Abstract
Pancreatitis is the most common complication after endoscopic retrograde cholangio-pancreatography (ERCP); the reported incidence of this complication varies from less than 1% to 40%, but a rate of 4%-8% is reported in most prospective studies involving non-selected patients. Differences in criteria for defining pancreatitis, methods of data collection, and patient populations (i.e. number of high-risk patients included in the published series) are factors that are likely to affect the varying rates of post-ERCP pancreatitis. The severity of post-ERCP pancreatitis (PEP) can range from a minor inconvenience with one or two days of added hospitalization with full recovery to a devastating illness with pancreatic necrosis, multiorgan failure, permanent disability, and even death. Although, most episodes of PEP are mild (about 90%), a small percentage of patients (about 10%) develop moderate or severe pancreatitis. In the past, PEP was often viewed as an unpredictable and unavoidable complication, with no realistic strategy for its avoidance. New data have aided in stratification of patients into PEP risk categories and new measures have been introduced to decrease the risk of PEP. As most ERCPs are performed on an outpatient basis, the majority of patients will not develop PEP and can be discharged. Alternatively, early detection of those patients who will go on to develop PEP can guide decisions regarding hospital admission and aggressive management. In the last decade, great efforts have been addressed toward prevention of this complication. Points of emphasis have included technical measures, pharmacological prophylaxis, and patient selection. This review provides a comprehensive, evidence-based assessment of published data on PEP and current suggestions for its avoidance.
Keywords: Endoscopic retrograde cholangiopan-creatography, Post-ERCP pancreatitis
DEFINITIONS
Different methodologies and definitions partially account for variations in post-endoscopic retrograde cholangio-pancreatography (ERCP) pancreatitis rates. Follow-up of patients (by phone) will detect some patients with pain who are hospitalized elsewhere or mild patients who stayed at home. Prospective studies commonly have post-ERCP pancreatitis (PEP) rates two to three times higher than those noted in retrospective studies. However, a variety of criteria have been used to define early or milder pancreatitis[1]. More severe forms of PEP are obvious and rarely escape notice. Up to 75% of asymptomatic patients have elevations in serum amylase and lipase after diagnostic or therapeutic ERCP, whereas, others develop significant abdominal pain without hyperamylasemia; neither of these entities are diagnosed as clinical pancreatitis[2,3]. Other patients fall into an overlap group, with pain that is consistent with pancreatitis but with borderline elevation of serum markers of pancreatitis or with substantial elevations of serum markers of pancreatitis combined with minimal pain that does not clearly indicate pancreatitis; these patients may be variably considered as having, or as not having, pancreatitis. In an effort to standardize the definition of PEP, a consensus conference held in 1991 proposed criteria that have become widely accepted. According to that consensus the pancreatitis is graded as mild, moderate, or severe, depending on the number of days of hospitalization required and on the level of necessary intervention[4]: (1) Mild: serum amylase at least three times normal at more than 24 h after the procedure, requiring admission or prolongation of planned admission to 2-3 d; (2) Moderate: hospitalization of 4-10 d; and (3) Severe: hospitalization of more than 10 d, or hemorrhagic pancreatitis, phlegmon or pseudocyst, or required intervention (percutaneous drainage or surgery).
The above definitions have been used in most but not all recent studies. If a serum amylase elevation of more than 5 times that of the upper normal limit is used to define PEP[5], this will reduce the apparent rate of pancreatitis. Serum lipase elevation has also been used, which may be less sensitive but more specific than serum amylase. If elevations of either lipase or amylase are considered, the apparent PEP rates may be higher[6].
Pathogenesis
In normal conditions, pancreatic digestive enzyme activation occurs mainly within the duodenum. In experimental models of acute pancreatitis, it has been suggested that digestive enzyme activation might occur within acinar cells. This has been shown in the early stages of acute pancreatitis induced by secretagogues or by diet[7]. There is co-localization of digestive enzymes and lysosomal hydrolases within large cytoplasm vacuoles[8]. As the lysosomal enzyme cathepsin B is known to be capable of activating trypsinogen and trypsin can activate the remaining digestive enzyme zymogens, the co-localization phenomenon could result in intravacuolar digestive enzyme activation[9].
During diagnostic and therapeutic ERCP, the pancreas is exposed to multiple potentially damaging factors. The exact mechanisms by which these factors trigger pancreatitis are unknown. Response to injury commonly occurs over the subsequent 2-12 h. These inciting factors include mechanical, hydrostatic, chemical, enzymatic, and microbiological etiologies.
RISK FACTORS AND PREVENTION OF POST-ERCP PANCREATITIS
Mechanical factors
Cannulation trauma. Direct contact from the endoscope rarely causes pancreatitis. Cannulation trauma to the papilla can cause edema and focal trauma, which may narrow the papillary orifice, thus creating an obstacle to the flow of pancreatic juice[10]. Moderate-to-difficult cannulation was an independent risk factor in the prospective, multicenter study of Freeman et al[11]. Difficulty found sometimes in cannulating the papillae may be due to specific pathology (scar, stone, tumor) or variant anatomy (such as with diverticula). Aggressive cannulation techniques are believed to be more traumatic and potential for duct or ampullary perforation or submucosal injection[12].
How to overcome cannulation trauma? Some cannulation-induced trauma is probably unavoidable. Gentle probing is of course advocated. Softer tipped instruments seem logical, but have never been formally compared to more rigid tipped ones. Once cannulation is begun, traumatic manipulation of the papilla should be kept to a minimum[12]. Visible bleeding or edema suggest excessive trauma. Prolonged probing in an unsuccessful direction should be avoided. If cannulation is not achieved quickly, instruments that may facilitate cannulation, such as tapered tipped cannulas, sphincterotomes, guidewires, and/or placement of a pancreatic guidewire or stent to assist biliary cannulation, should be considered. If cannulation is still not achieved, the endoscopist must balance the need for deep cannulation and use of more aggressive techniques such as precutting against the possible provocation of complications. Also, consideration should be given to terminate non-urgently indicated procedures.
Pancreatic stent placement has been shown to be the most effective technique to prevent PEP[13] (Figure 1). Pancreatic stents negate papillary orifice narrowing and maintain the flow of pancreatic juice[14]. Short-term pancreatic stent placement should be considered for higher-risk patients if pancreatic ductal anatomy is suitable, appropriate equipment is available, and the endoscopist is familiar with the technique. In particular, pancreatic stent placement should be considered in the following circumstances: before pre-cut (access) papillotomy; before or after biliary sphincterotomy for sphincter of Oddi dysfunction (SOD), pancreatic sphincterotomy, endoscopic papillectomy; after manometry or pancreatic instrumentation for suspected SOD, balloon dilation of the intact biliary sphincter, pancreatic brush cytology; and after a difficult cannulation or repeated pancreatic duct injections of contrast in patients with other risk factors. If pancreatic stent placement is advisable and the pancreatic duct is accessed first, we advocate stent placement early in the procedure. It is preferable that the prophylactic pancreatic stent should be of small diameter (3-4 French) and tailored to the diameter and configuration of the duct and to the depth to which a guidewire can be inserted. Placement of a longer stent is possible after guidewire placement to mid body or tail, whereas, a short stent (2-3 cm) can be placed if only 2-3 cm of the length of the guidewire can be inserted. In general, 3F or 4F stents are preferable for normal ducts as they include less ductitis than ≥ 5F stents. Prophylactic stents for insertion into normal ducts which we usually use are ≥ 6 cm long, small diameter (3F), unflanged on the inner end, with a duodenal pigtail to prevent inward migration. Alternatively, if the main pancreatic duct has an ansa contour and/or a deep guidance can not be passed, we advocate placement of a stent 2-3 cm up the duct with anchoring via intraductal ½ pigtail or a single inner flange. It is important that the inner tip of the stent should be in a relatively straight portion of the duct and not pushed into a bend or turn in the duct. If desired, longer stents can be partially pulled out after insertion to adjust the inner end of the stent to the configuration of the duct and to promote spontaneous passage. Pancreatic stents generally should be left in place for a minimum of 2-3 d but should be removed endoscopically (repeat pancreatography usually not required) within 2-3 wk from a normal duct if spontaneous passage is not documented by a plain abdominal radiograph. Use of such prophylactic pancreatic stents in large referral centers have shown a significant reduction in rates of PEP including severe cases (Table 1)[15-23].
Figure 1.
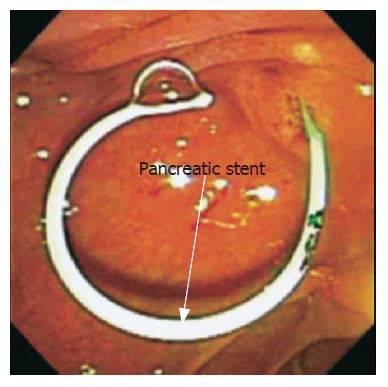
Endoscopic view of pancreatic 4 french stent after pancreatic sphincterotomy.
Table 1.
Studies assessing efficacy of pancreatic stents in prevention of post-ERCP pancreatitis
| First author/yr | Study design | Patients | n |
Pancreatitis rate without and
with pancreatic stent |
P | |
| Without (%) | With (%) | |||||
| Smithline 1993[15] | RCT | Pre-cut biliary ES, SOD, small ducts | 93 | 18 | 14 | 0.299 |
| Tarnasky 1998[16] | RCT | Biliary ES for SOD | 80 | 26 | 7 | 0.03 |
| Elton 1998[17] | Retrospective, case control | Pancreatic ES for all indications | 194 | 12.50 | 0.70 | < 0.003 |
| Fogel 2002[18] | Retrospective, case control | Biliary +/- pancreatic ES for SOD | 436 | 28.20 | 13.50 | < 0.05 |
| Norton 2002[19] | Retrospective, case control | Endosopic ampullectomy | 28 | 11.10 | 20 | > 0.05 |
| Fazel 2003[20] | RCT | Difficult cannulation, biliary ES, SOD | 76 | 28 | 5 | < 0.05 |
| Freeman 2004[21] | Prospective, case control | All attempted major papilla PD stents in high- risk therapeutic ERCP | 225 | 66.70 | 14.40 | 0.06 |
| Catalano 2004[22] | Retrospective, case control | Endoscopic ampullectomy | 103 | 16.70 | 3.30 | 0.10 |
| Harewood 2005[23] | Prospective, randomized, controlled | Endoscopic ampullectomy | 19 | 33 | 0 | 0.02 |
RCT: randomized controlled trial; ES: endoscopic sphincterotomy; SOD: sphincter of Oddi dysfunction; PD: pancreatic duct.
Endoscopic sphincterotomy (ES). During ES and stone removal, the ampullary area may be traumatized by the sphincterotome per se or by the associated extraction devices (balloons and baskets). This may obstruct the pancreatic duct orifice. Performance of biliary sphincterotomy for biliary stones or malignant biliary obstruction has not been associated with significant added independent risk of pancreatitis in most large-scale prospective multivariate analyses[11,24,25]. This was highlighted by a Japanese group[7], who showed that, although the frequency of biliary ES-induced pancreatitis was significantly higher than that of diagnostic PEP, the frequency of severe pancreatitis at 48-72 h was significantly lower within the group of patients who had undergone biliary ES. It is thought that biliary ES lowers the pancreatic intraductal pressure. However, there is some limited manometry data to support this concept.
Pancreatic sphincterotomy in the setting of SOD or idiopathic pancreatitis was associated with a higher rate of pancreatitis (up to 29%) and was found to be a significant risk factor for PEP in a large multivariate study (OR 3.1: 95% CI [1.6, 5.8])[11]. The risk of severe pancreatitis was low in that study (< 1%), probably because many patients had chronic pancreatitis and/or nearly all patients had a pancreatic stent placed to enhance drainage.
During biliary and pancreatic sphincterotomy, direct heat transfer to adjacent pancreatic parenchyma may cause injury. Thermo-couple analysis of adjacent parenchymal temperature has not been reported. Additionally, edema at the sphincterotomy site is obvious by endoscopic viewing up to 10 d after sphincterotomy[26]. Such edema in the pancreatic orifice may produce obstruction to the flow of pancreatic juice.
How to overcome? Biliary ES should be oriented towards 11:00 to 1:00 O'clock position (i.e. away from the pancreatic orifice). Excess cautery should be avoided near the pancreatic orifice (this tends to occur if too much cutting wire is inside the bile duct). Cutting then proceeds slowly with excessive local cautery. Pancreatic stents are recommended for all high risk biliary ES, PES or precutting patients.
Studies evaluating pure cutting current for biliary ES show slightly reduced PEP rates but slightly higher bleeding rates[26-28]. Overall the trade off has not been thought to be beneficial. Automatic current delivery systems are increasingly used to decrease rapid "zipper" cutting[29]. To date, they have not been shown to modify the rate of post-procedure pancreatitis.
Pre-cut (access) papillotomy. The pre-cut technique has many variations: standard needle-knife inserted at the papillary orifice with upward cutting, needle-knife ‘‘fistulotomy’’ by starting the incision above the papillary orifice, and wedging a pull-type sphincterotome into the papillary orifice or pancreatic duct. Any of these techniques has the potential to injure the pancreatic sphincter. There are different opinions as to whether the added risk of a pre-cut is because of the maneuver itself or because of the prolonged effort at cannulation that often precedes it[29-34]. However, in multivariate analyses, even after adjusting for other variables, such as the number of pancreatic injections and the difficulty level of cannulation, precut has been associated with a higher independent risk of pancreatitis, especially in multicenter studies that include endoscopists with varying experience and in which pancreatic stents are placed infrequently[24]. In a meta-analysis, pre-cut papillotomy was associated with an increased risk of pancreatitis[35]. By contrast, in many series from tertiary referral centers, the complication rate for pre-cut sphincterotomy was not different from that for standard sphincterotomy[30,33,36]. There are two possible explanations for this discrepancy. One is that an apparent low risk of pre-cut sphincterotomy may reflect case selection, such that pre-cutting is used only for patients at relatively low risk (e.g. older patients with obstructive jaundice) and with favorable anatomy, including a prominent papilla, whereas, standard traction sphincterotomy is performed in many other higher-risk circumstances, such as suspected SOD, only after free cannulation is achieved. The other explanation, also likely to be valid, is that the outcome of pre-cut papillotomy is highly operator dependent. However, an endoscopist found that, despite increasing success at pre-cutting as experience accumulated, the complication rate did not decrease (14%, mostly pancreatitis, including some severe ones) when pre-cutting was performed without pancreatic stent insertion in patients with an average risk[37]. Complications of pre-cut sphincterotomy may also vary with the indication for the procedure; in a multicenter study in which a pancreatic stent was seldom placed, precut papillotomy was associated with a higher overall rate of pancreatitis (15.3%); but, when performed for SOD, the rate was 35.3% (25% severe) compared with 11.3% (2% severe) for other indications (P = 0.01)[38]. In contrast, a subsequent study showed that needle-knife papillotomy over a pancreatic stent placed during the early stages of the procedure was shown to be substantially safer than conventional pull-type sphincterotomy without a pancreatic stent in patients with SOD[18]. Overall, the data thus suggest that traditional methods of pre-cut papillotomy are potentially injurious to the pancreas, albeit effective in experienced hands and probably safer than a protracted effort at cannulation. The benefit of pre-cut papillotomy should be weighed in each case against the risks arising from further attempts at cannulation and pancreatic manipulation, and in relation to the option of simply terminating the procedure and referring the patient to an endoscopist with more experience.
What should be done? Pre-cut should be used for biliary access only if the indication for therapy is relatively clear and the endoscopist is experienced in pre-cut techniques. Placement of temporary pancreatic stent before or after cutting should be strongly considered.
Balloon dilatation of the biliary sphincter. Balloon dilation of the biliary sphincter was introduced as an alternative to sphincterotomy for extraction of bile duct stones and is widely performed in some centers in Europe and Asia, although infrequently been used in the United States. A number of randomized trials from referral centers in countries other than the United States have shown total complication rates to be equivalent to or less than those for sphincterotomy. Most of the studies showed decreased bleeding rates, but increased pancreatitis rates. However, these studies generally involved older patients with larger stone burdens, a subgroup in which the risk of pancreatitis is relatively low[39-47]. In a multicenter, multivariate analysis from the United States, involving younger patients with bile duct stones (frequently identified in conjunction with laparoscopic cholecystectomy), balloon dilation was found to be one of only 4 independently significant risk factors, with OR 4.5: 95% CI [1.5, 13.5] and a frequency of post-ERCP pancreatitis of 16.1%[11]. In a randomized controlled trial of treatment of bile duct stones involving 337 patients in the United States, pancreatitis occurred in 15.4% (5.1% severe) after balloon dilation vs 0.8% (none severe) after pull-type sphincterotomy (P < 0.01), with two deaths from severe pancreatitis after balloon dilation[48]. A study from Germany also found a high rate of pancreatitis after papillary balloon dilation[49].
Recommendations. Balloon dilation (without prior biliary ES) for extraction of bile duct stones should not be recommended as a standard approach, especially in high risk subjects, unless there is a definite contraindication to sphincterotomy (e.g. patients with severe coagulopathy)[48]. Also, we recommend temporary pancreatic stent placement.
Endoscopic ampullectomy. Endoscopic papillectomy (ampullectomy) is increasingly performed for excision of adenomas and other lesions involving the papilla. Techniques include en bloc and piecemeal snare excision, with or without injection of saline solution to lift the lesion; biliary or pancreatic sphincterotomy; and pancreatic stent placement. The risk of pancreatitis as a result of thermal injury from papillectomy is substantial: rates in retrospective series range from 6% to 17%[50,51]. The pancreatitis can be severe and fatal.
Recommendations. In a retrospective study of papillectomy in 103 patients at 4 centers, placement of a pancreatic stent was associated with a lower frequency of pancreatitis (3.3% vs 17%; P = 0.1)[22]. Placing the stent before ampullectomy makes the resection more difficult. Placement after resection requires pancreas cannulation in a traumatized zone. Secretin and/or methylene blue assistance may be needed.
Manometry of the biliary and/or pancreatic sphincters. ERCP with manometry of the biliary and/or pancreatic sphincters is performed to diagnose sphincter of Oddi dysfunction. Initial studies reported a high rate of post-procedure pancreatitis. Continuous water perfusion (without aspiration) was used with early manometry catheters, and the duct entered was not determined or reported. These factors likely account for some cases of perfusion-related hydrostatic injury, particularly those occurring after placement of the catheter in the pancreatic duct[52]. In a randomized trial, use of the aspirating technique during pancreatic manometry significantly reduced the frequency of pancreatitis (4%) when compared with perfusion manometry without aspiration (31%)[53]. A similar study of biliary manometry found no difference in the frequency of hyperamylasemia or pancreatitis between aspirated and non-aspirated manometry groups, suggesting that perfusion injury is a factor only when the pancreatic duct is perfused[54]. Most authorities agree that only aspiration manometry should be done in the pancreas. Microtransducer (solid state) manometry catheters are an alternative to perfusion systems that eliminate the risk of perfusion injury to the pancreas. The frequency of pancreatitis when manometry was performed with a microtransducer catheter was 3.1% vs 13.8% for perfusion (non-aspiration) manometry in a randomized trial (P < 0.05)[55]. Microtransducer use is limited because of higher cost and aspiration catheters alternatives. Additionally, Freeman study showed that PEP was equally frequent when comparing suspected SOD treated empirically with manometry guided therapy[38].
In summary, aspiration type pancreatic manometry does not appear to add PEP risk to the patients at already high risk who can benefit from manometry evaluation.
Recommendation. Use any manometry catheters for the biliary studies. Use aspiration manometry catheter for pancreatic work. Temporarily stent the pancreatic duct, even if manometry is normal[18].
Contrast media
Hydrostatic factors. Injection pressure and the amount of contrast media or other fluid injection into the pancreatic duct, contribute to ductal epithelial or acinar injury. Acinarization occurs when the volume injected into the pancreatic duct exceeds the ductal capacity. Approximately 2 mL is adequate to fill the main pancreatic duct and secondary branches[56]. Quantitation of the volume injected appears to be of limited clinical value, as contrast tends to spill into the duodenum. However, the greatest elevations of total serum amylase activity and the highest frequencies of elevation occurred after complete filling of the acini[57,58]. Skude et al[57] in a series of 219 patients reported that pancreatic enzyme level elevation after ERCP correlated directly with the extent of opacification of the pancreatic duct system. Increases in intraductal pressure and filling of side branches may result in leakage of the pancreatic secretions into the circulation. In support of this proposal was the finding that serum amylase did not rise in patients with open pancreatojejunostomies. A high injection pressure and a larger injection volume into the pancreatic duct are known to cause higher and more prolonged hyperamylasemia in dogs[59]. The results of our large study[60] show clearly that increased frequency of PEP correlates directly with extent of opacification of the pancreatic duct system (Figure 2A and 2B). The highest frequency of PEP occurred after complete filling of the pancreatic duct. The extent of pancreatic duct opacification was a significant risk factor for PEP by uni- and multivariate analysis. In a relatively small study (n = 1223), there was a tendency toward increased PEP in patients in whom the pancreatic duct was opacified to the tail compared with those in whom only the duct in the head of the gland was visualized[61]. In another small study, Chen et al[62] reported that there was no statistically significant difference in the rate of pancreatitis between the patients who had a pancreatogram (n = 117) and those who did not (n = 93), 4.3 % vs 6.5%. They proposed that this was due to multiple attempts at cannulation of the bile duct alone which caused sufficient mechanical trauma at the pancreatic orifice without injection of the pancreatic duct.
Figure 2.
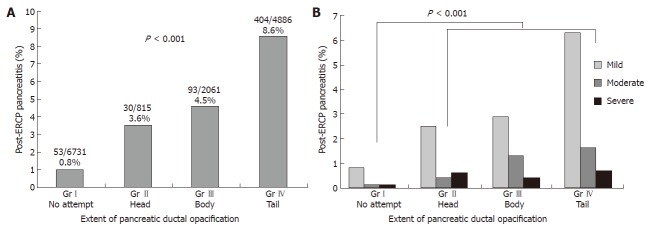
Severity of post-ERCP pancreatitis according to the extent of pancreatic duct opacification. Retrospective analysis of database with 14431 patients with no or normal pancreatogram (groupI: no pancreatogram or failed pancreatogram, group II: pancreatogram of the head only, group III: pancreatogram of head/body only, group IV: pancreatogram including tail)[69].
What to do? The endoscopist must balance the need for specific duct visualization against the possible provocation of complications. For biliary cases, the pancreas injections should be minimized. Entry into the pancreatic duct can not be avoided in many cases, but injection filling should be limited to the head. In setting with suboptimal pancreas visualization (obesity, overlying stool, gases or barium in right upper quadrant colon, etc), extra care should be taken to limit pancreas filling.
For repeated pancreas entry, aspirate out injected contrast. Guide-wire cannulation may be used to avoid repeated, undue opacification of the pancreatic ductal system. Placement of a pancreatic stent can help block pancreas re-entry. Reducing the injection pressure can minimize the risk of either submucosal injections or acinarization. Quality fluoroscopic imaging is needed to monitor pancreas filling.
Chemical factors. The contrast media used for pancreatography can provoke pancreatitis. Contrast media are differentially visualized from the surrounding tissue because of their iodine content. The osmolality and ionic nature of the contrast media are believed to be the major factors responsible for many of the adverse effects that occur after intravascular administration[63].
How to overcome? Investigators have used low-osmolality agents, usually non-ionic, in attempt to reduce PEP. Most studies including a meta-analysis failed to show reduced PEP rates from the newer, more expensive lower osmolality agents[63].
Allergy to contrast media. ERCP contrast media reactions are believed to be rare. The low frequency of allergic reactions is probably based on the slow absorption of the contrast media and also on the low dose of the agent administered. A multicenter study by Lasser et al[64] showed that the administration of methylprednisolone before the intravenous contrast injection lowered the incidence of adverse reactions from ionic agents to a level similar to that reported from other series with nonionic media. However, whether or not allergic reactions may cause pancreatitis is presently unknown.
How to overcome? Many centers give antihistamine and oral or IV corticosteroids to patients who report iodine allergies. However, there have been no randomized studies to evaluate the necessity or efficacy of this practice.
Enzymatic factors: According to the duodenal reflux pathogenic theory of acute pancreatitis, activated intestinal enzymes carried into the pancreatic ductal system by ERCP maneuvers would promote autodigestion. Current contrast agents do not activate trypsinogen in pancreatic juice. Previous prophylactic studies using old protease inhibitors failed to demonstrate any beneficial effects in preventing acute pancreatitis. More recently, gabexate mesilate, a low molecular weight protease inhibitor, has been shown to have a prophylactic effect on ERCP-induced pancreatitis[65].
Chemokines. Some studies[66-68] have indicated the potential usefulness of ERCP as a model for studying the early inflammatory response in acute pancreatitis. Kiviniemi et al[66] found that, in uncomplicated PEP cases, acute phase response is measured by serum C reactive protein (CRP) levels did not parallel the serum amylase or lipase levels. However, Blanchard et al[69] hypothesized that cytokines may be produced primarily by pancreatic parenchymal cells. Reasoning that ductal epithelium is the cell type most likely to be exposed to noxious stimuli in common causes of pancreatitis, such as ERCP and passage of a gallstone, they examined the response of well-differentiated pancreatic ductal adenocarcinoma cell lines to stimuli known to stimulate cytokine production in other cells. CAPAN-1 and CAPAN-2 cells were incubated with endotoxins or TNF-alpha and the supernatant was assayed for production of IL-1, IL-6, and IL-8 by ELISA. The cells were assayed for activation of the transcription factor NF-kappa B by electrophoretic mobility shift assay. These authors found no detectable production of IL-1 by either cell line. CAPAN-1 cells had a concentration-dependent production of IL-6 and IL-8 in response to both endotoxins and TNF-alpha. CAPAN-2 cells had a concentration-dependent production of IL-6 and IL-8 in response to TNF-alpha. They had a low-level expression of IL-8 which was unaffected by any concentration of lipopolysaccharide (LPS) and no detectable production of IL-6 in response to LPS. On the basis of these findings the authors concluded that pancreatic duct cells may play an active part in the pathogenesis of acute pancreatitis through the production of cytokines. More recently, Pezzilli et al[70], found that ERCP maneuvers significantly increase serum levels of CRP, amyloid A and IL-6 also in patients who did not develop acute pancreatitis, thus confirming the data of Blanchard et al[69]. Unfortunately, prospective double blinded randomized studies failed to show benefit of IL-10 in suppressing PEP. Further studies are needed.
Microbiological factors. Most studies do not find that infections play a role in PEP unless severe pancreatitis with necrosis occurs. Antibiotic prophylaxis is reserved for patients with high risk (immunosuppressed or cardiac prosthetic valves)[71,72].
Patient risk factors. Patient risk factors are now increasingly recognized. High-risk patients may develop PEP independent of the type of endoscopic procedure performed; moreover, the risk of pancreatitis escalates when multiple risk factors occur in the same patient or technique related risk factors occur during the procedure. High-risk patients include those with normal serum bilirubin, female gender, recurrent abdominal pain, absence of biliary ductal dilatation, and conditions suggesting possible sphincter of Oddi dysfunction, all increase the risk of pancreatitis by up to 10-fold[10]. In these patients, diagnostic ERCP should be avoided in routine practice and magnetic resonance cholangiopancreatography (MRCP) or detailed CT imaging should be alternatively used. When ERCP is clearly indicated, either diagnostic or therapeutic, these high-risk patients should be informed about the specific risk of post-procedure pancreatitis and the potential measures to decrease risks.
It is also important to use caution when performing pancreatography in patients with homozygeous alpha-1-anti trypsin deficiency[73]; two cases of hemorrhagic pancreatitis with one death following ERCP have been reported in patients with this genetic abnormality.
Physician risk factors. Adequate ERCP training is difficult to obtain in the average 2-3 years GI fellowship training program. Cannulation of the desired duct in more than 90% of cases and ability to provide biliary drainage is necessary for competence in ERCP. Trainees rarely achieve biliary cannulation competence with less than 200 cases[74]. Our experience is that 400-500 cases are needed to gain a high skill level. Nevertheless many trainees state that they plan to perform ERCP in private practice despite limited training[75]. PEP rates have not been reported from such settings. Univariate analysis showed that higher case volume per endoscopist was unexpectedly associated with a higher rather than a lower rate of pancreatitis. However, in the multivariate model, after adjustment for case mix, endoscopist case volume showed no effect on the rate of pancreatitis[11]. Previous multicenter studies have also failed to show a significant correlation between ERCP case volumes and pancreatitis rates, although they have shown a consistent correlation with bleeding rates, overall complication rates and rates of severe complications[38,76]. It is possible that none of the participating endoscopists in the study of Freeman et al[11] reached the threshold volume of ERCPs above which pancreatitis rates would diminish. The recent report by Shyham et al showed that patients who undergo ERCP in high-volume centers have a shorter length of stay and lower procedural failure rates than those undergoing ERCP at low-volume hospitals. They stated that these findings have important implications for health care policy decision-making and resource utilization[77].
Assessments for PEP. Bedside assessment alone has been shown to be suboptimal in assessing for development of pancreatitis[78]. A combined clinical and laboratory assessment has been shown to be most sensitive and specific[76-80]. Ideally, a test is needed which is readily available, inexpensive, with rapid results available, and patients are separated into patients with no PEP (for early discharge) and those with PEP (needing hospitalization).
Category 1: Pancreatic enzymes as markers of pancreatic injury
Serum amylase. Serum pancreatic enzymes rise in reaction to manipulations during ERCP in the majority of patients[81]. In the absence of clinical pancreatitis, serum amylase levels peak at 90 min to 4 h after ERCP and return to normal levels within 48 h. With PEP, the serum amylase is elevated more quickly and/or to a higher level. A test with high predictive value, helps rule out PEP and permits earlier hospital discharge. In the study by Gottlieb et al[78] prospectively evaluated 231 patients in whom serum amylase and lipase determinations were made 2 h after ERCP. Serum amylase below 276 IU/L (normal, 40-125 IU/L), and serum lipase below 1000 IU/L (normal, 4-24 IU/L) at 2 h after ERCP were highly predictive in ruling out pancreatitis with negative predictive values of 0.97 and 0.98 respectively. Testoni et al[79] evaluated 409 patients who underwent endoscopic sphincterotomy and measured serum amylase levels. Post-procedural hyperamylasemia (> 220 IU/L) occurred in 192 of the 409 patients (46.9%). Twenty-four hours after the procedure, the amylase level was still more than five times that of the upper normal limit (URL) in 26 (6.3%) patients. However, only 19 patients were considered to have developed post-procedural pancreatitis. Using serum amylase levels greater than 5 times the URL as a cut-off, they found that sensitivity of elevated serum amylase levels in predicting PEP was 26% at 2 h, 68% at 4 h and 100% at 8 h. In a study from Australia, 263 patients underwent ERCP and/or endoscopic sphincterotomy, and a 4-h post-ERCP, serum amylase level was tested and found to be a rapid and useful predictor of pancreatitis: Thomas and Sengupta[80] proposed an algorithm for patient management based on stratification by the 4-h serum amylase level. If the amylase level is less than 1.5 times the URL (negative predictive value 100%), the patient could be safely discharged home. If the amylase level is greater than 3.0 times the URL (positive predictive value 36.8%), the patient should be admitted to the hospital. If the value falls between 1.5 and 3.0 times the URL, clinical assessment, concerns or risk factors should govern decisions on management. Two studies have reported that serum amylase levels greater than 4-5 times the URL at 24 h and the presence of upper abdominal pain are more predictive of PEP. This does not help in patient management for the first 23 h[5,82].
In conclusion, a post-ERCP serum amylase level drawn 2-8 h after the procedure is a relatively rapid and reliable predictor of pancreatitis. The ideal time and cut-off is still uncertain, but studies suggest that a 4-h level greater than 4 times the URL is most practical and reliable. The above strategy is one that could be employed in the management of outpatient ERCPs[80].
A bedside urine amylase (RapignostTM) was evaluated in predicting acute pancreatitis. Kemppainen et al[83], found that the RapignostTM test was 79% sensitive and 89% specific for the diagnosis of acute pancreatitis. Hegewald et al[84] focused mainly on patients with hyperamylasemia after ERCP. They found that RapignostTM was highly specific for excluding pancreatitis at 0 h and 16-24 h (100%), but not as sensitive (78%) in predicting hyperamylasemia. At 4 h, the sensitivity and specificity were lower: 50% and 95%, respectively.
Category 2: Markers of proteolytic activation
Trypsinogen activation peptide. Trypsinogen activation peptide (TAP) is generated in the pancreas when trypsi-nogen is converted to its active form, trypsin. Plasma and urine levels of TAP have been found to be elevated and predictive of the development of acute pancreatitis[85,86]. However, this finding was not validated in other reports[87,88].
In a study looking specifically at post-ERCP patients, urinary TAP was not found to be useful in predicting mild PEP. Banks et al[89] prospectively enrolled 107 consecutive patients in a study to evaluate the utility of urine TAP assay 4 h after the procedure. Ten of the 107 patients developed mild PEP; urinary TAP levels were not significantly increased.
Trypsinogen-2. In patients with acute pancreatitis, trypsinogen-2 has been found to be markedly elevated in the serum and urine. Several studies have investigated rapid urinary tryspsinogen-2 test strips that utilize monoclonal antibodies and immunochromatography[90]. These studies have shown high sensitivities and negative predictive values, suggesting that the urinary trypsinogen-2 test can exclude pancreatitis with high probability. Kylanpaa et al[91] concluded that a negative urine dipstick test 6 h after the procedure was highly reliable for excluding PEP. Trypsinogen-2 levels in the serum as well as bound trypsin 2-alpha-1-antitrypsin complex (trypsin 2-AAT) have also been investigated as potential markers[67]. Kemppainen et al[92] prospectively evaluated 308 patients who underwent ERCP, 31 of whom developed PEP. Blood samples for the assay of trypsinogen-2, trypsin 2-AAT and amylase were collected at 1, 6, and 24 h after ERCP in all patients. The investigators found elevated trypsinogen-2 levels as early as 1 h after ERCP; this peaked at 6 h in patients with pancreatitis. Additionally, the rise in level seemed to correlate with the severity of the pancreatitis. The trypsin 2-AAT complex, however, did not show a clear rise until 24 h after ERCP. The sensitivity of a three-fold rise in trypsinogen 2 at 1 h was 74% and the specificity was 87%. These numbers were comparable to the 2-hour amylase and lipase elevations reported in the study by Gottlieb et al[78].
A drawback of using these markers is the lack of specificity, as many other conditions, including biliary and pancreatic malignancies, pseudocysts and cholangitis can cause elevations[93]. Despite this, an elevated serum trypsinogen-2 levels seen early in the course of PEP holds promise as a marker that can rapidly detect and reliably gauge the severity of PEP.
Category 3: Markers of systemic inflammation
Several studies have focused on markers that measure the degree of systemic inflammation as predictors of the development of PEP. Interleukins and CRP have been shown to be elevated in patients with acute pancreatitis[94]. These studies have shown that serum CRP is an accurate and readily available laboratory test for predicting severity of PEP, but it appears to be a late marker[66,68].
Oezcueruemez-Porsch et al[67] evaluated a number of inflammatory markers and acute phase reactants, including procalcitonin, serum amyloid A, interleukin-1 receptor antagonist, solubilized tumor necrosis factor alpha receptor II, interleukin-6, and interleukin-10 in 94 patients who underwent ERCP. Twelve patients developed PEP. The authors found that among all of the parameters that were evaluated, only peak IL-6 and IL-10 showed significant correlations with clinical data, i.e. pain score and duration of ERCP. They concluded that these two interleukins might prove useful for monitoring patients with PEP.
Pharmacological prevention. Ideally, an agent is needed to suppress or prevent an inflammatory response to the trauma of ERCP. Pharmacological prevention of pancreatitis after ERCP or sphincterotomy has been studied extensively. Prophylactic efficacy of octreotide, steroids, somatostatin, IL-10, gabexate mesilate, heparin, allopurinol, nifedipine, nitroglycerine and antibiotics has been studied. The ideal agent should be inexpensive, available and simple to administer before or immediately after ERCP.
The knowledge of the mechanisms involved in the early phases of acute pancreatitis plays a pivotal role in the search for pharmacological prophylaxis of PEP. As described, the inflammatory cascade in the acinar cells take place within a very short period of time and a delay of only a few hours exists between the pancreatic injury induced by ERCP and the onset of pancreatitis. Drugs must therefore be able to prevent the trypsinogen activation to trypsin or modulate the severity of pancreatitis within a short "therapeutic window". According to the suggested mechanism of PEP occurrence, pharmacological prevention has therefore been mainly addressed to reduce the pancreatic secretion, prevent the intra-acinar trypsinogen activation, interrupt the inflammatory cascades, relax the sphincter of Oddi, and prevent infection.
Reducing the amount of intrapancreatic enzymes. Inhibition of exocrine pancreatic secretion can be obtained by somatostatin and its synthetic analogue, octreotide. The hormone and its analogue affect the exocrine function both directly, by reducing the secretion of digestive enzymes, and indirectly, by inhibiting secretin and cholecystokinin production. In addition to their antisecretory effects, somatostatin and octreotide have been demonstrated to modulate the cytokine cascade and may also have a cytoprotective effect on pancreatic cells, although the mechanism whereby these agents exert their cytoprotective effect is unknown[95]. Octreotide has a longer biological half life. Experimental investigations have shown that both somatostatin and octreotide have a protective effect on animal models in experimental acute pancreatitis. Somatostatin has been administered for prophylactic purposes either by prolonged i.v. infusion or by a single bolus administration immediately before the ERCP procedure. In a large scale, multicenter, placebo controlled trial in 382 patients, Andriulli et al[96], found that a single dose of somatostatin at 750 μg, started 30 min before the procedure and continued for 2 h afterwards. Infusion was ineffective in preventing pancreatitis; pancreatitis occurred in 11.5% of patients who received somatostatin vs 6.5% of those given a placebo. A meta-analysis of somatostatin, in which data from short- and long-term infusion studies were pooled, found somatostatin to be ineffective (OR 0.68: 95% CI [0.44 1.04]; P = 0.075). Another study in 372 patients found that pancreatitis was significantly less frequent (1.7%) in patients treated with a bolus or a 12-h infusion of somatostatin compared with those given a placebo (9.8%)[97]. A recent study from Spain, showed that a bolus dose of 250 micrograms of somatostatin administration immediately before introducing the catheter in the papilla of Vater, does not help prevent post-ERCP acute pancreatitis[98].
Octreotide has the advantage of a simple admini-stration by subcutaneous injection. The simplest and cheapest prevention strategy, with a 100 μg subcutaneous bolus immediately before and one hour after ERCP and sphincterotomy, did not lower the incidence of post-ERCP hyperamylasemia or modify the risk of pancreatitis. This prophylactic approach aiming at inhibiting exocrine pancreatic secretion within the first hour after papillary manipulation did ensure a peak serum level of hormone at the time of papillary manipulation, and a subsequent subcutaneous dose was given to obtain a longer post-procedure effect. Subcutaneous injection of 0.2 mg of octreotide three times daily for three days effectively reduced both the incidence of post-ERCP hyperamylasemia and pain[99]; however, 24-h prophylaxis using octreotide 30 min before the procedure did not reduce the incidence of pancreatitis in selected patients at high risk of PEP[5]. From 1991 up to now, all randomized clinical trials have been published with mainly disappointing results. A meta-analysis of 10 clinical trials published before the year 2000 by Andriulli et al[100] concluded that octreotide was only associated with a reduced risk of post-ERCP hyperamylasemia but had no effect on acute pancreatitis and pain. The overall evidence in the literature suggests that somatostatin is likely to be effective in reducing the frequency of post-ERCP pancreatitis, whereas octreotide is not[101]. Whether the difference is related to the different effects of the two agents on the motor function of sphincter of Oddi or to other reasons is unclear. Unfortunately, somatostatin is not available in the USA.
Preventing co-localization of enzymes and lysosomal hydrolases. Prevention of intra-acinar trypsinogen activation to trypsin and the subsequent inflammatory cascade may be achieved by using antiprotease agents. One of the most promising agents regarding this issue is gabexate mesilate which was shown to be effective in preventing post-ERCP pancreatitis. In a prospective, multicenter, controlled trial involving 276 patients[102]: the incidence of pancreatitis was reduced four-fold in the treatment group compared with the placebo group (2% vs 8%). Another multicenter study by a Japanese group has demonstrated that a 6-h infusion was as effective as a 12-h infusion[65]. Overall, a meta-analysis study by Andriulli et al[100] evaluating six clinical trials published between 1978 and 1996 showed that gabexate mesilate was effective in preventing post-ERCP pancreatitis. However, the same author did not find any beneficial effect of the drug administered in high-risk patients over a two-hour period, starting 30 min before the procedure[96]. A second meta-analysis from the investigators who performed the first meta-analysis suggested that when all studies were combined, gabexate was minimally effective (OR 0.58: 95% CI [0.34, 0.99]), with the number needed to treat being 35; in addition, gabexate given as a short-term infusion (< 4 h) was found to be ineffective[97].
Another new antiprotease agent is ulinastatin. A recent study from Japan concluded that administration of low- and high-dose ulinastatin has similar effects to high-dose gabexate in the prevention of PEP[103]. Further studies are awaited.
Based on their mechanisms of action, both anti-secretory and anti-protease agents may be beneficial only when administered before the procedure but do not seem to be able to prevent the inflammatory cascade, once activated, and, therefore, are likely to be ineffective if used "on demand" when technique-related high-risk conditions have occurred. Moreover, available data show that these drugs are ineffective in high-risk subjects. Gabexate and ulinastatin are not available in the USA.
Blocking some steps of the enzyme-activated inflammatory cascade. A variety of agents that interrupt the inflammatory cascade at various points have been investigated. A role for corticosteroid in various forms has been investigated. Two studies (without adjustment for confounding variables or multivariate analysis) suggested that corticosteroids might be protective[104,105]. Subsequently, 5 randomized controlled trials with large numbers of patients demonstrated no benefit, nor any trend toward a benefit, for various corticosteroid formulations, including methylprednisolone, hydrocortisone, and prednisone[106-110].
In a similar approach, it was hoped that xanthine oxidase inhibitors, such as allopurinol, might prevent PEP by inhibiting generation of oxygen-derived free radicals. To date, there have been 3 large-scale, randomized, placebo-controlled studies of allopurinol for prevention of PEP in human subjects reported in the medical literature. Katsinelos et al[111], reported that pretreatment with high-dose, orally administered allopurinol decreases the frequency of PEP (3% in allopurinol group compared to 18% in placebo group). Moseler and colleagues, in another large-scale trial found no statistical difference in the frequency of PEP in patients given allopurinol (13%) compared with those given a placebo (12%)[112]. Budzynska et al[106] reported similar rates of pancreatitis in 200 patients who received either allopurinol (12%) or placebo (8%). If one was to pool the results of all 3 studies in a crude meta-analysis, total rates of pancreatitis in the allopurinol groups (62 of 579, 10.7%) and the placebo groups (71 of 565, 12.6%) are not statistically different.The allopurinol dosage and the timing of administration differ among all 3 studies: 600 mg at 15 and 3 h before ERCP in the Katsinelos study, 600 mg at 4 h and 300 mg 1 hour before ERCP in the Mosler study, and 200 mg at 15 h and 3h efore ERCP in the Budzynska study.
N-acetylcysteine a free radical scavenger inhibits capillary endothelial injury mediated by oxygen-derived free radicals. Two recent studies from Poland and Greece concluded that N-acetylcysteine failed to demonstrate any significant preventive effect on PEP, nor asymptomatic serum or urine amylase elevations[113,114].
An inhibitor of the platelet activating factor diminished experimental acute pancreatitis. Unfortunately, human trials did not indicate any reduction in PEP.
Heparin has a direct inhibitory effect on pancreatic proteases in both plasma and pancreatic tissue and also improves pancreatic microcirculation during experimental pancreatitis. Administration of heparin before the ERCP appeared to reduce the risk of post-ERCP pancreatitis without an increased risk of bleeding. Unfortunately, results from a well-designed prospective randomized controlled trial in 438 patients found that low-molecular weight heparin given 2 h before and 22 h after ERCP did not result in even a trend toward a protective effect against post-ERCP pancreatitis (8.8% vs 8.1%; P = 0.87); there also were two episodes of bleeding in the treatment group[115].
More recently, attempts to block the inflammatory cascade have been carried out by using an anti-inflammatory cytokine, recombinant interleukin-10 with encouraging results in experimental models[116,117]. A randomized trial that included 144 higher-risk patients undergoing ERCP found lower rates of pancreatitis in each of two treatment groups (3% and 5%) vs the control group (11%) (P < 0.05); the distribution of risk factors was somewhat imbalanced between the groups[118]. In contrast, another study of average-risk patients in which a lower dose of IL-10 (8 mcg/kg) was administered failed to demonstrate any significant difference, or any trend toward a difference, in the frequency of pancreatitis in treated patients (11%) vs those given a placebo (9%)[119].
A simple agent for interrupting the inflammatory cascade, based partly on its ability to inhibit phospho-lipase-A2, prostaglandins, or endothelial neutrophil attachment during acute pancreatitis is diclofenac, a nonsteroidal anti-inflammatory agent. In a prospective, randomized study of average risk patients, diclofenac given as a rectal suppository immediately after ERCP decreased PEP[120]. Another prospective randomized study involving 223 predominantly high-risk patients, the oral diclofenac form showed no benefit[121].
Reducing sphincter of Oddi post-procedure pressure. Several agents have been used in an effort to relax the sphincter of Oddi, promote pancreatic drainage and, thereby, prevent pancreatitis. Nifedipine, a calcium channel antagonist, was ineffective in two randomized control trials in average risk patients[122,123]. A topical spray of lidocaine on the papilla has been proposed to have a relaxing effect, but a randomized trial in average-risk patients did not demonstrate efficacy[124]. Nitroglycerine was used in an attempt to relax the sphincter of Oddi, facilitate cannulation, and prevent PEP. The results with nitroglycerine in two randomized trials were more encouraging. In one study, 186 patients at average risk for pancreatitis were randomized to sublingual nitroglycerine or placebo before ERCP, and a significant reduction in the frequency of pancreatitis was observed in the nitroglycerine group (7.7% vs 17.8%; P < 0.05); the drug was primarily effective in patients undergoing diagnostic ERCP and in those who had cholangiography alone, which generally is of low risk[125]. In the other study, 144 patients at average risk were randomized to a nitroglycerine patch or a placebo[126]. There was a significant reduction in the frequency of pancreatitis in the nitroglycerine group (4% vs 15%; P = 0.03) and by multivariate analysis treatment with nitroglycerine was independently significant. Problems with both of these studies include background rates of pancreatitis in the control groups that seemed unusually high for low-to average-risk patient groups and limited assessment of efficacy in high-risk patients. A recent prospective, randomized, placebo-controlled trial of transdermal glyceryl trinitrate involving 318 patients did not show any improvement in the rate of success in ERCP cannulation or prevention of PEP in either average or high-risk patient groups[127].
Reducing the risk of post-procedure infection. Administration of antibiotics was postulated by one group of investigators to prevent pancreatitis by limiting secondary infection. A single randomized trial in 321 predominantly average risk patients compared prophylactically administered ceftazidime to placebo: the frequency of PEP was significantly lower in the antibiotics-treated group (2.6% vs 9.4%; P = 0.009), a finding sustained in a multivariate analysis with a limited number of potentially confounding variables[128]. There has been no confirmatory study of this intriguing hypothesis. According to the guidelines of the European Society of Gastrointestinal Endoscopy[129], antibiotic prophylaxis is recommended even in average risk patients in the case of therapeutic retrograde cholangiopancreatography. However, the American Society of Gastrointestinal Endoscopy does not recommend prophylactic antibiotics for average risk patients (Table 2).
Table 2.
Pharmacalogical prevention of post-ERCP pancreatitis: targeted mechanisms, drug tested, quality of evidence, and overall results1
| Mechanism | Drug | Evidence | Average risk patients | High risk patients |
| Sphincter spasm | Ca++ channel blocker Lidocaine (topical) Nitroglycerine | B B B | Ineffective Ineffective Possibly effective in high dose | No data No data Ineffective |
| Infection | Antibiotics | B | Possible effective | No data |
| Contrast | Nonionic contrast | A | Ineffective | Ineffective |
| Toxicity | Corticosteroids | A | Ineffective | Ineffective |
| Inflammatory | Allopurinol | B | Ineffective | Ineffective |
| PAF inhibitors | A | Ineffective | Ineffective | |
| Cascade | IL-10 | B | Ineffective | Ineffective |
| Heparin derivatives | A | Ineffective | Ineffective | |
| NSAID | B | Possibly effective | No data | |
| Gabexate | A | Ineffective (≤ 6 h infusion) | Ineffective | |
| A | Effective (12-h infusion) | No data | ||
| Pancreatic secretions | Octreotide | A | Ineffective | No data |
| Somatostatin | A | Ineffective (≤ 6-h infusion) | No data | |
| B | Possibly effective (12-24 h infusion) |
PAF: Platelet activating factor; IL-10: interleukin 10; NSAID: non-steroidal anti-inflammatory drug. The level of evidence was graded by using a system adapted from Cook et al 17 as follows: Grade A supported by two or more randomized trials with P values < 0.05, appropriate methodology, and no conflicting results in the trials; Grade B supported by randomized trials with P values > 0.05, and/or inappropriate methodology, and/or inadequate sample sizes, and/or conflicting results among the trials; Grade C supported by non-randomized trials. 1Adapted from Freeman and Guda[5].
CAPSULE SUMMARY
Which test for early prediction of PEP?
Although, no single available test has been shown to be 100% reliable, serum amylase or lipase levels at 2-4 h greater than 4-5 times the URL in conjunction with clinical assessment are practical predictors. We request these tests mainly the morning after ERCP on hospitalized patients. We do not routinely draw post-ERCP inflammatory markers.
Which prophylactic medical therapy?
At present there are no drugs in widespread use among those drugs mentioned above. A strategy of routine chemoprevention in average risk patients would require a low cost agent to be cost-effective. However, a strategy of chemoprevention only in high-risk cases could more readily be cost-effective, but up to now no drug has been definitely proven effective. Somatostatin and gabexate (not available in USA) six-hour infusion, suppository diclofenac and high dose transdermal or sublingual glyceryl trinitrate appear promising but need further studies in high- and low-risk patients.
Suggested strategies for avoiding post-ERCP pancreatitis
They are shown in Table 3.
Table 3.
Suggested strategies for avoiding post-ERCP pancreatitis
| Risk factors | Recommendations |
| Suspected SOD (sphincter of Oddi manometry is to be performed) | - Usage of an aspirating catheter technique is strongly recommended, particularly for pancreatic manometry. - Stent the pancreas after. - Do in high volume referral centers. |
| Difficult cannulation | - Once cannulation is begun, traumatic manipulation of the papilla should be kept to a minimum. - Placement of a pancreatic stents to assist biliary cannulation, should be considered. |
| Pre-cut sphincterotomy | - Should be used for biliary access only if the indication for therapy is relatively clear and the endoscopist is experienced in pre-cut techniques. - Strongly consider placement of temporary pancreatic stent before or after cutting. |
| During traction biliary and pancreatic sphincterotomy | - Biliary sphincterotomy should be oriented toward the region from 11:00 to 1:00 o’clock on the papilla (i.e., away from the pancreatic orifice). - Consider use of pure-cut current for pancreatic ES. |
| Balloon dilation of the intact biliary sphincter for stone extraction | - Should be avoided in routine practice, unless the risk of sphincterotomy is unusually high (e.g., patients with severe coagulopathy). - Stent the pancreas after. |
| Biliary stents | - Generally should not be placed through an intact biliary sphincter in patients with suspected SOD - When placing a plastic biliary stent > 7F for any reason, consider biliary ES first, to help prevent pancreatic orifice occlusion. |
| Pancreatic brush cytology | - Consider temporary pancreatic stent placement. |
| Patients with hilar tumors | - Biliary sphincterotomy is recommended before placement of transpapillary biliary stents. |
| Pancreatic duct injection | - Pancreatic injection should be avoided if the indication for ERCP pertains to the biliary tract alone. |
| and/or Pancreatic acinarization | - Avoid filling of the body and tail of the pancreas unless clinically needed. - Over injection (acinarization) of the pancreas should be avoided. - Use guidewire to aid view of duct entered (instead of repeat dye injection. - Limit pancreatic filling in obese patients or other settings with suboptimal fluoroscopic viewing. |
| High risk patients (normal serum bilirubin, female gender, recurrent abdominal pain, absence of biliary dilatation, conditions suggesting possible sphincter of Oddi dysfunction, prior post-ERCP pancreatitis, recurrent pancreatitis or absent chronic pancreatitis) | - Consider non-invasive imaging techniques such as MRCP, EUS, or laparoscopic cholecystectomy with intra-operative cholangiography. - Once a decision for ERCP need is made, the endoscopist should assess the risk profile of the patient and plan maneuvers and modify technique accordingly. - Consider referral to a high volume centers with the capability to reliably place protective small caliber pancreatic stents |
Footnotes
S- Editor Liu Y L- Editor Ma JY E- Editor Chen GJ
References
- 1.Adler DG, Lichtenstein D, Baron TH, Davila R, Egan JV, Gan SL, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, et al. The role of endoscopy in patients with chronic pancreatitis. Gastrointest Endosc. 2006;63:933–937. doi: 10.1016/j.gie.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Testoni PA, Bagnolo F, Natale C, Primignani M. Incidence of post-endoscopic retrograde-cholangiopancreatography/sphincterotomy pancreatitis depends upon definition criteria. Dig Liver Dis. 2000;32:412–418. doi: 10.1016/s1590-8658(00)80262-5. [DOI] [PubMed] [Google Scholar]
- 3.Murray WR. Reducing the incidence and severity of post ERCP pancreatitis. Scand J Surg. 2005;94:112–116. doi: 10.1177/145749690509400206. [DOI] [PubMed] [Google Scholar]
- 4.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 5.Testoni PA, Bagnolo F. Pain at 24 hours associated with amylase levels greater than 5 times the upper normal limit as the most reliable indicator of post-ERCP pancreatitis. Gastrointest Endosc. 2001;53:33–39. doi: 10.1067/mge.2001.111390. [DOI] [PubMed] [Google Scholar]
- 6.Yang RW, Shao ZX, Chen YY, Yin Z, Wang WJ. Lipase and pancreatic amylase activities in diagnosis of acute pancreatitis in patients with hyperamylasemia. Hepatobiliary Pancreat Dis Int. 2005;4:600–603. [PubMed] [Google Scholar]
- 7.Akashi R, Kiyozumi T, Tanaka T, Sakurai K, Oda Y, Sagara K. Mechanism of pancreatitis caused by ERCP. Gastrointest Endosc. 2002;55:50–54. doi: 10.1067/mge.2002.118964. [DOI] [PubMed] [Google Scholar]
- 8.van Acker GJ, Perides G, Steer ML. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J Gastroenterol. 2006;12:1985–1990. doi: 10.3748/wjg.v12.i13.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CL, Sherman S, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, et al. Risk factors for post-ERCP pancreatitis: a prospective multicenter study. Am J Gastroenterol. 2006;101:139–147. doi: 10.1111/j.1572-0241.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 11.Freeman ML, DiSario JA, Nelson DB, Fennerty MB, Lee JG, Bjorkman DJ, Overby CS, Aas J, Ryan ME, Bochna GS, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54:425–434. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]
- 12.Freeman ML, Guda NM. ERCP cannulation: a review of reported techniques. Gastrointest Endosc. 2005;61:112–125. doi: 10.1016/s0016-5107(04)02463-0. [DOI] [PubMed] [Google Scholar]
- 13.Freeman ML. Role of pancreatic stents in prevention of post-ERCP pancreatitis. JOP. 2004;5:322–327. [PubMed] [Google Scholar]
- 14.Tarnasky PR. Mechanical prevention of post-ERCP pancreatitis by pancreatic stents: results, techniques, and indications. JOP. 2003;4:58–67. [PubMed] [Google Scholar]
- 15.Smithline A, Silverman W, Rogers D, Nisi R, Wiersema M, Jamidar P, Hawes R, Lehman G. Effect of prophylactic main pancreatic duct stenting on the incidence of biliary endoscopic sphincterotomy-induced pancreatitis in high-risk patients. Gastrointest Endosc. 1993;39:652–657. doi: 10.1016/s0016-5107(93)70217-5. [DOI] [PubMed] [Google Scholar]
- 16.Tarnasky PR, Palesch YY, Cunningham JT, Mauldin PD, Cotton PB, Hawes RH. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–1524. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 17.Elton E, Howell DA, Parsons WG, Qaseem T, Hanson BL. Endoscopic pancreatic sphincterotomy: indications, outcome, and a safe stentless technique. Gastrointest Endosc. 1998;47:240–249. doi: 10.1016/s0016-5107(98)70320-7. [DOI] [PubMed] [Google Scholar]
- 18.Fogel EL, Eversman D, Jamidar P, Sherman S, Lehman GA. Sphincter of Oddi dysfunction: pancreaticobiliary sphincterotomy with pancreatic stent placement has a lower rate of pancreatitis than biliary sphincterotomy alone. Endoscopy. 2002;34:280–285. doi: 10.1055/s-2002-23629. [DOI] [PubMed] [Google Scholar]
- 19.Norton ID, Gostout CJ, Baron TH, Geller A, Petersen BT, Wiersema MJ. Safety and outcome of endoscopic snare excision of the major duodenal papilla. Gastrointest Endosc. 2002;56:239–243. doi: 10.1016/s0016-5107(02)70184-3. [DOI] [PubMed] [Google Scholar]
- 20.Fazel A, Quadri A, Catalano MF, Meyerson SM, Geenen JE. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–294. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 21.Freeman ML, Overby C, Qi D. Pancreatic stent insertion: consequences of failure and results of a modified technique to maximize success. Gastrointest Endosc. 2004;59:8–14. doi: 10.1016/s0016-5107(03)02530-6. [DOI] [PubMed] [Google Scholar]
- 22.Catalano MF, Linder JD, Chak A, Sivak MV, Raijman I, Geenen JE, Howell DA. Endoscopic management of adenoma of the major duodenal papilla. Gastrointest Endosc. 2004;59:225–232. doi: 10.1016/s0016-5107(03)02366-6. [DOI] [PubMed] [Google Scholar]
- 23.Harewood GC, Pochron NL, Gostout CJ. Prospective, randomized, controlled trial of prophylactic pancreatic stent placement for endoscopic snare excision of the duodenal ampulla. Gastrointest Endosc. 2005;62:367–370. doi: 10.1016/j.gie.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 25.Vandervoort J, Soetikno RM, Tham TC, Wong RC, Ferrari AP, Montes H, Roston AD, Slivka A, Lichtenstein DR, Ruymann FW, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56:652–656. doi: 10.1067/mge.2002.129086. [DOI] [PubMed] [Google Scholar]
- 26.Stefanidis G, Karamanolis G, Viazis N, Sgouros S, Papadopoulou E, Ntatsakis K, Mantides A, Nastos H. A comparative study of postendoscopic sphincterotomy complications with various types of electrosurgical current in patients with choledocholithiasis. Gastrointest Endosc. 2003;57:192–197. doi: 10.1067/mge.2003.61. [DOI] [PubMed] [Google Scholar]
- 27.Gorelick A, Cannon M, Barnett J, Chey W, Scheiman J, Elta G. First cut, then blend: an electrocautery technique affecting bleeding at sphincterotomy. Endoscopy. 2001;33:976–980. doi: 10.1055/s-2001-17918. [DOI] [PubMed] [Google Scholar]
- 28.Macintosh DG, Love J, Abraham NS. Endoscopic sphincterotomy by using pure-cut electrosurgical current and the risk of post-ERCP pancreatitis: a prospective randomized trial. Gastrointest Endosc. 2004;60:551–556. doi: 10.1016/s0016-5107(04)01917-0. [DOI] [PubMed] [Google Scholar]
- 29.Cotton PB. Precut papillotomy--a risky technique for experts only. Gastrointest Endosc. 1989;35:578–579. doi: 10.1016/s0016-5107(89)72921-7. [DOI] [PubMed] [Google Scholar]
- 30.Freeman ML. Precut (access) sphincterotomy. Techniques in Gastrointestinal Endoscopy. 1999;1:40–48. [Google Scholar]
- 31.Vandervoort J, Carr-Locke DL. Needle-knife access papillotomy: an unfairly maligned technique? Endoscopy. 1996;28:365–366. doi: 10.1055/s-2007-1005482. [DOI] [PubMed] [Google Scholar]
- 32.Rabenstein T, Ruppert T, Schneider HT, Hahn EG, Ell C. Benefits and risks of needle-knife papillotomy. Gastrointest Endosc. 1997;46:207–211. doi: 10.1016/s0016-5107(97)70087-7. [DOI] [PubMed] [Google Scholar]
- 33.Baillie J. Needle-knife papillotomy revisited. Gastrointest Endosc. 1997;46:282–284. [PubMed] [Google Scholar]
- 34.Katsinelos P, Mimidis K, Paroutoglou G, Christodoulou K, Pilpilidis I, Katsiba D, Kalomenopoulou M, Papagiannis A, Tsolkas P, Kapitsinis I, et al. Needle-knife papillotomy: a safe and effective technique in experienced hands. Hepatogastroenterology. 2004;51:349–352. [PubMed] [Google Scholar]
- 35.Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: a meta-analysis. Endoscopy. 2003;35:830–834. doi: 10.1055/s-2003-42614. [DOI] [PubMed] [Google Scholar]
- 36.Cotton PB, Geenen JE, Sherman S, Cunningham JT, Howell DA, Carr-Locke DL, Nickl NJ, Hawes RH, Lehman GA, Ferrari A, et al. Endoscopic sphincterotomy for stones by experts is safe, even in younger patients with normal ducts. Ann Surg. 1998;227:201–204. doi: 10.1097/00000658-199802000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haber GB. Prevention of post-ERCP pancreatitis. Gastrointest Endosc. 2000;51:100–103. doi: 10.1016/s0016-5107(00)70402-0. [DOI] [PubMed] [Google Scholar]
- 38.Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 39.Bergman JJ, Rauws EA, Fockens P, van Berkel AM, Bossuyt PM, Tijssen JG, Tytgat GN, Huibregtse K. Randomised trial of endoscopic balloon dilation versus endoscopic sphincterotomy for removal of bileduct stones. Lancet. 1997;349:1124–1129. doi: 10.1016/S0140-6736(96)11026-6. [DOI] [PubMed] [Google Scholar]
- 40.Fujita N, Maguchi H, Komatsu Y, Yasuda I, Hasebe O, Igarashi Y, Murakami A, Mukai H, Fujii T, Yamao K, et al. Endoscopic sphincterotomy and endoscopic papillary balloon dilatation for bile duct stones: A prospective randomized controlled multicenter trial. Gastrointest Endosc. 2003;57:151–155. doi: 10.1067/mge.2003.56. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu Y, Kawabe T, Toda N, Ohashi M, Isayama M, Tateishi K, Sato S, Koike Y, Yamagata M, Tada M, et al. Endoscopic papillary balloon dilation for the management of common bile duct stones: experience of 226 cases. Endoscopy. 1998;30:12–17. doi: 10.1055/s-2007-993721. [DOI] [PubMed] [Google Scholar]
- 42.Mathuna PM, White P, Clarke E, Merriman R, Lennon JR, Crowe J. Endoscopic balloon sphincteroplasty (papillary dilation) for bile duct stones: efficacy, safety, and follow-up in 100 patients. Gastrointest Endosc. 1995;42:468–474. doi: 10.1016/s0016-5107(95)70052-8. [DOI] [PubMed] [Google Scholar]
- 43.Minami A, Nakatsu T, Uchida N, Hirabayashi S, Fukuma H, Morshed SA, Nishioka M. Papillary dilation vs sphincterotomy in endoscopic removal of bile duct stones. A randomized trial with manometric function. Dig Dis Sci. 1995;40:2550–2554. doi: 10.1007/BF02220440. [DOI] [PubMed] [Google Scholar]
- 44.Ochi Y, Mukawa K, Kiyosawa K, Akamatsu T. Comparing the treatment outcomes of endoscopic papillary dilation and endoscopic sphincterotomy for removal of bile duct stones. J Gastroenterol Hepatol. 1999;14:90–96. doi: 10.1046/j.1440-1746.1999.01798.x. [DOI] [PubMed] [Google Scholar]
- 45.Prat F, Fritsch J, Choury AD, Meduri B, Pelletier G, Buffet C. Endoscopic sphincteroclasy: a useful therapeutic tool for biliary endoscopy in Billroth II gastrectomy patients. Endoscopy. 1997;29:79–81. doi: 10.1055/s-2007-1004079. [DOI] [PubMed] [Google Scholar]
- 46.Ueno N, Ozawa Y. Pancreatitis induced by endoscopic balloon sphincter dilation and changes in serum amylase levels after the procedure. Gastrointest Endosc. 1999;49:472–476. doi: 10.1016/s0016-5107(99)70045-3. [DOI] [PubMed] [Google Scholar]
- 47.Vlavianos P, Chopra K, Mandalia S, Anderson M, Thompson J, Westaby D. Endoscopic balloon dilatation versus endoscopic sphincterotomy for the removal of bile duct stones: a prospective randomised trial. Gut. 2003;52:1165–1169. doi: 10.1136/gut.52.8.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron TH, Harewood GC. Endoscopic balloon dilation of the biliary sphincter compared to endoscopic biliary sphincterotomy for removal of common bile duct stones during ERCP: a metaanalysis of randomized, controlled trials. Am J Gastroenterol. 2004;99:1455–1460. doi: 10.1111/j.1572-0241.2004.30151.x. [DOI] [PubMed] [Google Scholar]
- 49.Arnold JC, Benz C, Martin WR, Adamek HE, Riemann JF. Endoscopic papillary balloon dilation vs. sphincterotomy for removal of common bile duct stones: a prospective randomized pilot study. Endoscopy. 2001;33:563–567. doi: 10.1055/s-2001-15307. [DOI] [PubMed] [Google Scholar]
- 50.Mutignani M, Tringali A, Costamagna G. Therapeutic biliary endoscopy. Endoscopy. 2004;36:147–159. doi: 10.1055/s-2004-814182. [DOI] [PubMed] [Google Scholar]
- 51.Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, Howell DA. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001;54:202–208. doi: 10.1067/mge.2001.116564. [DOI] [PubMed] [Google Scholar]
- 52.Maldonado ME, Brady PG, Mamel JJ, Robinson B. Incidence of pancreatitis in patients undergoing sphincter of Oddi manometry (SOM) Am J Gastroenterol. 1999;94:387–390. doi: 10.1111/j.1572-0241.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 53.Sherman S, Troiano FP, Hawes RH, Lehman GA. Sphincter of Oddi manometry: decreased risk of clinical pancreatitis with use of a modified aspirating catheter. Gastrointest Endosc. 1990;36:462–466. doi: 10.1016/s0016-5107(90)71115-7. [DOI] [PubMed] [Google Scholar]
- 54.Sherman S, Hawes RH, Troiano FP, Lehman GA. Pancreatitis following bile duct sphincter of Oddi manometry: utility of the aspirating catheter. Gastrointest Endosc. 1992;38:347–350. doi: 10.1016/s0016-5107(92)70430-1. [DOI] [PubMed] [Google Scholar]
- 55.Wehrmann T, Stergiou N, Schmitt T, Dietrich CF, Seifert H. Reduced risk for pancreatitis after endoscopic microtransducer manometry of the sphincter of Oddi: a randomized comparison with the perfusion manometry technique. Endoscopy. 2003;35:472–477. doi: 10.1055/s-2003-39677. [DOI] [PubMed] [Google Scholar]
- 56.Sherman S, Lehman GA. ERCP- and endoscopic sphincterotomy-induced pancreatitis. Pancreas. 1991;6:350–367. doi: 10.1097/00006676-199105000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Skude G, Wehlin L, Maruyama T, Ariyama J. Hyperamylasaemia after duodenoscopy and retrograde cholangiopancreatography. Gut. 1976;17:127–132. doi: 10.1136/gut.17.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okuno M, Himeno S, Kurokawa M, Shinomura Y, Kuroshima T, Kanayama S, Tsuji K, Higashimoto Y, Tarui S. Changes in serum levels of pancreatic isoamylase, lipase, trypsin, and elastase 1 after endoscopic retrograde pancreatography. Hepatogastroenterology. 1985;32:87–90. [PubMed] [Google Scholar]
- 59.Waldron RL, Luse SA, Wollowick HE, Seaman WB. Demonstration of a retrograde pancreatic pathway: correlation of roentgenographic and electron microscopic studies. Am J Roentgenol Radium Ther Nucl Med. 1971;111:695–699. doi: 10.2214/ajr.111.4.695. [DOI] [PubMed] [Google Scholar]
- 60.Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Lehman GA. Frequency and severity of post-ERCP pancreatitis correlated with extent of pancreatic ductal opacification. Gastrointest Endosc. 2007;65:385–393. doi: 10.1016/j.gie.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 61.Ciocirlan M, Ponchon T. Diagnostic endoscopic retrograde cholangiopancreatography. Endoscopy. 2004;36:137–146. doi: 10.1055/s-2004-814181. [DOI] [PubMed] [Google Scholar]
- 62.Chen YK, Foliente RL, Santoro MJ, Walter MH, Collen MJ. Endoscopic sphincterotomy-induced pancreatitis: increased risk associated with nondilated bile ducts and sphincter of Oddi dysfunction. Am J Gastroenterol. 1994;89:327–333. [PubMed] [Google Scholar]
- 63.George S, Kulkarni AA, Stevens G, Forsmark CE, Draganov P. Role of osmolality of contrast media in the development of post-ERCP pancreatitis: a metanalysis. Dig Dis Sci. 2004;49:503–508. doi: 10.1023/b:ddas.0000020511.98230.20. [DOI] [PubMed] [Google Scholar]
- 64.Lasser EC, Berry CC, Mishkin MM, Williamson B, Zheutlin N, Silverman JM. Pretreatment with corticosteroids to prevent adverse reactions to nonionic contrast media. AJR Am J Roentgenol. 1994;162:523–526. doi: 10.2214/ajr.162.3.8109489. [DOI] [PubMed] [Google Scholar]
- 65.Masci E, Cavallini G, Mariani A, Frulloni L, Testoni PA, Curioni S, Tittobello A, Uomo G, Costamagna G, Zambelli S, et al. Comparison of two dosing regimens of gabexate in the prophylaxis of post-ERCP pancreatitis. Am J Gastroenterol. 2003;98:2182–2186. doi: 10.1111/j.1572-0241.2003.07698.x. [DOI] [PubMed] [Google Scholar]
- 66.Kiviniemi H, Juvonen T, Mäkelä J. Acute phase response in patients with uncomplicated and complicated endoscopic retrogradic cholangiopancreaticography. HPB Surg. 1994;8:129–131. doi: 10.1155/1994/69467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oezcueruemez-Porsch M, Kunz D, Hardt PD, Fadgyas T, Kress O, Schulz HU, Schnell-Kretschmer H, Temme H, Westphal S, Luley C, et al. Diagnostic relevance of interleukin pattern, acute-phase proteins, and procalcitonin in early phase of post-ERCP pancreatitis. Dig Dis Sci. 1998;43:1763–1769. doi: 10.1023/a:1018887704337. [DOI] [PubMed] [Google Scholar]
- 68.Kaw M, Singh S. Serum lipase, C-reactive protein, and interleukin-6 levels in ERCP-induced pancreatitis. Gastrointest Endosc. 2001;54:435–440. doi: 10.1067/mge.2001.117763. [DOI] [PubMed] [Google Scholar]
- 69.Blanchard JA, Barve S, Joshi-Barve S, Talwalker R, Gates LK. Cytokine production by CAPAN-1 and CAPAN-2 cell lines. Dig Dis Sci. 2000;45:927–932. doi: 10.1023/a:1005573024448. [DOI] [PubMed] [Google Scholar]
- 70.Pezzilli R, Gabbrielli A, Labate AM, D'Alessio P, Barakat B, Costamagna G, Dibenedetti F, Massa M, Merlini G, d'Eril GM. Does gabexate mesilate affect serum concentrations of acute phase proteins after endoscopic retrograde cholangiopancreatography examination? Hepatogastroenterology. 2003;50:851–855. [PubMed] [Google Scholar]
- 71.Sauter G, Grabein B, Huber G, Mannes GA, Ruckdeschel G, Sauerbruch T. Antibiotic prophylaxis of infectious complications with endoscopic retrograde cholangiopancreatography. A randomized controlled study. Endoscopy. 1990;22:164–167. doi: 10.1055/s-2007-1012830. [DOI] [PubMed] [Google Scholar]
- 72.Campos GM, Herani Filho B, Pereira CA, Machado AM, Baretta MC. Bacteremia after endoscopic retrograde cholangiopancreatography with and without therapeutic procedure: frequency, associated factors and clinical significance. Rev Assoc Med Bras. 1997;43:326–334. doi: 10.1590/s0104-42301997000400009. [DOI] [PubMed] [Google Scholar]
- 73.Svenberg T, Häggmark T, Strandvik B, Slezak P. Haemorrhagic pancreatitis after ERCP in patients with alpha 1-antitrypsin deficiency. Lancet. 1988;1:772. doi: 10.1016/s0140-6736(88)91585-1. [DOI] [PubMed] [Google Scholar]
- 74.Jowell PS, Baillie J, Branch MS, Affronti J, Browning CL, Bute BP. Quantitative assessment of procedural competence. A prospective study of training in endoscopic retrograde cholangiopancreatography. Ann Intern Med. 1996;125:983–989. doi: 10.7326/0003-4819-125-12-199612150-00009. [DOI] [PubMed] [Google Scholar]
- 75.Sivak MV. "Trained in ERCP". Gastrointest Endosc. 2003;58:412–414. doi: 10.1067/s0016-5107(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 76.Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 77.Varadarajulu S, Kilgore ML, Wilcox CM, Eloubeidi MA. Relationship among hospital ERCP volume, length of stay, and technical outcomes. Gastrointest Endosc. 2006;64:338–347. doi: 10.1016/j.gie.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Gottlieb K, Sherman S, Pezzi J, Esber E, Lehman GA. Early recognition of post-ERCP pancreatitis by clinical assessment and serum pancreatic enzymes. Am J Gastroenterol. 1996;91:1553–1557. [PubMed] [Google Scholar]
- 79.Testoni PA, Caporuscio S, Bagnolo F, Lella F. Twenty-four-hour serum amylase predicting pancreatic reaction after endoscopic sphincterotomy. Endoscopy. 1999;31:131–136. doi: 10.1055/s-1999-13660. [DOI] [PubMed] [Google Scholar]
- 80.Thomas PR, Sengupta S. Prediction of pancreatitis following endoscopic retrograde cholangiopancreatography by the 4-h post procedure amylase level. J Gastroenterol Hepatol. 2001;16:923–926. doi: 10.1046/j.1440-1746.2001.02547.x. [DOI] [PubMed] [Google Scholar]
- 81.Matull WR, Pereira SP, O'Donohue JW. Biochemical markers of acute pancreatitis. J Clin Pathol. 2006;59:340–344. doi: 10.1136/jcp.2002.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Christoforidis E, Goulimaris I, Kanellos I, Tsalis K, Demetriades C, Betsis D. Post-ERCP pancreatitis and hyperamylasemia: patient-related and operative risk factors. Endoscopy. 2002;34:286–292. doi: 10.1055/s-2002-23630. [DOI] [PubMed] [Google Scholar]
- 83.Kemppainen EA, Hedström JI, Puolakkainen PA, Sainio VS, Haapiainen RK, Perhoniemi V, Osman S, Kivilaakso EO, Stenman UH. Rapid measurement of urinary trypsinogen-2 as a screening test for acute pancreatitis. N Engl J Med. 1997;336:1788–1793. doi: 10.1056/NEJM199706193362504. [DOI] [PubMed] [Google Scholar]
- 84.Hegewald MJ, Isenberg G, Sterling RK, Cooper GS, Chak A, Sivak MV. Evaluation of a rapid urine amylase test using post-ERCP hyperamylasemia as a model. Am J Gastroenterol. 2001;96:2640–2645. doi: 10.1111/j.1572-0241.2001.04118.x. [DOI] [PubMed] [Google Scholar]
- 85.Tenner S, Fernandez-del Castillo C, Warshaw A, Steinberg W, Hermon-Taylor J, Valenzuela JE, Hariri M, Hughes M, Banks PA. Urinary trypsinogen activation peptide (TAP) predicts severity in patients with acute pancreatitis. Int J Pancreatol. 1997;21:105–110. doi: 10.1007/BF02822381. [DOI] [PubMed] [Google Scholar]
- 86.Lempinen M, Stenman UH, Halttunen J, Puolakkainen P, Haapiainen R, Kemppainen E. Early sequential changes in serum markers of acute pancreatitis induced by endoscopic retrograde cholangiopancreatography. Pancreatology. 2005;5:157–164. doi: 10.1159/000085267. [DOI] [PubMed] [Google Scholar]
- 87.Gudgeon AM, Heath DI, Hurley P, Jehanli A, Patel G, Wilson C, Shenkin A, Austen BM, Imrie CW, Hermon-Taylor J. Trypsinogen activation peptides assay in the early prediction of severity of acute pancreatitis. Lancet. 1990;335:4–8. doi: 10.1016/0140-6736(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 88.Sáez J, Martínez J, Trigo C, Sánchez-Payá J, Compañy L, Laveda R, Griñó P, García C, Pérez-Mateo M. Clinical value of rapid urine trypsinogen-2 test strip, urinary trypsinogen activation peptide, and serum and urinary activation peptide of carboxypeptidase B in acute pancreatitis. World J Gastroenterol. 2005;11:7261–7265. doi: 10.3748/wjg.v11.i46.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banks PA, Carr-Locke DL, Slivka A, Van Dam J, Lichtenstein DR, Hughes M. Urinary trypsinogen activation peptides (TAP) are not increased in mild ERCP-induced pancreatitis. Pancreas. 1996;12:294–297. doi: 10.1097/00006676-199604000-00013. [DOI] [PubMed] [Google Scholar]
- 90.Chen YT, Chen CC, Wang SS, Chang FY, Lee SD. Rapid urinary trypsinogen-2 test strip in the diagnosis of acute pancreatitis. Pancreas. 2005;30:243–247. doi: 10.1097/01.mpa.0000153618.48137.ef. [DOI] [PubMed] [Google Scholar]
- 91.Kylänpää-Bäck M, Kemppainen E, Puolakkainen P, Hedström J, Haapiainen R, Perhoniemi V, Kivilaakso E, Korvuo A, Stenman U. Reliable screening for acute pancreatitis with rapid urine trypsinogen-2 test strip. Br J Surg. 2000;87:49–52. doi: 10.1046/j.1365-2168.2000.01298.x. [DOI] [PubMed] [Google Scholar]
- 92.Hedström J, Kemppainen E, Andersén J, Jokela H, Puolakkainen P, Stenman UH. A comparison of serum trypsinogen-2 and trypsin-2-alpha1-antitrypsin complex with lipase and amylase in the diagnosis and assessment of severity in the early phase of acute pancreatitis. Am J Gastroenterol. 2001;96:424–430. doi: 10.1111/j.1572-0241.2001.03457.x. [DOI] [PubMed] [Google Scholar]
- 93.Lempinen M, Stenman UH, Puolakkainen P, Hietaranta A, Haapiainen R, Kemppainen E. Sequential changes in pancreatic markers in acute pancreatitis. Scand J Gastroenterol. 2003;38:666–675. doi: 10.1080/00365520310000357. [DOI] [PubMed] [Google Scholar]
- 94.Jiang CF, Shiau YC, Ng KW, Tan SW. Serum interleukin-6, tumor necrosis factor alpha and C-reactive protein in early prediction of severity of acute pancreatitis. J Chin Med Assoc. 2004;67:442–446. [PubMed] [Google Scholar]
- 95.Poon RT, Fan ST. Antisecretory agents for prevention of post-ERCP pancreatitis: rationale for use and clinical results. JOP. 2003;4:33–40. [PubMed] [Google Scholar]
- 96.Andriulli A, Clemente R, Solmi L, Terruzzi V, Suriani R, Sigillito A, Leandro G, Leo P, De Maio G, Perri F. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002;56:488–495. doi: 10.1067/mge.2002.128130. [DOI] [PubMed] [Google Scholar]
- 97.Andriulli A, Caruso N, Quitadamo M, Forlano R, Leandro G, Spirito F, De Maio G. Antisecretory vs. antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. JOP. 2003;4:41–48. [PubMed] [Google Scholar]
- 98.Vila JJ, Jiménez FJ, Prieto C, Borobio E, Juanmartiñena JF, Borda F. Utility of bolus somatostatin administration in preventing pancreatitis after endoscopic retrograde cholangiopancreatography: a controlled, non-randomized study. Gastroenterol Hepatol. 2006;29:231–236. doi: 10.1157/13085969. [DOI] [PubMed] [Google Scholar]
- 99.Testoni PA, Lella F, Bagnolo F, Caporuscio S, Cattani L, Colombo E, Buizza M. Long-term prophylactic administration of octreotide reduces the rise in serum amylase after endoscopic procedures on Vater's papilla. Pancreas. 1996;13:61–65. doi: 10.1097/00006676-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Andriulli A, Leandro G, Niro G, Mangia A, Festa V, Gambassi G, Villani MR, Facciorusso D, Conoscitore P, Spirito F, et al. Pharmacologic treatment can prevent pancreatic injury after ERCP: a meta-analysis. Gastrointest Endosc. 2000;51:1–7. doi: 10.1016/s0016-5107(00)70377-4. [DOI] [PubMed] [Google Scholar]
- 101.Arvanitidis D, Anagnostopoulos GK, Giannopoulos D, Pantes A, Agaritsi R, Margantinis G, Tsiakos S, Sakorafas G, Kostopoulos P. Can somatostatin prevent post-ERCP pancreatitis? Results of a randomized controlled trial. J Gastroenterol Hepatol. 2004;19:278–282. doi: 10.1111/j.1440-1746.2003.03297.x. [DOI] [PubMed] [Google Scholar]
- 102.Cavallini G, Tittobello A, Frulloni L, Masci E, Mariana A, Di Francesco V. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. Gabexate in digestive endoscopy--Italian Group. N Engl J Med. 1996;335:919–923. doi: 10.1056/NEJM199609263351302. [DOI] [PubMed] [Google Scholar]
- 103.Fujishiro H, Adachi K, Imaoka T, Hashimoto T, Kohge N, Moriyama N, Suetsugu H, Kawashima K, Komazawa Y, Ishimura N, et al. Ulinastatin shows preventive effect on post-endoscopic retrograde cholangiopancreatography pancreatitis in a multicenter prospective randomized study. J Gastroenterol Hepatol. 2006;21:1065–1069. doi: 10.1111/j.1440-1746.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- 104.Weiner GR, Geenen JE, Hogan WJ, Catalano MF. Use of corticosteroids in the prevention of post-ERCP pancreatitis. Gastrointest Endosc. 1995;42:579–583. doi: 10.1016/s0016-5107(95)70014-5. [DOI] [PubMed] [Google Scholar]
- 105.Kwanngern K, Tiyapattanaputi P, Wanitpukdeedecha M, Navicharern P. Can a single dose corticosteroid reduce the incidence of post-ERCP pancreatitis? A randomized, prospective control study. J Med Assoc Thai. 2005;88 Suppl 4:S42–S45. [PubMed] [Google Scholar]
- 106.Budzyńska A, Marek T, Nowak A, Kaczor R, Nowakowska-Dulawa E. A prospective, randomized, placebo-controlled trial of prednisone and allopurinol in the prevention of ERCP-induced pancreatitis. Endoscopy. 2001;33:766–772. doi: 10.1055/s-2001-16520. [DOI] [PubMed] [Google Scholar]
- 107.De Palma GD, Catanzano C. Use of corticosteriods in the prevention of post-ERCP pancreatitis: results of a controlled prospective study. Am J Gastroenterol. 1999;94:982–985. doi: 10.1111/j.1572-0241.1999.999_u.x. [DOI] [PubMed] [Google Scholar]
- 108.Dumot JA, Conwell DL, O'Connor JB, Ferguson DR, Vargo JJ, Barnes DS, Shay SS, Sterling MJ, Horth KS, Issa K, et al. Pretreatment with methylprednisolone to prevent ERCP-induced pancreatitis: a randomized, multicenter, placebo-controlled clinical trial. Am J Gastroenterol. 1998;93:61–65. doi: 10.1111/j.1572-0241.1998.061_c.x. [DOI] [PubMed] [Google Scholar]
- 109.Sherman S, Blaut U, Watkins JL, Barnett J, Freeman M, Geenen J, Ryan M, Parker H, Frakes JT, Fogel EL, et al. Does prophylactic administration of corticosteroid reduce the risk and severity of post-ERCP pancreatitis: a randomized, prospective, multicenter study. Gastrointest Endosc. 2003;58:23–29. doi: 10.1067/mge.2003.307. [DOI] [PubMed] [Google Scholar]
- 110.Manolakopoulos S, Avgerinos A, Vlachogiannakos J, Armonis A, Viazis N, Papadimitriou N, Mathou N, Stefanidis G, Rekoumis G, Vienna E, et al. Octreotide versus hydrocortisone versus placebo in the prevention of post-ERCP pancreatitis: a multicenter randomized controlled trial. Gastrointest Endosc. 2002;55:470–475. doi: 10.1067/mge.2002.122614. [DOI] [PubMed] [Google Scholar]
- 111.Katsinelos P, Kountouras J, Chatzis J, Christodoulou K, Paroutoglou G, Mimidis K, Beltsis A, Zavos C. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest Endosc. 2005;61:407–415. doi: 10.1016/s0016-5107(04)02647-1. [DOI] [PubMed] [Google Scholar]
- 112.Mosler P, Sherman S, Marks J, Watkins JL, Geenen JE, Jamidar P, Fogel EL, Lazzell-Pannell L, Temkit M, Tarnasky P, et al. Oral allopurinol does not prevent the frequency or the severity of post-ERCP pancreatitis. Gastrointest Endosc. 2005;62:245–250. doi: 10.1016/s0016-5107(05)01572-5. [DOI] [PubMed] [Google Scholar]
- 113.Milewski J, Rydzewska G, Degowska M, Kierzkiewicz M, Rydzewski A. N-acetylcysteine does not prevent post-endoscopic retrograde cholangiopancreatography hyperamylasemia and acute pancreatitis. World J Gastroenterol. 2006;12:3751–3755. doi: 10.3748/wjg.v12.i23.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katsinelos P, Kountouras J, Paroutoglou G, Beltsis A, Mimidis K, Zavos C. Intravenous N-acetylcysteine does not prevent post-ERCP pancreatitis. Gastrointest Endosc. 2005;62:105–111. doi: 10.1016/s0016-5107(05)01574-9. [DOI] [PubMed] [Google Scholar]
- 115.Rabenstein T, Fischer B, Wiessner V, Schmidt H, Radespiel-Tröger M, Hochberger J, Mühldorfer S, Nusko G, Messmann H, Schölmerich J, et al. Low-molecular-weight heparin does not prevent acute post-ERCP pancreatitis. Gastrointest Endosc. 2004;59:606–613. doi: 10.1016/s0016-5107(04)00159-2. [DOI] [PubMed] [Google Scholar]
- 116.Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960–967. doi: 10.1053/gast.1997.v112.pm9041259. [DOI] [PubMed] [Google Scholar]
- 117.Van Laethem JL, Marchant A, Delvaux A, Goldman M, Robberecht P, Velu T, Devière J. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology. 1995;108:1917–1922. doi: 10.1016/0016-5085(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 118.Devière J, Le Moine O, Van Laethem JL, Eisendrath P, Ghilain A, Severs N, Cohard M. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology. 2001;120:498–505. doi: 10.1053/gast.2001.21172. [DOI] [PubMed] [Google Scholar]
- 119.Dumot JA, Conwell DL, Zuccaro G, Vargo JJ, Shay SS, Easley KA, Ponsky JL. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol. 2001;96:2098–2102. doi: 10.1111/j.1572-0241.2001.04092.x. [DOI] [PubMed] [Google Scholar]
- 120.Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–1791. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 121.Cheon YK, Cho KB, Watkins JL, McHenry L, Fogel EL, Sherman S, Schmidt S, Lazzell-Pannell L, Lehman GA. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007;66:1126–1132. doi: 10.1016/j.gie.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 122.Prat F, Amaris J, Ducot B, Bocquentin M, Fritsch J, Choury AD, Pelletier G, Buffet C. Nifedipine for prevention of post-ERCP pancreatitis: a prospective, double-blind randomized study. Gastrointest Endosc. 2002;56:202–208. doi: 10.1016/s0016-5107(02)70178-8. [DOI] [PubMed] [Google Scholar]
- 123.Sand J, Nordback I. Prospective randomized trial of the effect of nifedipine on pancreatic irritation after endoscopic retrograde cholangiopancreatography. Digestion. 1993;54:105–111. doi: 10.1159/000201021. [DOI] [PubMed] [Google Scholar]
- 124.Schwartz JJ, Lew RJ, Ahmad NA, Shah JN, Ginsberg GG, Kochman ML, Brensinger CM, Long WB. The effect of lidocaine sprayed on the major duodenal papilla on the frequency of post-ERCP pancreatitis. Gastrointest Endosc. 2004;59:179–184. doi: 10.1016/s0016-5107(03)02540-9. [DOI] [PubMed] [Google Scholar]
- 125.Sudhindran S, Bromwich E, Edwards PR. Prospective randomized double-blind placebo-controlled trial of glyceryl trinitrate in endoscopic retrograde cholangiopancreatography-induced pancreatitis. Br J Surg. 2001;88:1178–1182. doi: 10.1046/j.0007-1323.2001.01842.x. [DOI] [PubMed] [Google Scholar]
- 126.Moretó M, Zaballa M, Casado I, Merino O, Rueda M, Ramírez K, Urcelay R, Baranda A. Transdermal glyceryl trinitrate for prevention of post-ERCP pancreatitis: A randomized double-blind trial. Gastrointest Endosc. 2003;57:1–7. doi: 10.1067/mge.2003.29. [DOI] [PubMed] [Google Scholar]
- 127.Kaffes AJ, Bourke MJ, Ding S, Alrubaie A, Kwan V, Williams SJ. A prospective, randomized, placebo-controlled trial of transdermal glyceryl trinitrate in ERCP: effects on technical success and post-ERCP pancreatitis. Gastrointest Endosc. 2006;64:351–357. doi: 10.1016/j.gie.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 128.Räty S, Sand J, Pulkkinen M, Matikainen M, Nordback I. Post-ERCP pancreatitis: reduction by routine antibiotics. J Gastrointest Surg. 2001;5:339–345; discussion 345. doi: 10.1016/s1091-255x(01)80059-7. [DOI] [PubMed] [Google Scholar]
- 129.Rey JR, Axon A, Budzynska A, Kruse A, Nowak A. Guidelines of the European Society of Gastrointestinal Endoscopy (E.S.G.E.) antibiotic prophylaxis for gastrointestinal endoscopy. European Society of Gastrointestinal Endoscopy. Endoscopy. 1998;30:318–324. [PubMed] [Google Scholar]


