Abstract
Whether due to therapeutic or belligerent exposure, the gastrointestinal effects of irradiation produce symptoms dreaded by a majority of the population. Nausea, vomiting, diarrhea and abdominal cramping are hallmarks of the prodromal phase of radiation sickness, occurring hours to days following radiation exposure. The prodromal phase is distinct from acute radiation sickness in that the absorptive, secretory and anatomic changes associated with radiation damage are not easily identifiable. It is during this phase of radiation sickness that gastrointestinal motility significantly changes. In addition, there is evidence that motor activity of the gut contributes to some of the acute and chronic effects of radiation.
Keywords: Electrical control activity, Retrograde giant contraction, Giant migrating contraction, Migrating motor Complex, Radiation therapy
GASTROINTESTINAL MOTILITY
The gastrointestinal tract facilitates absorption of ingested nutrients with specific spatial and temporal patterns of contractile activity or motility. The contractions mix and propel ingested food, keep the intestinal tract clear of debris, secretions and bacteria between meals and, in special situations, propel intestinal contents rapidly over great distances. Contractile activity is under myogenic, neural and chemical control. Myogenic control refers to the electrical activity generated by the smooth muscle of the gut. Electrical control activity (ECA)[1-4] is the omnipresent rhythmic depolarization of the cell membranes of the smooth muscle of the small intestine. With neural or chemical stimulation, membrane depolarization exceeds an excitation threshold and a contraction results. The electrical correlate of a contraction is called electrical response activity (ERA)[1-4]. These bursts of electrical response activity have a 1:1 relationship with contractions. Because ERA occurs only during the depolarization phase of the ECA cycle, the frequency of contractions is limited to and determined by the frequency of ECA. Neural and chemical stimulation may not be present during each depolarization of ECA, and thus contractions then do not occur at the maximum possible frequency (Figure 1). Recordings of gastrointestinal motility can either be made with devices that record physical contractions (manometry catheter or strain gauges) or motility can be implied from electrical activity or transit times.
Figure 1.
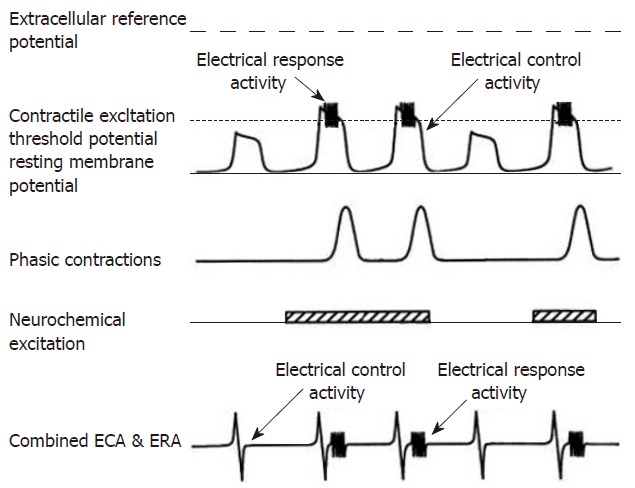
The top electrical tracing illustrates the relationship of intracellularly recorded myoelectric activity and neurochemical excitation with contractions. In the absence of neurochemical excitation, the ECA depolarizations do not exceed the contractile excitation threshold potential. Consequently, no ERA burst is recorded and no contraction occurs during the first and fourth depolarization. When neurochemical stimulation occurs, ECA depolarization exceeds the excitation threshold, an ERA burst occurs and the smooth muscle contracts (cycle 2, 3 and 5). The extracellular electrode recordings are shown in the last tracing and record the follow of currents from a large numbers of smooth muscle cells. The relationship between ECA, neurochemical excitation ERA and contractions is the same as with intracellular recordings. (From Sarna, SK: In vivo myoelectric activity: Methods, analysis and interpretation. In Wood JD (ed): Handbook of Physiology: A Critical, Comprehensive Presentation of Physiological Knowledge and Concepts. Bethesda, MD, American Physiological Society, 1989: 817-863).
Single dose (938 Gy) in a canine model disrupted normal electrical patterns[5]. Jejunal myoelectric activity decreased in frequency, duration and length of migration of ERA postprandially. ECA was irregular with nonuniform morphology and occasionally migrated in an orad direction. ECA was occasionally uncoupled; coupling is a phenomenon in which corresponding ECAs recorded from different areas maintain a consistent time shift[6]. In spite of these functional abnormalities, the histology was minimally altered. These changes in electrical activity suggest a profound change in the physiology of the smooth muscle as well as the nerves controlling the muscle.
Gastrointestinal smooth muscle contractile activity can also be influenced by extrinsic autonomic (parasympathetic and sympathetic) from the CNS and the intrinsic neurons of the enteric nervous system. The stomach, small intestine and proximal colon derive their parasympathetic innervation through the vagus nerve which contains both afferent and efferent fibers. Relative to the intrinsic enteric neurons, the efferent potion of the vagus is really quite small; however, each vagal efferent fiber may influence about 2000 enteric neurons[7,8]. The sensory component of the vagus nerve is much greater. Sensory fibers account for 80% of all vagal fibers[9]. Vagal afferents detect both the mechanical and chemical stimulation of the small intestine and relay this information centrally for processing. Sympathetic innervation of the intestine arises from the thoracic and lumbar spinal nerves. These nerves pass through the paravertebral ganglia and form the splanchnic nerves, which go to the prevertebral ganglia the celiac, superior mesenteric and inferior mesenteric. Within these ganglia, cell bodies receive synaptic input from interganglionic mesenteric neurons. These ganglia intercommunicate and relay sensory information from the gut to the central nervous system, allowing interactions between different areas of the gut. The stimulation of vagus fibers produces contractile activity within the upper small intestine[7,9] while stimulation of the mesenteric sympathetic nerves inhibit small intestinal contractions[10].
The enteric nervous system, or the “little brain” of the gut[11], consists of an intricately coordinated network composed of all neurons having their cell bodies within the bowel wall. Though less well studied than the central nervous system, the enteric nervous system is quite complicated and contains nearly as many neurons as the CNS[12]. The myenteric plexus, located between the longitudinal and circular muscle layers, integrates sensory, extrinsic and enteric neural information and is thought to control gastrointestinal motility. Chemical control involves the stimulation or inhibition of smooth muscle contractile activity by humoral substances[2] that may act either through neurocrine, paracrine or endocrine mode. Examples of these regulatory substances include serotonin, histamine, opioids, cholecystokinin, motilin, somatostatin, vasoactive intestinal peptide (VIP) and substance P. These and many other putative neuroregulatory substances administered exogenously can modulate contractile activity within the small intestine. Prostaglandins may also contribute to the severity illness, histological injury and changes in small intestinal myoelectric activity induced by irradiation[13]. A major component of the excitatory neural input to the gut is cholinergic; VIP is an inhibitory neural transmitter; substance P is an excitatory peptide, peptide YY (PYY), an endocrine peptide, is elevated in the serum following a meal, and motilin, another endocrine peptide, cycles in the fasted state with the MMC. In a canine model during fractionated irradiation, cholinergic activity increased in some segments of the small bowel and colon and remained unchanged in other regions[14]. VIP and substance P tissue levels increased while tissue motilin levels decreased after radiation exposure. Colonic tissue PYY levels remained unchanged but serum PYY levels decreased after irradiation. These differences suggest that there are variations in the sensitivity of gut endocrine cells to irradiation. Radiation also increased the plasma levels of substance P while altering binding sites and contractile activity in a rat model[15]. There is an intimate relationship between the immune system of the gut and the enteric nervous system that may extend to the motor activity of the small intestine. The best-recognized immune modulator is histamine, found within the mast cells of the gut. When mast cells degranulate in response to antigenic stimulation, the enteric nervous system is activated and specific patterns of contractile activity may be initiated[16-19]. While the precise mechanism by which inflammatory cells and mediators affect small intestine motility in unknown, it is clear that an interaction exists. Their interaction following irradiation has not been determined.
ORGANIZATION OF CONTRACTILE ACTIVITY
Contractions of the small intestine may be divided into individual phasic contractions, organized groups of contractions, and special propulsive contractions.
Individual phasic contractions
Individual phasic contractions are the basic contractile activity of the stomach and intestine. They occur in both the fasted and the fed state. In the proximal small intestine, contractions occur regularly and propagate caudal over a variable distance that tends to be greater than what occurs in the distal small intestine. Contractions in the distal small intestine are much less coordinated and consequently, the rate of propulsion in the distal small bowel is less than that in the proximal small intestine.
In the stomach, the antrum has contractions of greater amplitude and duration than those which occur within the small intestine. They serve to grind and break down ingested food. In contrast, the gastric fundus serves as a reservoir and has much greater compliance than the antrum. Fundic contractions are of greater duration and when they occur in coordination with the antrum and pylorus, enhance gastric emptying[20].
The contractile activity of the colon is more complicated than that of the stomach and small bowel. The contractions are of longer duration and greater amplitude to be able to propel semisolid material through the colon and out of the body in the event of defecation. Using a combination of electrical and contractile recordings in a canine model, cyclic bursts of contractions called colonic contractile states can be measured[21]. Colonic muscle contracts at two frequencies during a contractile state, long-duration contractions at 0.5-2 cycles/min and short-duration contractions at 4-6 cycles/min. As in the small intestine, contractile activity is associated with ERA.
Postprandial contractile activity of the small intestine is more complicated to analyze and requires computer modeling. Following fractionated irradiation, proximal small intestinal contractile amplitude did not change but the frequency and duration of these contractions decreased during radiation[22], returning to baseline values post-irradiation. Decreased strength and frequency of contractions in the mid and distal small intestine also occurred and these subtle differences may alter intestinal transit time. With high single dose irradiation (938 cGy) the myoelectric activity returns to normal after several days however, multiple exposures cause the animals to develop chronic diarrhea, profound weight loss and progressive changes in myoelectric activity. ECA became variable, had an irregular rhythm, and was frequently uncoupled[23]. ERA, duration and length of migration were reduced.
ORGANIZED GROUPS OF CONTRACTIONS
Migrating motor complex
The migrating motor complex (MMC) is a cyclic pattern of phasic contractile activity that occurs in the interdigestive state (Figure 2). The MMC originates in the proximal small intestine and migrates to the distal ileum, cycling every 90 to 120 min[3,24]. The MMC cycle is divided into four distinct phases. PhaseIis an interval of contractile quiescence; phase II consists of intermittent contractions, which eventuate into phase III: regular phasic contractions of large amplitude that occur at maximum frequency for approximately 6 to 8 min. Phase IV of the MMC consists of a short transition of intermittent contractions. In humans, the MMC occurs only during the fasted state. Its purported function is to cleanse the small intestine of residential food, desquamated cells and enteric secretions and to keep bacterial growth to a minimum[25]. The overall control of the MMC appears to reside in the periodic activation of the enteric nervous system.
Figure 2.
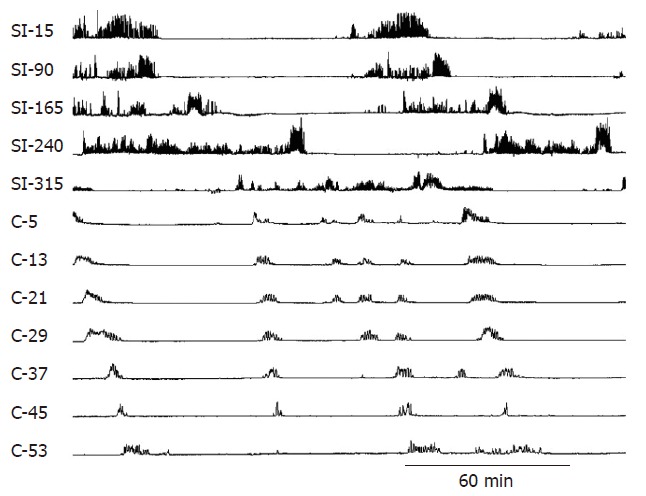
Normal small intestinal and colonic MMCs. Note how the phasic contractions of the small intestine have a shorter duration and different character from the contractions of the colon. The numbers on the left-side indicate the distance in centimeters of the small intestine (SI) from the pylorus. The letter C refers to the colon and the numbers are the distance in cm from the ileocolonic junction.
Within the stomach, contractile patterns similar to what is seen in the small intestine are also found with contractile groups being divided into phaseI, II, III and IV. Because the contractions within the stomach do not “migrate” from the stomach to the small intestine, the activity is sometimes called cyclic motor activity (CMA)[26]. Similar cyclic contractile activity also occurs within the gallbladder[27,28] and within the lower esophageal sphincter[24].
In the stomach, a single dose of 8 Gy significantly delayed gastric emptying in both a canine and primate model[29,30]. The ECA was transiently suppressed by radiation and this was thought to contribute to delayed gastric emptying by decreasing the maximum number of contractions per minute. Similar results were reported in rodent studies[31]. There have been some attempts to treat the radiation-induced delay in gastric emptying[32], but most efforts have been focused upon emesis which may not be associated with the same contractile patterns.
In the small intestine with smaller doses of radiation, MMC cycling persisted and cycle length was unchanged from normal with smaller doses of radiation (2.5 Gy) but was transiently disrupted at higher doses (9 Gy)[23]. The persistence and stability of the MMC suggests that, at least in the short term, the enteric neurons controlling the MMC are functioning. Serum motilin levels also continued to cycle with MMCs[14] suggesting that enteric endocrine function persists.
Normal colonic motor activity is characterized by rhythmic bursts of contractions. The duration of this contractile activity is 7.0 to 11.5 min[33]. When contractions migrate over at least half the distance of the colon, they are called colonic migrating motor complexes (CMMCs). Colonic MMCs occur less frequently that small intestinal MMCs and, unlike small intestinal MMCS, some migrate in an orad direction[21].
As in the small intestine, irradiation did not alter the frequency of colonic MMCs at lower doses[34]. However, a single high dose decreased the duration of CMMCs and diminished the amplitude of contractions[35]. The rate of non-migrating colonic contractions increased with higher doses.
Serotonin (5-hydroxytryptamine, 5-HT) is known to be released following irradiation and this may contribute to motility changes[36,37]. In a rat high dose model, colonic motility and fluid absorption were reduced after irradiation, producing diarrhea and increased colonic mucosal 5-HT levels. Administration of a 5-hydroxytryptamine receptor 3 (5-HT3) antagonist prevented the diarrhea, attenuated colonic motility changes and reduced 5-HT levels early after irradiation, although fluid absorption was only slightly improved. By 1 wk following irradiation, colonic motility and fluid absorption were restored in treated animals.
Other studies have confirmed that, at high dose (10 Gy), abdominal irradiation decreased and disrupted CMMC frequency early after irradiation[38] and diminished colonic myoelectric activity. Myeloperoxidase activity in the small intestine and colon was increased transiently and was associated with diarrhea. Myeloperoxidase is present in inflammatory cells and suggests an interaction between these cells and motility following irradiation.
Colon specimens from human patients[39] after irradiation showed overall innervation, VIP and substance P nerve fiber densities were increased early (1 wk). In contrast, VIP and substance P nerve fiber densities later decreased. No peptide changes were seen in the plasma. The decreased VIP and substance P neuronal supply seen may contribute to late intestinal malfunction and alterations in motility.
Neurotensin is another peptide thought to contribute to the control of intestinal muscle activity. In rats, 6 Gy irradiation increased the intestinal muscle content of neurotensin-like immunoreactivity and altered receptor sites transiently but it returned to normal by 1 wk postirradiation[40].
Using a guinea pig model, 10 Gy did not alter neurally mediated relaxations of isolated gut preparations[41]. Contractions in response to direct muscle stimulation with a cholinergic muscarinic agonist or ganglionic stimulation of intrinsic cholinergic motor neurons were increased in some segments of the gut, but not all. This increased sensitivity to cholinergic stimulation was associated with in increased contractility. Histamine-evoked contractions were unaffected. Cholinergic stimulation increased propulsive motility. Pretreatment of animals with a 5-HT3 receptor antagonist prevented the effect of radiation and reduced propulsive activity to below normal. This suggests that gastrointestinal motility disturbances may be due to an increased sensitivity of the cholinergic system.
The data from these animal experiments is somewhat difficult to interpret as the doses and models differ significantly however, it can be summarized that the effects of single high dose irradiation differ from the sum of fractionated lower doses of radiation. The role of dose rate upon gastrointestinal motility is unclear. Many studies have demonstrated transient profound alterations that return to normal with minor functional alterations after a period of approximately 1 wk.
Migrating clustered contractions
Migrating or discrete clustered contractions occur in the small intestine, last 1 to 3 min and migrate caudad over distances of 10 to 30 cm[43-46]. Because they do not occur as regularly and predictably as MMCs, the mechanisms of initiation of propagation of the contractions are less well studied. These migrating clustered contractions are highly effective at propulsion[47]. Single dose radiation in a canine model[5] produced clusters of migrating ERA probably associated with these migrating clustered contractions.
SPECIAL PROPULSIVE CONTRACTIONS
The gastrointestinal tract usually propels chyme slowly in the caudad direction. In special situations, it is advantageous to the organism to expel ingested material rapidly. The small intestine is capable of generating special propulsive contractions to achieve rapid movement of chyme. If orad propulsion is necessary, retrograde giant contractions (RGCs) occur. These small intestinal contractions immediately precede vomiting. When rapid caudad evacuation of the small bowel is necessary, giant migrating contractions (GMCs) rapidly propel chyme into the colon. Giant migrating contractions may propagate into the colon and, when they reach the distal colon are associated with defecation.
Retrograde giant contractions
The RGC is a large-amplitude, long-duration contraction that originates in the mid small bowel and rapidly propels intestinal contents in the stomach for subsequent expulsion. It is one of the gastrointestinal motor correlates of vomiting (Figure 3). The RGC travels at a rapid velocity of 8 to 10 cm/s[48]. By contrast, the MMC migrates at 2 to 8 cm/min[3,24]. The RGC has been extensively studied in the canine model and is under the control of the vagus nerve[48]. This contraction also occurs in primates but has not yet been documented in the human gastrointestinal tract. The powerful and rapid nature of this contraction may preclude recording with the manometric or solid-state methods utilized currently in human studies. Retrograde giant contractions precede emetic episodes but may also occur without subsequent vomiting[48] in a dose-dependent fashion. For example, with low stimulation, the RGC is initiated without emesis; higher doses generate an RGC followed by vomitus expulsion.
Figure 3.
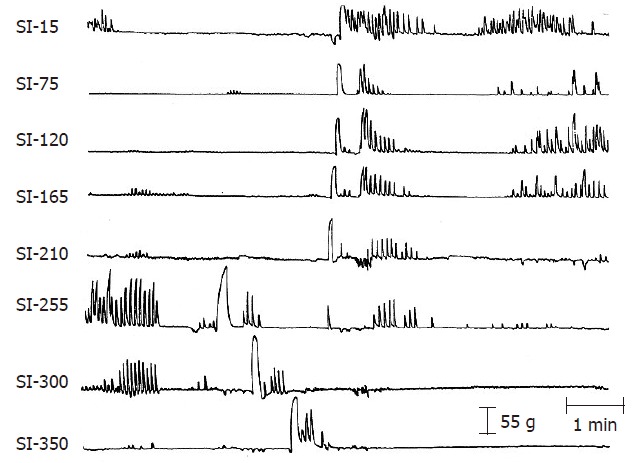
A RGC and a GMC following radiation. The two giant contractions originated about 255 cm from the pylorus and migrated in opposite directions. The duration of the GMC is longer than that of the RGC but the RGC migrates more rapidly. The numbers on the left-side indicate the distance in centimeters of the small intestine (SI) from the pylorus. (From Otterson MF, Sarna SK, Moulder JE. Effects of fractionated doses of ionizing radiation on small intestinal motor activity. Gastroenterology 1988; 95: 1249-1257.).
The relation between radiation-induced vomiting and delayed gastric emptying is not straight forward. Dubois et al[30] explored the effect of irradiation on gastric emptying in a canine model. At a dose of 8 Gy total body irradiation, 9 of 10 dogs vomited. While domperidone was able to suppress emesis but it was unable to normalize the delayed gastric emptying. In a similar experiment, the gastric ECA, fractional gastric emptying rate and acid output was measured in non human primates[29]. In addition to causing vomiting, radiation transiently suppressed gastric ECA, gastric emptying and secretion. In smaller doses on humans (1.8 Gy single dose or after 2 wk of fractionated therapy), no delayed gastric emptying was observed[49]. It is unclear whether there is a threshold dose of radiation which induces delayed gastric emptying since the doses utilized in human patients were much less than had been employed in the animal models. While uncommon, humans with significant exposure can develop delayed gastric emptying, which may respond to pharmacologic intervention[50]. The mechanisms of delayed gastric emptying are separate from those which produce emesis.
Small intestinal motility has been difficult to study in humans because of the cumbersome nature of recording devices. The canine model has similar ECA frequency, contractions and contractile coordination, and this has made it the dominant model for small intestinal motility. The rat has the disadvantage of not being capable of emesis and this limits its usefulness when emesis is an important component to be studied. In a canine model exposed to fractionated irradiation, vomiting developed as early as the first dose of radiation[42] and was associated with a five fold increase of retrograde giant contractions (RGCs) during irradiation[42]. RGCs peaked on the day of the first fraction of radiation and were more likely to be associated with emesis than the rare spontaneous RGC that occurred prior to radiation. This may reflect the level of the stimulus to vomit.
A human trial concerning radiation-induced emesis [51] identified prior experience with chemotherapy as the only patient-related risk factor for radiation-induced emesis. The irradiated site and field size were the only radiation factors associated with increased emesis. A higher percentage of radiation-induced emesis occurred in upper abdomen radiotherapy and with radiotherapy fields > 400 cm2. Patients receiving radiotherapy to the thorax, head and neck also trended toward a higher incidence of radiation-induced emesis. The 5-HT3-receptor antagonists have been proved to provide effective antiemetic therapy in patients undergoing highly emetogenic radiotherapy[52]. In spite of this, optimal treatments are not always employed. The animal experimental data suggests that the peak stimulus for emesis occurs at the first dose of radiation[42]. It is important to use the opportunity to premedicate the patient when they are most likely to vomit and not wait for emesis to occur.
The widespread availability and low cost make corticosteroids attractive drugs for suppression of radiation induced emesis but no prospective trial has been performed comparing the 5-HT3 antagonists to corticosteroids[53]. No study has examined the motility of patients exposed to radiation and experiencing nausea and vomiting.
Giant migrating contractions
Giant migrating contractions (Figure 4), are large-amplitude, long-duration contractions that propagate rapidly in a caudad direction. Their amplitude is approximately 1.5 times that the phasic contractions of the MMC[45,54]. Once initiated, these contractions usually propagate uninterruptedly to the ileocolonic junction and are more propulsive than the MMC[47]. In the normal, healthy state these contractions occur intermittently in the distal small intestine and are never seen postprandially. In pathologic states or after administration of certain drugs, GMCs are more frequent and originate more proximally in the small intestine[17,42,54-57]. In human patients with irritable bowel syndrome, GMCs are associated with the sensation of abdominal cramping[58]. These powerful contractions may generate pain because they stimulate nociceptive receptors within the bowel wall above their threshold level or because propulsion of intestinal chyme produced by GMCs distends the distal intestine, producing pain.
Figure 4.
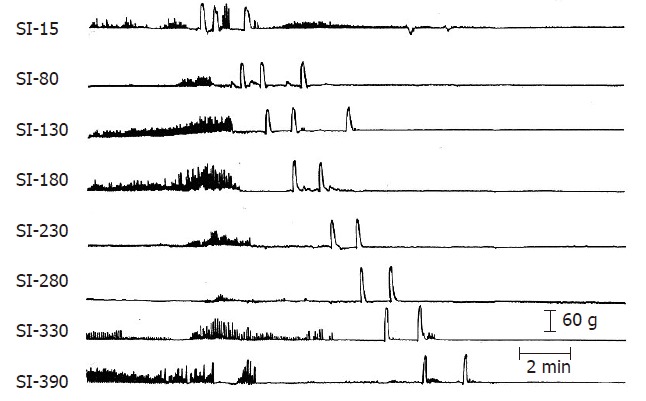
Three GMCs originating in the duodenum following irradiation. The first two GMCs migrated to the ileocolonic junction but the third stopped at 130 cm from the pylorus. See Figure 3 caption for details. (From Otterson MF, Sarna SK, Moulder JE. Effects of fractionated doses of ionizing radiation on small intestinal motor activity. Gastroenterology 1988; 95: 1249-1257).
In pathologic states, frequent GMCs may contribute to diarrhea by propelling bile and intestinal secretions rapidly through the gastrointestinal tract without allowing sufficient time for reabsorption[17,42]. Postprandial GMCs that propel undigested food into the colon would contribute to diarrhea[42]; partially digested food exposed to bacterial degradation in the colon would result in gas production and increase the colonic osmotic load.
There is a myoelectric correlate of GMCs in the small intestine which is not seen in colonic recordings[54]. The velocity of GMCs is not bound by the normal constraints of ECA. These contractions require the enteric nervous system for propagation and to generate the descending inhibition associated with them. Interestingly, there is ascending inhibition associated with GMCs which requires neural input extrinsic to the bowel wall[57].
In a canine model exposed to fractionated irradi-ation, diarrhea developed as early as the first dose of radiation[42,59]. GMCs occurred infrequently prior to irradiation, usually originating in the distal small bowel. However, following irradiation, the incidence of these contractions increased dramatically. They were more likely to originate in the more proximal small intestine. The incidence of GMCs peaked after the second dose of radiation. The characteristics of there contractions, including the amplitude, duration and velocity of migration, were the same as had occurred in normal animals, suggesting that the neural mechanisms for these contractions remained the same. In addition, following irradiation GMCs were identified in the postprandial state[22]. With food in the intestine, these powerful contractions push bile and partially digested material into the colon where it is subjected to bacterial degradation, compounding the diarrhea and discomfort experienced. In an isolated loop of the small intestine, GMCs can be stimulated in any section of the small intestine and they propagate uninterruptedly[57]. The propagation velocity of GMCs increased in the distal small intestine, whereas that of MMCs decreased distally, suggesting different mechanisms of propagation between these types of contractions. In the intact small intestine, the GMCs produce ascending and descending inhibition contractions but do not interrupt the propagation of an ongoing MMC, suggesting that the mechanism of initiation and propagation of GMCs and MMCs is different. The ability of the GMC to generate descending inhibition is important for the coordinated propagation of these powerful contractions as a large bolus of intestinal contents precedes the contraction.
The GMC, in the absence of irradiation, is powerful enough to produce mucosal irritation and bleeding[57]. Prior studies have shown that irradiation produces changes in small intestinal villous length which were thought to be secondary only to changes in the crypt epithelial compartment[59]. Atropine, which decreases gut motility, decreased the villous damage caused by irradiation in mice, implying that altered small intestinal motility may influence villous morphology.
Intestinal segments from rats were studied after treatment with 10 Gy abdominal irradiation[60]. Irradiation had no effect on the overall number of contractions, but increased an in vitro contraction which may be the equivalent of the GMC. In control animals, these contractions were localized at a single recording site but after radiotherapy, three quarters of them occurred at multiple recording sites.
In vitro ileal pressure recordings from a ferret after fractionated irradiation showed an initial increase in frequency followed by a non-significant reduction in the frequency, but not the amplitude of contractions[61]. The frequency of pressure waves showed an inverse relationship with time after radiation with no relationship between motility and histology. This is another example that abdominal irradiation is associated with a time-dependent alteration in motility not correlated well with histology.
The colonic motor correlates of defecation is the GMC[62], whose amplitude is 2.5-2.8 times greater than the amplitude of phasic contractions during CMMCs. GMCs migrate either over the entire colon or a part of its length. GMCs can occur almost simultaneously at different recording sites at the time of defecation or may occur only at a single site. Defecation is usually preceded by colonic GMCs; however, evacuation of contents such as a balloon from the colon is possible without GMCs with a Valsalva mechanism.
Spontaneous colonic GMCs occur once every 6-7 h in normal animals, infrequently originating in the small bowel and propagating into the colon[34]. Abdominal irradiation increased colonic GMCs to once every 2 h with more than half of these contractions originating in the small intestine (Figure 5). GMCs during the radiation were associated with explosive diarrhea when they propagated into the distal colon. Interestingly, there is no electrical correlate for GMCs in the colon as exists in the small intestine. This limits the ability of researchers to use this alternate technique for recording of motility. Both small intestinal and colonic GMCs reverted to normal after cessation of radiation exposure.
Figure 5.
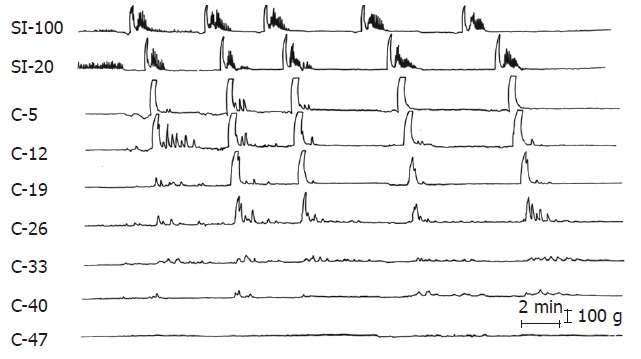
Five GMCs originating in the small intestine at greater than 100 cm from the ileocolonic junction are migrating into the proximal colon following radiation. SI refers to the small intestine; C to colonic strain gauges and the numbers are the distance in cm from the ileocolonic junction. (From Otterson MF et al, Effects of fractionated doses of ionizing radiation on colonic motor activity. Am J Physiol 1992; 263(4 Pt 1): G518-G526.).
Colonic GMCs were rarely observed prior to irra-diation[35]. In contrast, repetitive clusters of giant contractions were observed for up to a week after exposure and were associated with discomfort and passage of diarrheal stools (sometimes bloody) with nearly every occurrence. However, the study reinforces the important role of colonic GMCs in irradiation injury. Other models of colonic inflammation have demonstrated similar motility patterns in the dog colon[36].
HUMAN MOTILITY INCLUDING PSEUDOOBSTRUCTION
The syndrome of intestinal pseudo-obstruction is a complex of the signs and symptoms of intestinal obstruction without evidence of mechanical obstruction. Several investigators have described patients with chronic diarrhea, recurrent crampy abdominal pain, nausea and vomiting years after being irradiated years[63,64]. Perino et al[65] described a case of intestinal pseudoobstruction occurring 30 years after radiation therapy in which mechanical causes of obstruction were excluded by laparotomy. Histology of full-thickness sections of the small bowel revealed vascular ectasia and sclerosis, serosal fibrosis, neuronal proliferation within the submucosa, and degeneration of the muscle fibers of the circular layer of the muscularis propria, suggesting that the pseudoobstruction was due to muscular and neuronal injury from abdominal irradiation.
Two to 10 years following irradiation, there was an increase in gastrointestinal complaints and stool frequency and gastric emptying[66]. Vitamin B12, bile acid, lactose or fat absorption, small intestinal transit or whole gut transit was not altered, although fecal fat excretion was greater than normal in several of the patients. Interestingly, patients with right-sided irradiation had greater bowel frequency when compared to those with left-sided irradiation. At least one parameter of gastrointestinal function was abnormal in three quarters of patients.
In a prospective study in humans, increased stool frequency, decreased bile acid and vitamin B12 absorption, increased fecal fat excretion, increased lactose malabsorption, and more rapid small-intestinal and whole-gut transit were observed[67]. Although changes improved with time, at 1 to 2 years after the completion of irradiation, the frequency of bowel movements was greater, bile acid absorption was less and small-intestinal transit was more rapid when compared to baseline and normal subjects. Stool weight was greater and whole-gut transit faster in patients who received both pelvic and abdominal irradiation compared with those who received pelvic irradiation alone. Stool frequency and fecal fat excretion were greater in patients with surgery prior to radiation therapy. Pelvic irradiation produces persistent effects on gastrointestinal function, many of which are associated with altered motility.
Another study examined patients who had had pelvic irradiation 1 and 6 years prior to treatment of carcinoma of the cervix[68]. Gastric emptying and small intestinal transit were more rapid and both small intestinal and whole gut transit were inversely related to stool frequency. Either bowel frequency, bile acid absorption, vitamin B12 absorption was outside the control range in nearly two thirds of patients. Loperamide was able to slow small intestinal transit, increase bile acid absorption, and decrease the diarrhea associated with chronic radiation damage[69].
Husebye et al[70] performed a prospective study to address whether alterations in proximal intestinal motility could predict the clinical severity of late radiation dysfunction of the intestine. Selecting for patients with persistent chronic abdominal complaints after radiotherapy, impaired fasting motility was found in about one third of patients, and attenuated contractile response after a meal was seen in one quarter of patients. Postprandial delay of the MMC was a good predictor of the degree of malnutrition, and changes in the MMC and postprandial motility index explained two thirds of the malnutrition experienced by these patients. The clinical symptoms of these patients were intestinal pseudoobstruction, malnutrition, failure of a liquid-solid meal to induce postprandial motility, and delayed initiation and reduced intensity of MMC during nocturnal fasting. This study suggested that impaired motility of proximal small intestine may be a key factor in the pathogenesis of severe late effects of radiation.
HUMAN ANORECTAL FUNCTION
Anorectal function can be disturbed after rectal surgery with or without radiotherapy[71] due to decreased rectal compliance. With time however, partial improvement occurred if the patients had not received radiation therapy suggesting that radiation produces sustained decrease in rectal compliance. Some patients treated with radiotherapy for rectal cancer had increased bowel movements, loose or liquid stool, incontinence[72]. Nearly half of the patients in one study needed to wear a pad. Patients also experienced fecal urgency and were unable to differentiate stool from gas more often. Anorectal manometry showed reduced rectal capacity and diminished maximum squeeze pressure of the sphincters in the radiotherapy group as well as diminished rectal distensibility.
Patients complained of rectal bleeding, urgency, frequency, tenesmus, discomfort or pain, fecal inco-ntinence, change in bowel habit, weight loss, vomiting, steatorrhea, nocturnal defecation and obstructive symptoms after pelvic radiotherapy[73]. One-third of all diagnoses were unrelated to the previous radiation therapy with many patients having more than one diagnoses. Many of the abnormalities were treatable but the symptoms did not predict the final diagnosis, with the exception of pain, which was associated with undiagnosed neoplasia. “Radiation enteritis” may not be a single disease entity.
A significant correlation has been found between radiation to the anal-sphincter region and the risk of fecal leakage when the patients received 45-55 Gy[74]. Radiation to the rectum increases the risk of defecation urgency and diarrhea or loose stools when the patients received 25-42 Gy.
Others have noted an increase in bowel frequency, urgency and fecal incontinence[75].
After radiation therapy, there were progressive reduc-tions of basal anal pressures, anal pressures in response to squeeze and increased intra-abdominal pressure, rectal compliance, and the rectal volumes associated with sensory perception and the desire to defecate. The thickness of the external anal sphincter increased with time after radiation therapy.
Sphincter injury following irradiation was evaluated from anal canal specimens collected from patients undergoing abdominoperineal resection[76]. Increased fibrosis and nerve density in the chemoradiotherapy group compared to the control group increased as the interval between radiation and surgery increased. A trend toward increased collagen deposition also was observed.
CONCLUSION
Radiation produces a variety of changes in gastrointestinal motility. Most of the changes observed in patients and animal models occur in other pathologic states. These include delayed gastric emptying, retrograde giant contractions (RGCs) and vomiting, giant migrating contractions (GMCs) and abdominal cramping and diarrhea. The threshold for these contractile events to occur and their control mechanisms are incompletely understood. Many studies suggest that treatment prior to exposure may be the best method to prevent the contractions from occurring. The role of dose rate is unclear. Within hours of a significant exposure to radiation, these contractions begin to occur and contribute significantly to the early stages of radiation illness. Later in the course of radiation injury, contractile changes continue to contribute to symptoms experienced by people who have been irradiated.
Footnotes
Supported by a cooperative agreement with NIAID, AI067734
S- Editor Liu Y L- Editor Negro F E- Editor Ma WH
References
- 1.Sarna SK. Gastrointestinal electrical activity: terminology. Gastroenterology. 1975;68:1631–1635. [PubMed] [Google Scholar]
- 2.Sarna SK, Otterson MF. Small intestinal physiology and pathophysiology. Gastroenterol Clin North Am. 1989;18:375–404. [PubMed] [Google Scholar]
- 3.Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 4.Weisbrodt NW. Patterns of intestinal motility. Annu Rev Physiol. 1981;43:21–31. doi: 10.1146/annurev.ph.43.030181.000321. [DOI] [PubMed] [Google Scholar]
- 5.Summers RW, Flatt AJ, Prihoda MJ, Mitros FA. Effect of irradiation on morphology and motility of canine small intestine. Dig Dis Sci. 1987;32:1402–1410. doi: 10.1007/BF01296667. [DOI] [PubMed] [Google Scholar]
- 6.Smout AJ. Myoelectric activity of the stomach. Gastroelectro-myography and electrogastrography. Delft, The Netherlands: Delft University Press; 1980. [Google Scholar]
- 7.Gidda JS, Goyal RK. Influence of vagus nerves on electrical activity of opossum small intestine. Am J Physiol. 1980;239:G406–G410. doi: 10.1152/ajpgi.1980.239.5.G406. [DOI] [PubMed] [Google Scholar]
- 8.Mir SS, Mason GR, Ormsbee HS. Vagal influence on duodenal motor activity. Am J Surg. 1978;135:97–101. doi: 10.1016/0002-9610(78)90017-x. [DOI] [PubMed] [Google Scholar]
- 9.Evans DH, Murray JG. Histological and functional studies on the fibre composition of the vagus nerve of the rabbit. J Anat. 1954;88:320–337. [PMC free article] [PubMed] [Google Scholar]
- 10.Euler C. Autonomic neuroeffector transmission. In: Magoun H, editor. Handbook of Physiology, Section I: Neurophysiology. Washington DC: American Physiology Society; 1959. pp. 217–237. [Google Scholar]
- 11.Cooke HJ. Role of the "little brain" in the gut in water and electrolyte homeostasis. FASEB J. 1989;3:127–138. doi: 10.1096/fasebj.3.2.2464517. [DOI] [PubMed] [Google Scholar]
- 12.Telford GL, Go VL, Szurszewski JH. Effect of central sympathectomy on gastric and small intestinal myoelectric activity and plasma motilin concentrations in the dog. Gastroenterology. 1985;89:989–995. doi: 10.1016/0016-5085(85)90198-2. [DOI] [PubMed] [Google Scholar]
- 13.Summers RW, Glenn CE, Flatt AJ, Elahmady A. Radiation and indomethacin effects on morphology, prostaglandins, and motility in dog jejunum. Am J Physiol. 1991;261:G145–G151. doi: 10.1152/ajpgi.1991.261.1.G145. [DOI] [PubMed] [Google Scholar]
- 14.Otterson MF, Koch TR, Zhang Z, Leming SC, Moulder JE. Fractionated irradiation alters enteric neuroendocrine products. Dig Dis Sci. 1995;40:1691–1702. doi: 10.1007/BF02212690. [DOI] [PubMed] [Google Scholar]
- 15.Esposito V, Linard C, Maubert C, Aigueperse J, Gourmelon P. Modulation of gut substance P after whole-body irradiation. A new pathological feature. Dig Dis Sci. 1996;41:2070–2077. doi: 10.1007/BF02093612. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh H, Castro GA, Weems WA. Intrinsic jejunal propulsion in the guinea pig during parasitism with Trichinella spiralis. Gastroenterology. 1987;93:784–790. doi: 10.1016/0016-5085(87)90441-0. [DOI] [PubMed] [Google Scholar]
- 17.Cowles VE, Sarna SK. Effect of T. spiralis infection on intestinal motor activity in the fasted state. Am J Physiol. 1990;259:G693–G701. doi: 10.1152/ajpgi.1990.259.5.G693. [DOI] [PubMed] [Google Scholar]
- 18.Nemeth PR, Ort CA, Wood JD. Intracellular study of effects of histamine on electrical behaviour of myenteric neurones in guinea-pig small intestine. J Physiol. 1984;355:411–425. doi: 10.1113/jphysiol.1984.sp015427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer JM, Castro GA. Anamnestic stimulus-specific myoelectric responses associated with intestinal immunity in the rat. Am J Physiol. 1986;250:G266–G273. doi: 10.1152/ajpgi.1986.250.2.G266. [DOI] [PubMed] [Google Scholar]
- 20.Read NW, Houghton LA. Physiology of gastric emptying and pathophysiology of gastroparesis. Gastroenterol Clin North Am. 1989;18:359–373. [PubMed] [Google Scholar]
- 21.Sarna SK. Myoelectric correlates of colonic motor complexes and contractile activity. Am J Physiol. 1986;250:G213–G220. doi: 10.1152/ajpgi.1986.250.2.G213. [DOI] [PubMed] [Google Scholar]
- 22.Otterson MF, Sarna SK, Lee MB. Fractionated doses of ionizing radiation alter postprandial small intestinal motor activity. Dig Dis Sci. 1992;37:709–715. doi: 10.1007/BF01296427. [DOI] [PubMed] [Google Scholar]
- 23.Summers RW, Glenn CE, Flatt AJ, Elahmady A. Does irradiation produce irreversible changes in canine jejunal myoelectric activity. Dig Dis Sci. 1992;37:716–722. doi: 10.1007/BF01296428. [DOI] [PubMed] [Google Scholar]
- 24.Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology. 1985;89:894–913. doi: 10.1016/0016-5085(85)90589-x. [DOI] [PubMed] [Google Scholar]
- 25.Code CF, Schlegel JF. The gastrointestinal interdigestive housekeeper motor correlates of the interdigestive myoelectric complex in the dog. Fourth International Symposium on GI Motility. Vancouver: Mitchell; 1973. [Google Scholar]
- 26.Itoh Z, Sekiguchi T. Interdigestive motor activity in health and disease. Scand J Gastroenterol Suppl. 1983;82:121–134. [PubMed] [Google Scholar]
- 27.Scott RB, Strasberg SM, El-Sharkawy TY, Diamant NE. Fasting canine biliary secretion and the sphincter of Oddi. Gastroenterology. 1984;87:793–804. [PubMed] [Google Scholar]
- 28.Mochinaga N, Sarna SK, Condon RE, Dodds WJ, Matsumoto T. Gastroduodenal regulation of common duct bile flow in the dog. Gastroenterology. 1988;94:755–761. doi: 10.1016/0016-5085(88)90251-x. [DOI] [PubMed] [Google Scholar]
- 29.Danquechin Dorval E, Mueller GP, Eng RR, Durakovic A, Conklin JJ, Dubois A. Effect of ionizing radiation on gastric secretion and gastric motility in monkeys. Gastroenterology. 1985;89:374–380. doi: 10.1016/0016-5085(85)90339-7. [DOI] [PubMed] [Google Scholar]
- 30.Dubois A, Jacobus JP, Grissom MP, Eng RR, Conklin JJ. Altered gastric emptying and prevention of radiation-induced vomiting in dogs. Gastroenterology. 1984;86:444–448. [PubMed] [Google Scholar]
- 31.Chmelar V, Grossmann V, Hais IM, Deml F. Gastric retention and its changes in mice and rats during the first six days following x-irradiation. Radiat Res. 1969;37:627–635. [PubMed] [Google Scholar]
- 32.Hulse EV, Patrick G. A model for treating post-irradiation nausea and vomiting in man: the action of insulin in abolishing radiation-induced delay in gastric emptying in the rat. Br J Radiol. 1977;50:645–651. doi: 10.1259/0007-1285-50-597-645. [DOI] [PubMed] [Google Scholar]
- 33.Sarna SK, Condon R, Cowles V. Colonic migrating and nonmigrating motor complexes in dogs. Am J Physiol. 1984;246:G355–G360. doi: 10.1152/ajpgi.1984.246.4.G355. [DOI] [PubMed] [Google Scholar]
- 34.Otterson MF, Sarna SK, Leming SC, Moulder JE, Fink JG. Effects of fractionated doses of ionizing radiation on colonic motor activity. Am J Physiol. 1992;263:G518–G526. doi: 10.1152/ajpgi.1992.263.4.G518. [DOI] [PubMed] [Google Scholar]
- 35.Summers RW, Hayek B. Changes in colonic motility following abdominal irradiation in dogs. Am J Physiol. 1993;264:G1024–G1030. doi: 10.1152/ajpgi.1993.264.6.G1024. [DOI] [PubMed] [Google Scholar]
- 36.Sethi AK, Sarna SK. Colonic motor response to a meal in acute colitis. Gastroenterology. 1991;101:1537–1546. doi: 10.1016/0016-5085(91)90389-3. [DOI] [PubMed] [Google Scholar]
- 37.Picard C, Ksas B, Griffiths NM, Fioramonti J. Effect of granisetron on radiation-induced alterations of colonic motility and fluid absorption in rats. Aliment Pharmacol Ther. 2002;16:623–631. doi: 10.1046/j.1365-2036.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- 38.Picard C, Wysocki J, Fioramonti J, Griffiths NM. Intestinal and colonic motor alterations associated with irradiation-induced diarrhoea in rats. Neurogastroenterol Motil. 2001;13:19–26. doi: 10.1046/j.1365-2982.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 39.Höckerfelt U, Franzén L, Norrgård O, Forsgren S. Early increase and later decrease in VIP and substance P nerve fiber densities following abdominal radiotherapy: a study on the human colon. Int J Radiat Biol. 2002;78:1045–1053. doi: 10.1080/09553000210158047. [DOI] [PubMed] [Google Scholar]
- 40.Linard C, Griffiths NM, Esposito V, Aigueperse J, Gourmelon P. Changes in gut neurotensin and modified colonic motility following whole-body irradiation in rat. Int J Radiat Biol. 1997;71:581–588. doi: 10.1080/095530097143914. [DOI] [PubMed] [Google Scholar]
- 41.Otterson MF, Sarna SK, Moulder JE. Effects of fractionated doses of ionizing radiation on small intestinal motor activity. Gastroenterology. 1988;95:1249–1257. doi: 10.1016/0016-5085(88)90358-7. [DOI] [PubMed] [Google Scholar]
- 42.Krantis A, Rana K, Harding RK. The effects of gamma-radiation on intestinal motor activity and faecal pellet expulsion in the guinea pig. Dig Dis Sci. 1996;41:2307–2316. doi: 10.1007/BF02100119. [DOI] [PubMed] [Google Scholar]
- 43.Cowles VE, Sarna SK. Effect of cholera toxin on small intestinal motor activity in the fed state. Dig Dis Sci. 1990;35:353–359. doi: 10.1007/BF01537414. [DOI] [PubMed] [Google Scholar]
- 44.Kellow JE, Borody TJ, Phillips SF, Tucker RL, Haddad AC. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386–395. doi: 10.1016/0016-5085(86)90573-1. [DOI] [PubMed] [Google Scholar]
- 45.Quigley EM, Phillips SF, Dent J. Distinctive patterns of interdigestive motility at the canine ileocolonic junction. Gastroenterology. 1984;87:836–844. [PubMed] [Google Scholar]
- 46.Summers RW, Anuras S, Green J. Jejunal manometry patterns in health, partial intestinal obstruction, and pseudoobstruction. Gastroenterology. 1983;85:1290–1300. [PubMed] [Google Scholar]
- 47.Kruis W, Azpiroz F, Phillips SF. Contractile patterns and transit of fluid in canine terminal ileum. Am J Physiol. 1985;249:G264–G270. doi: 10.1152/ajpgi.1985.249.2.G264. [DOI] [PubMed] [Google Scholar]
- 48.Lang IM, Sarna SK, Condon RE. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology. 1986;90:40–47. doi: 10.1016/0016-5085(86)90072-7. [DOI] [PubMed] [Google Scholar]
- 49.Makrauer FL, Oates E, Becker J, Abrams R, O'Connor T, McCallum R, Shumaker J. Does local irradiation affect gastric emptying in humans. Am J Med Sci. 1999;317:33–37. doi: 10.1097/00000441-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Layer P, Demol P, Hotz J, Goebell H. Gastroparesis after radiation. Successful treatment with carbachol. Dig Dis Sci. 1986;31:1377–1380. doi: 10.1007/BF01299817. [DOI] [PubMed] [Google Scholar]
- 51.Radiation-induced emesis: a prospective observational multicenter Italian trial. The Italian Group for Antiemetic Research in Radiotherapy. Int J Radiat Oncol Biol Phys. 1999;44:619–625. doi: 10.1016/s0360-3016(99)00055-3. [DOI] [PubMed] [Google Scholar]
- 52.Horiot JC. Prophylaxis versus treatment: is there a better way to manage radiotherapy-induced nausea and vomiting. Int J Radiat Oncol Biol Phys. 2004;60:1018–1025. doi: 10.1016/j.ijrobp.2004.07.722. [DOI] [PubMed] [Google Scholar]
- 53.Kirkbride P, Bezjak A, Pater J, Zee B, Palmer MJ, Wong R, Cross P, Gulavita S, Blood P, Sun A, et al. Dexamethasone for the prophylaxis of radiation-induced emesis: a National Cancer Institute of Canada Clinical Trials Group phase III study. J Clin Oncol. 2000;18:1960–1966. doi: 10.1200/JCO.2000.18.9.1960. [DOI] [PubMed] [Google Scholar]
- 54.Sarna SK. Giant migrating contractions and their myoelectric correlates in the small intestine. Am J Physiol. 1987;253:G697–G705. doi: 10.1152/ajpgi.1987.253.5.G697. [DOI] [PubMed] [Google Scholar]
- 55.Kamath PS, Hoepfner MT, Phillips SF. Short-chain fatty acids stimulate motility of the canine ileum. Am J Physiol. 1987;253:G427–G433. doi: 10.1152/ajpgi.1987.253.4.G427. [DOI] [PubMed] [Google Scholar]
- 56.Otterson MF, Sarna SK. Gastrointestinal motor effects of erythromycin. Am J Physiol. 1990;259:G355–G363. doi: 10.1152/ajpgi.1990.259.3.G355. [DOI] [PubMed] [Google Scholar]
- 57.Otterson MF, Sarna SK. Neural control of small intestinal giant migrating contractions. Am J Physiol. 1994;266:G576–G584. doi: 10.1152/ajpgi.1994.266.4.G576. [DOI] [PubMed] [Google Scholar]
- 58.Kellow JE, Phillips SF. Altered small bowel motility in irritable bowel syndrome is correlated with symptoms. Gastroenterology. 1987;92:1885–1893. doi: 10.1016/0016-5085(87)90620-2. [DOI] [PubMed] [Google Scholar]
- 59.Erickson BA, Otterson MF, Moulder JE, Sarna SK. Altered motility causes the early gastrointestinal toxicity of irradiation. Int J Radiat Oncol Biol Phys. 1994;28:905–912. doi: 10.1016/0360-3016(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 60.Fraser R, Frisby C, Blackshaw LA, Schirmer M, Howarth G, Yeoh E. Small intestinal dysmotility following abdominal irradiation in the rat small intestine. Neurogastroenterol Motil. 1998;10:413–419. doi: 10.1046/j.1365-2982.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- 61.Fraser R, Frisby C, Schirmer M, Blackshaw A, Langman J, Yeoh E, Rowland R, Horowitz M. Effects of fractionated abdominal irradiation on small intestinal motility--studies in a novel in vitro animal model. Acta Oncol. 1997;36:705–710. doi: 10.3109/02841869709001341. [DOI] [PubMed] [Google Scholar]
- 62.Karaus M, Sarna SK. Giant migrating contractions during defecation in the dog colon. Gastroenterology. 1987;92:925–933. doi: 10.1016/0016-5085(87)90966-8. [DOI] [PubMed] [Google Scholar]
- 63.Conklin JL, Anuras S. Radiation-induced recurrent intestinal pseudo-obstruction. Am J Gastroenterol. 1981;75:440–444. [PubMed] [Google Scholar]
- 64.Vidal A, de la Cuerda C, Luis Escat J, Bretón I, Camblor M, García-Peris P. Chronic radiation enteritis after ovarian cancer: from home parenteral nutrition to oral diet. Clin Nutr. 2006;25:701–704. doi: 10.1016/j.clnu.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Perino LE, Schuffler MD, Mehta SJ, Everson GT. Radiation-induced intestinal pseudoobstruction. Gastroenterology. 1986;91:994–998. doi: 10.1016/0016-5085(86)90705-5. [DOI] [PubMed] [Google Scholar]
- 66.Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Chatterton B. The effects of abdominal irradiation for seminoma of the testis on gastrointestinal function. J Gastroenterol Hepatol. 1995;10:125–130. doi: 10.1111/j.1440-1746.1995.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 67.Yeoh E, Horowitz M, Russo A, Muecke T, Robb T, Maddox A, Chatterton B. Effect of pelvic irradiation on gastrointestinal function: a prospective longitudinal study. Am J Med. 1993;95:397–406. doi: 10.1016/0002-9343(93)90309-d. [DOI] [PubMed] [Google Scholar]
- 68.Yeoh E, Horowitz M, Russo A, Muecke T, Ahmad A, Robb T, Chatterton B. A retrospective study of the effects of pelvic irradiation for carcinoma of the cervix on gastrointestinal function. Int J Radiat Oncol Biol Phys. 1993;26:229–237. doi: 10.1016/0360-3016(93)90202-7. [DOI] [PubMed] [Google Scholar]
- 69.Yeoh EK, Horowitz M, Russo A, Muecke T, Robb T, Chatterton BE. Gastrointestinal function in chronic radiation enteritis--effects of loperamide-N-oxide. Gut. 1993;34:476–482. doi: 10.1136/gut.34.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Husebye E, Hauer-Jensen M, Kjørstad K, Skar V. Severe late radiation enteropathy is characterized by impaired motility of proximal small intestine. Dig Dis Sci. 1994;39:2341–2349. doi: 10.1007/BF02087648. [DOI] [PubMed] [Google Scholar]
- 71.van Duijvendijk P, Slors JF, Taat CW, van Tets WF, van Tienhoven G, Obertop H, Boeckxstaens GE. Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol. 2002;97:2282–2289. doi: 10.1111/j.1572-0241.2002.05782.x. [DOI] [PubMed] [Google Scholar]
- 72.Lundby L, Krogh K, Jensen VJ, Gandrup P, Qvist N, Overgaard J, Laurberg S. Long-term anorectal dysfunction after postoperative radiotherapy for rectal cancer. Dis Colon Rectum. 2005;48:1343–1349; discussion 1349 -1352; author reply 1352. doi: 10.1007/s10350-005-0049-1. [DOI] [PubMed] [Google Scholar]
- 73.Andreyev HJ, Vlavianos P, Blake P, Dearnaley D, Norman AR, Tait D. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist. Int J Radiat Oncol Biol Phys. 2005;62:1464–1471. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 74.al-Abany M, Helgason AR, Cronqvist AK, Lind B, Mavroidis P, Wersäll P, Lind H, Qvanta E, Steineck G. Toward a definition of a threshold for harmless doses to the anal-sphincter region and the rectum. Int J Radiat Oncol Biol Phys. 2005;61:1035–1044. doi: 10.1016/j.ijrobp.2004.07.706. [DOI] [PubMed] [Google Scholar]
- 75.Yeoh EE, Holloway RH, Fraser RJ, Botten RJ, Di Matteo AC, Moore JW, Schoeman MN, Bartholomeusz FD. Anorectal dysfunction increases with time following radiation therapy for carcinoma of the prostate. Am J Gastroenterol. 2004;99:361–369. doi: 10.1111/j.1572-0241.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 76.Da Silva GM, Berho M, Wexner SD, Efron J, Weiss EG, Nogueras JJ, Vernava AM, Connor JT, Gervaz P. Histologic analysis of the irradiated anal sphincter. Dis Colon Rectum. 2003;46:1492–1497. doi: 10.1007/s10350-004-6800-1. [DOI] [PubMed] [Google Scholar]


