Rossi J. Hepatocellular adenoma displaying a HNF1alpha inactivation in a patient with familial adenomatous polyposis coli. J Hepatol. 2006;45:883–886. doi: 10.1016/j.jhep.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepreux S, Laurent C, Blanc JF, Trillaud H, Le Bail B, Trouette H, Saric J, Zucman-Rossi J, Balabaud C, Bioulac-Sage P. The identification of small nodules in liver adenomatosis. J Hepatol. 2003;39:77–85. doi: 10.1016/s0168-8278(03)00145-4. [DOI] [PubMed] [Google Scholar]
- 17.Jeannot E, Poussin K, Chiche L, Bacq Y, Sturm N, Scoazec JY, Buffet C, Van Nhieu JT, Bellanné-Chantelot C, de Toma C, et al. Association of CYP1B1 germ line mutations with hepatocyte nuclear factor 1alpha-mutated hepatocellular adenoma. Cancer Res. 2007;67:2611–2616. doi: 10.1158/0008-5472.CAN-06-3947. [DOI] [PubMed] [Google Scholar]
- 18.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, Rullier A, Cubel G, Couchy G, Imbeaud S, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–748. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 19.Belghiti J, Dokmak S, Paradis V, Vilgrain V, Durand F, Valla D. Specific management for multiple liver cell adenoma: is it justified. Hepatology. 2005;42 Suppl:297A. [Google Scholar]
- 20.Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, Boney A, Sullivan J, Frush DP, Chen YT, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–162. doi: 10.1007/s10545-005-7500-2. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez I, Alter BP. Androgens and liver tumors: Fanconi's anemia and non-Fanconi's conditions. Am J Hematol. 2004;77:257–267. doi: 10.1002/ajh.20183. [DOI] [PubMed] [Google Scholar]
- 22.Foster JH, Donohue TA, Berman MM. Familial liver-cell adenomas and diabetes mellitus. N Engl J Med. 1978;299:239–241. doi: 10.1056/NEJM197808032990508. [DOI] [PubMed] [Google Scholar]
- 23.Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P, Fabre M, Oberti F, Fatome A, et al. Hepatocyte nuclear factor-1 alpha gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab. 2004;89:1476–1480. doi: 10.1210/jc.2003-031552. [DOI] [PubMed] [Google Scholar]


