Abstract
AIM: Anti-Saccharomyces cerevisiae antibodies (ASCA), anti-nuclear associated anti-neutrophil antibodies (NANA) and antibodies to exocrine pancreas (PAB), are serological tools for discriminating Crohn’s disease (CrD) and ulcerative colitis (UC). Like CrD, coeliac disease (CoD) is an inflammatory bowel disease (IBD) associated with (auto) antibodies. Performing a multicenter study we primarily aimed to determine the performance of ASCA, NANA and PAB tests for IBD diagnosis in children and adults, and secondarily to evaluate the prevalence of these markers in CoD.
METHODS: Sera of 109 patients with CrD, 78 with UC, 45 with CoD and 50 healthy blood donors were retrospectively included. ASCA, NANA and PAB were detected by indirect immunofluorescence (IIF).
RESULTS: ASCA+/NANA- profile displayed a positive predictive value of 94.2% for CrD. Detection of ASCA was correlated with a more severe clinical profile of CrD and treatment of the disease did not influence their serum levels. ASCA positivity was found in 37.9% of active CoD. PAB were found in 36.7% CrD and 13.3% CoD patients and were not correlated with clinical features of CrD, except with an early onset of the disease. Fifteen CrD patients were ASCA negative and PAB positive.
CONCLUSION: ASCA and PAB detected by IIF are specific markers for CrD although their presence does not rule out a possible active CoD. The combination of ASCA, NANA and PAB tests improves the sensitivity of immunological markers for CrD. Repeating ASCA, NANA, and PAB testing during the course of CrD has no clinical value.
Keywords: Inflammatory bowel disease, Coeliac disease, Anti-Saccharomyces cerevisiae antibodies, Anti-neutrophil cytoplasmic antibodies, Anti-pancreatic antibodies
INTRODUCTION
Combined measurement of anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear anti-neutrophil cytoplasmic auto-antibodies (pANCA) has been widely described as valuable serological tools for differential diagnosis between Crohn’s disease (CrD) and ulcerative colitis (UC), especially for indeterminate colitis (IC)[1-3]. ASCA are directed against Saccharomyces cerevisiae wall oligomannosidic epitopes while pANCA observed during UC recognize a nuclear membrane antigen of 50 kDa and are so called “NANA” for anti-Nuclear Associated Neutrophil Antibodies[4]. These immunological tests permit us to distinguish two different serologic profiles: (1) “ASCA+/NANA-” which correlates with CrD and (2) “ASCA-/NANA+”, associated to UC. Similarly, antibodies to pancreatic juice and the exocrine pancreas (PAB) have also been proposed as serologic markers for CrD. However, theses antibodies have a low sensitivity and are found in only 27%-40% of patients with CrD[5,6].
CrD and UC are inflammatory bowel diseases (IBD) characterized by a chronic inflammation of the gut mucosa in which environmental factors play an important role, a chronic diarrhoea and the presence of distinct (auto) antibodies. Even though coeliac disease (CoD) displays similar characteristics, only two teams have studied the prevalence of serological markers for IBD in CoD[7,8].
In the current study we primarily assessed the perfor-mance of ASCA, NANA and PAB in a population of IBD and secondly we evaluated their prevalence in CoD patients. We analysed the correlation between ASCA and/or PAB seropositivities and: (1) clinical features and (2) therapeutic parameters in the CrD population. Our study is the first cross-sectional multicenter study analysing a cohort of patients and healthy individuals, composed of adults and children and avoiding biases of recruitment by including patients from seven different centers.
MATERIALS AND METHODS
Patients
Patients suffering from CrD, UC, or CoD and healthy blood donors (HBD) were selected retrospectively for this multicenter study. All samples have been collected from gastroenterology, internal medicine or pediatric units of 6 University Hospitals in France (CHU of Marseilles, Paris, Montpellier, Strasbourg, Lyon, and Dijon) and one in Luxemburg (CH of Luxembourg). Children and adults of both sexes were included. Clinical informations have been collected from medical charts establishing a patient profile. The diagnosis of CrD and UC was made using previously described criteria[9,10]. The activity of the Crohn’s disease was evaluated according to the Crohn’s Disease Activity Index (CDAI) or Best Index for the adult population and according to the Pediatric Crohn’s disease activity index (PCDAI) for the children population. A CDAI > 150 or a PCDAI > 30 defined an active disease, while a CDAI ≤ 150 or a PCDAI ≤ 10 defined a quiescent disease. Some of the patients of the CrD group had suffered from pancreatitis, defined as abdominal pain associated with an increased value of amylasemia (> 3 × N) and/or lipasemia (> 3 × N).
All patients suffering from CoD fulfilled the following diagnostic criteria: 1- presence of sub-total villous atrophy at duodenal biopsy, 2- clinical remission on a gluten-free diet, 3- detection of auto-antibodies associated with CoD (Antiendomysium and/or anti-tissular transglutaminase antibodies, antigliadin antibodies) in their serum at the time of diagnosis. They were divided into two subgroups according to clinical, histological and serological parameters of disease activity at the time of sampling: 1- active disease, 2- remitting disease. After serum separation, blood samples were stored at -80°C until further analysis.
ASCA Indirect Immunofluorescence
ASCA IgA and IgG were detected by indirect immuno-fluorescence (IIF) using a commercially available detection kit (Euroimmun, Germany). Sera diluted in phosphate buffer (1:500 for IgG and 1:50 for IgA) were incubated for 30 min on slides with smears of Saccharomyces cerevisiae. After a washing step, fluorescein-conjugated goat anti-human IgG or IgA detected ASCA IgG and IgA respectively (Figure 1A).
Figure 1.
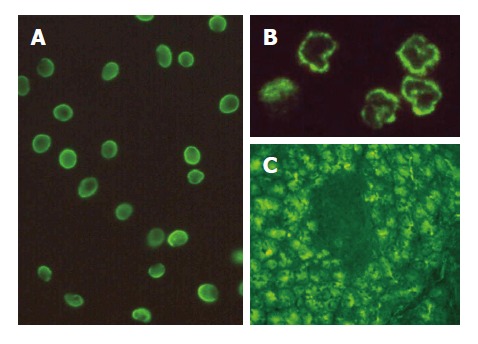
Detection by IIF of (A) ASCA on smears of Saccharomyces cerevisiae, (B) NANA on ethanol-fixed human neutrophils and (C) PAB on human pancreas (× 400). IIF: indirect immunofluorescence; ASCA: anti-Saccharomyces cerevisiae antibodies; NANA: anti-Nuclear Associated Neutrophil Antibodies; PAB: anti-pancreatic antibodies.
NANA (also called x-ANCA or atypical pANCA) indirect Immunofluorescence
ANCA were determined by IIF using ethanol-fixed human neutrophil slides home-prepared[11] or commercially available (Inova Diagnostics, San Diego, CA/EuroImmun, Germany), depending on the laboratory. Sera were incubated at a 1/40 dilution for 30 min , washed and incubated with fluorescein-labelled antihuman IgG immunoglobulin (Inova Diagnostics/Bio-Rad Marnes-la-Coquette, France). For perinuclear (pANCA) staining, myeloperoxydase (MPO) antigenic specificity of antibodies was examined by ELISA (Inova Diagnostics, San Diego, CA/EuroImmun, Germany). Interference by antinuclear antibodies, which may mimic the pANCA pattern, was ruled out by using formalin fixation. Sera reactivities displaying the pANCA pattern and associated with (1) negative anti-MPO ELISA, (2) negative pattern using formalin fixation, and (3) perinuclear pattern using methanol fixation, determined the NANA pattern associated with IBD (Figure 1B).
Auto-antibodies to exocrine pancreas Indirect Immunofluorescence
Sections of human pancreas (blood group O) (Fluotest, Angers, France) were used for detection of auto-antibodies to pancreas (PAB) by indirect immunofluorescence. The sections were incubated for 40 min in a moist chamber at 20°C with 50 μL serum samples [diluted 1: 10 in phosphate buffered saline (PBS)]. After washing with PBS, sections were incubated with polyvalent fluorescein conjugated donkey antihuman immunoglobulin (Bio-Rad Laboratories, Hercules, CA, USA) for 30 min (Figure 1C).
Statistical analysis
Sensitivity was measured as the probability of a positive ASCA in a patient with CrD; specificity was measured as the probability of a negative ASCA in a patient with UC or in HBD. The positive predictive value (PPV) was the probability of having CrD and a positive ASCA while the negative predictive value was the probability of having UC or being HBD and a negative ASCA. Comparison of different groups was done using the chi-square test or Fisher’s exact test when appropriate. Data were analysed using GraphPad Prism® version 3 (GraphPad software, San Diego, CA, USA) and the threshold for statistical significance was set at P = 0.05.
RESULTS
One hundred and nine patients with CrD, 78 patients with UC, 45 patients with CoD (29 active disease and 16 remitting disease) and 50 HBD were selected retrospectively for this study. Among the CrD group, 14 children (under 15 years) and 95 adults were included [mean age: 31 years (9-67)] with a sex ratio of 1 man for every 1.4 woman. The mean CrD duration at the sample time was 7.5 years (1-30).
Serologic profile ASCA+/NANA- detected by IIF displayed a positive predictive value of 94.2% for CrD diagnosis
According to data widely described for ELISA-detected ASCA, ASCA IgA and/or ASCA IgG were significantly more frequent in CrD (49.5%) compared with UC (5.1%) or HBD (4%) (P < 0.001, Figure 2). Conversely, NANA were found with a statistically higher frequency in patients suffering from UC (71.8%) compared to CrD patients (11%) and HBD (0%) (P < 0.001, Figure 2). Serologic profiles of ASCA+/NANA- displayed the following performances for CrD diagnosis: sensitivity: 46.2%, specificity: 97.6%, positive predictive value: 94.2% and negative predictive value: 68.7%. Within the CrD population under scrutiny, 14/109 patients were in childhood at the time of sampling. Among these children, 10/14 displayed IgA or IgG or IgA and IgG ASCA (71.4% versus 46.3% in the adult group, P < 0.05). In both adult and child groups, both IgA and IgG isotypes of ASCA were associated with IBD (Table 1).
Figure 2.
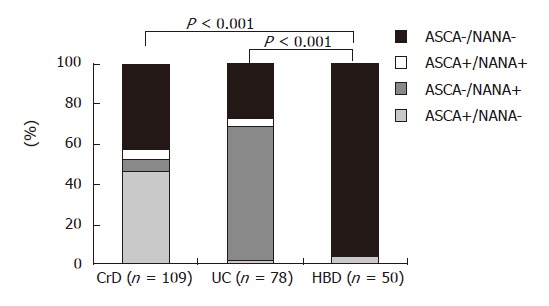
Detection of ASCA and NANA in CrD and UC patient groups and HBD. ASCA: anti-Saccharomyces cerevisiae antibodies; NANA: anti-Nuclear Associated Neutrophil Antibodies; CrD: Crohn’s disease; UC: ulcerative colitis; HBD: healthy blood donors.
Table 1.
ASCA detected in CrD and UC patient groups and HBD: frequency of IgA and/or IgG isotype
| ASCA + isotype | CrD (n = 54) | UC (n = 4) | HBD (n = 2) |
| IgA and IgG | 22 | 1 | 1 |
| IgA and not IgG | 23 | 2 | 0 |
| IgG and not IgA | 9 | 1 | 1 |
ASCA: anti-Saccharomyces cerevisiae antibodies; CrD: Crohn’s disease; HBD: healthy blood donors.
ASCA were found by IIF in 37.9% of active CoD
We analysed the presence of ASCA in the serum of 45 patients suffering from CoD. Among them, 29 displayed an active form of the disease and 16 a remitting form. In 11 CoD patients (24.5%), we detected ASCA by IIF (Figure 3), significantly more than in HBD (P < 0.01). Both IgA and IgG isotypes were represented: 7 patients had ASCA IgA and ASCA IgG, 1 had only ASCA IgA and 3 had only ASCA IgG. We found NANA in the serum of only 1 patient suffering from CoD. Interestingly, all the patients who were found positive for ASCA suffered from an active form of the CoD (37.9% versus 0% under gluten free diet, P < 0.01). Within the CoD population, 28/45 patients were under 15 years and among these children, 5 were ASCA positive (17.8% versus 35.3% in the adult group, P < 0.05).
Figure 3.
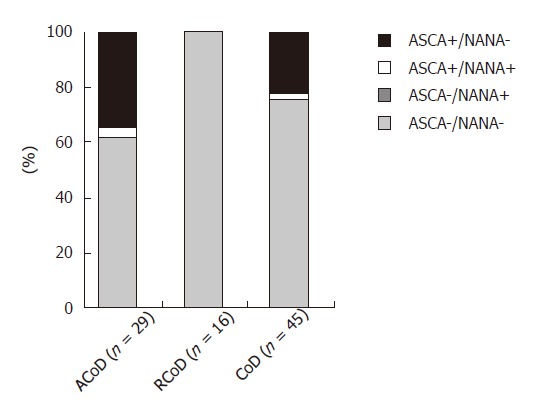
Detection of ASCA and NANA in CoD. Data obtained for patients with an active (AcoD) or a remitting (RcoD) form of the disease are represented. ASCA: anti-Saccharomyces cerevisiae antibodies; NANA: anti-Nuclear associated neutrophil antibodies; CoD: coeliac disease.
Detection of ASCA by IIF was correlated with a more severe clinical profile of CrD characterised by an early onset and the presence of anal complications
In our CrD cohort, we did not find any correlation between ASCA positivity and clinical features such as disease activity or disease location (small bowel versus colon) (Table 2). Similarly, no correlation between presence of ASCA and diagnosis of extra-intestinal manifestations of CrD such as pancreatitis, liver diseases, cutaneous, rheumatologic or ocular involvement was found (Table 2).
Table 2.
Study of the correlation between clinical features of CrD and detection of ASCA
| Clinical features of CrD | Number of patients | Number of patients positive for ASCA (%) | P |
| Age at diagnosis | < 0.001 | ||
| ≤ 20 yr | 47 | 34 (72) | |
| > 20 yr | 58 | 20 (34) | |
| Disease activity | NS | ||
| Quiescent | 29 | 10 (34) | |
| Active | 71 | 40 (56) | |
| Disease location | NS | ||
| Small bowel | 17 | 11 (65) | |
| Colon | 28 | 10 (36) | |
| Small bowel and colon | 49 | 25 (51) | |
| Anal complications | < 0.001 | ||
| Presence | 31 | 22 (71) | |
| Absence | 63 | 22 (35) | |
| Extra intestinal manifestations | NS | ||
| Presence (except pancreatitis) | 45 | 19 (42) | |
| Pancreatitis | 14 | 6 (43) | |
| Absence | 45 | 23 (51) | |
NS: no statistically significant difference; CrD: Crohn’s disease; ASCA: anti Saccharomyces cerevisiae antibodies. The total number of patients is different in each clinical feature because patients with one or two unknown items were nevertheless included.
Conversely, we showed that patients under 20 years at the time of CrD diagnosis exhibited a higher frequency of serum ASCA (P < 0.001) than patients who were diagnosed at an older age (Table 2). We also found that anal complications of CrD such as perianal abscess, anal fissure and anal fistula are statistically (P < 0.001) associated with detection of ASCA by IIF (Table 2).
Treatment of CrD did not influence serum levels of ASCA
Next, we analysed the influence of CrD therapy on ASCA expression in the serum. None of the medical (corticosteroids, immunosuppressive drugs, anti-TNF, 5-aminosalicylic acid, enteral feeding) or surgical treatments significantly altered the prevalence of ASCA in our CrD cohort. Specifically, we did not observe a statistically significant difference in serum ASCA levels between treated and not treated patients (Table 3).
Table 3.
Study of the correlation between therapeutic modalities of CrD and detection of ASCA
| Treatment of CrD patients | Number of patients | Number of positive patients for ASCA (%) | P |
| Medical treatment | NS | ||
| Corticosteroids | 27 | 15 (55) | |
| Immunosuppressive | 19 | 8 (42) | |
| Drugs (IS) | |||
| Corticosteroids + IS | 18 | 10 (55) | |
| Other treatment | 25 | 9 (36) | |
| No treatment | 11 | 5 (45) | |
| Surgical treatment | NS | ||
| Presence | 21 | 11 (52) | |
| Absence | 79 | 40 (51) |
NS: no statistically significant difference; CrD: Crohn’s disease; ASCA: anti-Saccharomyces cerevisiae antibodies. The total number of patients is different in each clinical feature because patients with one or two unknown items were nevertheless included.
PAB are found in 36.7% of CrD patients and 13.3% of CoD patients
We studied the prevalence of PAB in CrD, UC, CoD patients and HBD. PABs were present in 36.7% of CrD sera, compared with 2% in HBD, 7.7% in UC and 13.3% in CoD (Figure 4). Sensitivity, specificity, predictive positive value and negative predictive value of PAB for CrD diagnosis were respectively: 36.7%, 94.5%, 85.1% and 63.7%. Focusing on CrD patients suffering from pancreatitis (n = 14), we detected PAB positivity in 50% of cases (versus 38.8% in the CrD population without pancreatitis, P > 0.05), demonstrating that PAB did not arise more often in patients with CrD and acute pancreatitis than in CrD patients without pancreatitis. Of note is the PAB positivity in 15 patients who suffered from CrD and displayed negative ASCA profile. In CoD patients, PAB were found in both active and remitting forms of the disease (16% versus 8%, NS) (Figure 4).
Figure 4.
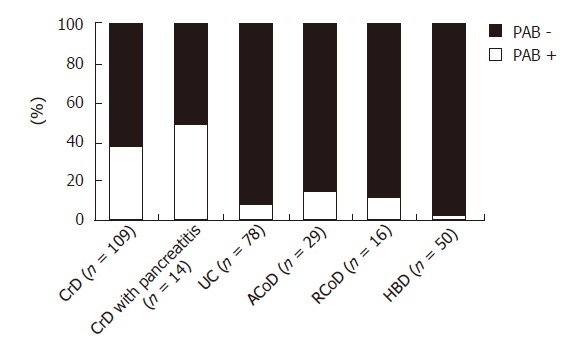
Detection of PAB in CrD (with or without pancreatitis), UC and CoD (active or remitting form) patient groups and HBD. PAB: anti-pancreatic antibodies; CrD: Crohn’s disease; UC: ulcerative colitis; CoD: coeliac disease; HBD: healthy blood donors.
PAB detection was not correlated with clinical features of CrD except an early onset of the disease
In our study, PAB detection did not increase with either disease activity or disease location but was correlated with an early onset of the disease (P < 0.05) (Table 4). Occurrence of anal or extra-intestinal manifestations of CrD did not affect antibody prevalence in the sera. Moreover, neither medical nor surgical treatment of CrD patients influenced PAB serum levels (Table 4).
Table 4.
Study of the correlation between clinical features and therapeutic modalities of CrD and detection of PAB
| Clinical features of CrD | Number of patients | Number of patients positive for PAB (%) | P |
| Age at diagnosis | 0.028 | ||
| ≤ 20 yr | 47 | 25 (53) | |
| > 20 yr | 58 | 18 (31) | |
| Disease activity | NS | ||
| Quiescent | 29 | 14 (48) | |
| Active | 71 | 27 (38) | |
| Disease location | NS | ||
| Small bowel | 17 | 6 (35) | |
| Colon | 28 | 14 (50) | |
| Small bowel and colon | 49 | 19 (39) | |
| Anal complications | NS | ||
| Presence | 31 | 17 (55) | |
| Absence | 63 | 22 (35) | |
| Extra intestinal manifestations | NS | ||
| Presence (except pancreatitis) | 45 | 16 (36) | |
| Pancreatitis | 14 | 7 (50) | |
| Absence | 45 | 21 (47) | |
| Medical treatment | NS | ||
| Corticosteroids | 27 | 8 (30) | |
| Immunosuppressive drugs (IS) | 19 | 8 (42) | |
| Corticosteroids + IS | 18 | 10 (55) | |
| Other treatment | 25 | 12 (48) | |
| No treatment | 11 | 5 (45) | |
| Surgical treatment | NS | ||
| Presence | 21 | 7 (33) | |
| Absence | 79 | 35 (44) | |
NS: No statistically significant difference; CrD: Crohn’s disease; PAB: Anti-pancreatic antibodies. The total number of patients is different in each clinical feature because patients with one or two unknown items were nevertheless included.
DISCUSSION
In the present study, we assessed the clinical value of ASCA, NANA and PAB tests for the diagnosis of IBD and CoD, both characterised by gut inflammation, chronic diarrhoea and the presence of distinct auto-antibodies. This is the first study in which the performance of ASCA detected by indirect immunofluorescence have been tested in a cohort of patients issued from different medical centers and composed of adults and children. The results reveal that (1) the ASCA+/NANA- serologic profile, detected by IIF, provides a PPV of 94.2% for CrD diagnosis and (2) detection of ASCA by IIF was correlated with a more severe clinical profile of CrD, as suggested by an age of onset under 20 years and the presence of anal complications. Moreover, serum levels of ASCA were not influenced by any treatment of CrD. PAB detection, positive in 36.7% CrD, was not correlated with clinical features of CrD except with an age of onset under 20 years. Concerning the CoD cohort, we describe an unexpectedly high percentage (37.9%) of ASCA positivity by IIF in the active but not the remitting form of CoD.
In accordance with other studies using home-made or commercially available ELISA methods, we found that detection of circulating ASCA by IIF, even though it was associated with NANA test, has a poor sensitivity for CrD diagnosis[1,2,12]. Therefore, the use of a smear of Saccharomyces cerevisiae as a source of native antigens for IIF did not allow us to increase the sensitivity of the test when compared to ELISA technique.
The ASCA testing displays a good positive predictive value (94.2%)[3]. Israeli et al[13], recently showed that ASCA and pANCA precede the clinical diagnosis of IBD. We have no data concerning this parameter but it would be of great interest to follow-up the 2 HBD that were found ASCA-positive.
ASCA specificity is also very good (97.5%) and should be increased up to > 99% by combination of IIF and ELISA as suggested by Klebl et al[14]. However, many studies have recently shown that patients suffering from diseases other than CrD have a higher frequency of ASCA seropositivity than the general population. For example, adults with Behcet disease, ankylosing spondylitis or primary biliary cirrhosis as well as children with cystic fibrosis can display circulating ASCA[15-19]. Moreover, two teams have described a high prevalence of ASCA in patients with CoD[7,8]. We confirm here these data: 11/45 (24.5%) coeliac patients had circulating ASCA of both IgG and IgA isotypes. We show that this seropositivity is linked to the active phase of the disease since none of the patients with a remitting form of CoD was ASCA-positive. We can postulate that this seropositivity is linked to common physiopathological features in CrD and CoD, such as (1) inflammation of small intestine mucosa and/or (2) loss of oral tolerance of alimentary antigen(s) and/or (3) alteration of gut permeability. Nevertheless, several studies showed that the ASCA test was a stable marker of CrD and was not a second phenomenon due to increased intestinal permeability[20,21]. It would be of great interest to assess whether the ASCA-positive CoD patients became negative under a gluten-free diet. It is worth noting that bread, a major source of gluten in France, is also a great source of Saccharomyces cerevisiae.
For the first time, we have analysed sera from both children and adults. We included 42 children (14 CrD and 28 CoD) and observed that CrD patients under 15 years displayed more often ASCA than adults. This was not true for CoD patients. Therefore, the ASCA test is of great interest for CrD young patients. We can argue that in children, IBD are more severe than in adults and that ASCA are correlated with disease severity. This correlation is described in the literature[22-25] and is in accordance with our results showing an increased frequency of ASCA in patients who (1) were under 20 years at the time of CrD diagnosis and/or (2) suffered from anal complications. These two parameters are in favour of a severe CrD. Early onset Crohn’s disease may reflect a subgroup of patients characterized by a particular sensitivity to modifications of the intestinal flora associated to an immaturity of the mucosa-associated immune system. These parameters could be associated to an immunization, like for a pathogen, against Saccharomyces cerevisiae instead of the induction of a tolerance state. Amre DK and colleagues have recently shown that infection-related exposures seem to enhance risk for CD in children[26]. Moreover ASCA have been described to be correlated to the presence of NOD2/CARD15 mutations implicated in IBD and this genetic predisposition favours early-onset IBD development[27,28].
Several studies have suggested that ASCA expression is significantly associated with ileal CrD[1,23,29,30]. However, in accordance with the findings of Saibeni et al[31], we did not observe any correlation between ASCA positivity and location of the disease.
In our study, we also tested pancreatic antibodies that have been reported in patients with IBD, without any correlation with clinical characteristics of the disease such as involvement of intestine, previous surgical interventions, drug therapy or disease activity[6,32]. These antibodies recognise a large macromolecular trypsine-sensitive protein in pancreatic secretion[33]. Even though their specificity for CD is high (94.5%), their sensitivity (36.7%) is too low to be used in clinical practice. Moreover, we show that PAB autoreactivity against pancreas is not correlated with occurrence of pancreatitis during CrD. We propose that PAB detection is interesting for documenting a CrD diagnosis in clinically highly CrD suspected patients without circulating ASCA. Nevertheless, Lawrance et al[34] showed that (1) the PAB test is more sensitive in Chinese than Caucasian patients with CrD and (2) the combination of PAB, pANCA and ASCA testing improved the differentiation between UC and CrD, particularly in isolated colonic disease, when compared to pANCA and ASCA test used individually. Our study is in agreement with these findings: 15 patients suffering from CrD were negative for ASCA but displayed PAB positivity.
In conclusion, ASCA and PAB are specific markers for CrD but their presence in the serum does not allow us to eliminate a diagnosis of CoD. The combination of the three antibodies and the detection of both IgG and IgA isotypes for ASCA improve the sensitivity of immunological markers for CrD diagnosis. Finally, observed correlations between ASCA positivity and clinical features of CrD lead to the conclusion that repeating ASCA, NANA, and PAB detection during the clinical course of the disease has no clinical value.
ACKNOWLEDGMENTS
The authors would like to thank all the physicians who have contributed to this study. S Desplat-Jégo, C Johanet, A Escande, J Goetz, N Fabien, N Olsson, E Ballot and RL Humbel are members of the Groupe d’Etude de l’Auto-Immunité.
COMMENTS
Background
Some patients suffering from inflammatory bowel diseases cannot be easily classified into either Crohn’s disease (CrD) or ulcerative colitis (UC). Making an accurate diagnosis is important as the management of CrD and UC is different, especially when surgery is planned. Antibodies to oligomannosidic epitopes of the yeast saccharomyces cerevisiae (ASCA) are a marker previously described as associated with CrD. The clinical relevance and the diagnostic role of ASCA are still debated and multicenter study on this topic is lacking.
Research frontiers
This study is a cross-sectional multicenter study.
Innovations and breakthroughs
The detection of ASCA by indirect immunofluorescence shows similar performances than those previously described with ELISA. ASCA are found in 37.9% of active celiac disease. The sensitivity of ASCA for CrD is higher for children than for adults. ASCA are associated with early-onset of CrD and presence of anal complications during CrD.
Applications
The presence of ASCA and/or PAB does not rule out the diagnosis of celiac disease. The testing of pancreatic antibodies is interesting when ASCA are negative in a patient suspected of CrD.
Peer review
This study looks at the usefulness of ASCA, NANA and PAB antibodies as biomarkers of inflammatory bowel disease including coeliac disease. Although this is an area which has been extensively covered in the literature, this study provides some interesting observations in a reasonable large cohort of patients.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Chen GJ
References
- 1.Quinton JF, Sendid B, Reumaux D, Duthilleul P, Cortot A, Grandbastien B, Charrier G, Targan SR, Colombel JF, Poulain D. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeters M, Joossens S, Vermeire S, Vlietinck R, Bossuyt X, Rutgeerts P. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease. Am J Gastroenterol. 2001;96:730–734. doi: 10.1111/j.1572-0241.2001.03613.x. [DOI] [PubMed] [Google Scholar]
- 3.Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, Geboes K, Bossuyt X, Vandewalle P, Oberhuber G, et al. The value of serologic markers in indeterminate colitis: a prospective follow-up study. Gastroenterology. 2002;122:1242–1247. doi: 10.1053/gast.2002.32980. [DOI] [PubMed] [Google Scholar]
- 4.Terjung B, Spengler U, Sauerbruch T, Worman HJ. "Atypical p-ANCA" in IBD and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310–322. doi: 10.1053/gast.2000.9366. [DOI] [PubMed] [Google Scholar]
- 5.Seibold F, Mörk H, Tanza S, Müller A, Holzhüter C, Weber P, Scheurlen M. Pancreatic autoantibodies in Crohn's disease: a family study. Gut. 1997;40:481–484. doi: 10.1136/gut.40.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seibold F, Weber P, Jenss H, Wiedmann KH. Antibodies to a trypsin sensitive pancreatic antigen in chronic inflammatory bowel disease: specific markers for a subgroup of patients with Crohn's disease. Gut. 1991;32:1192–1197. doi: 10.1136/gut.32.10.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damoiseaux JG, Bouten B, Linders AM, Austen J, Roozendaal C, Russel MG, Forget PP, Tervaert JW. Diagnostic value of anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies for inflammatory bowel disease: high prevalence in patients with celiac disease. J Clin Immunol. 2002;22:281–288. doi: 10.1023/a:1019926121972. [DOI] [PubMed] [Google Scholar]
- 8.Barta Z, Csípõ I, Szabó GG, Szegedi G. Seroreactivity against Saccharomyces cerevisiae in patients with Crohn's disease and celiac disease. World J Gastroenterol. 2003;9:2308–2312. doi: 10.3748/wjg.v9.i10.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Truelove SC, WITTS LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984;86:249–266. [PubMed] [Google Scholar]
- 11.Claise C, Johanet C, Bouhnik Y, Kapel N, Homberg JC, Poupon R. Antineutrophil cytoplasmic autoantibodies in autoimmune liver and inflammatory bowel diseases. Liver. 1996;16:28–34. doi: 10.1111/j.1600-0676.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 12.Gupta SK, Fitzgerald JF, Croffie JM, Pfefferkorn MD, Molleston JP, Corkins MR. Comparison of serological markers of inflammatory bowel disease with clinical diagnosis in children. Inflamm Bowel Dis. 2004;10:240–244. doi: 10.1097/00054725-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Israeli E, Grotto I, Gilburd B, Balicer RD, Goldin E, Wiik A, Shoenfeld Y. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54:1232–1236. doi: 10.1136/gut.2004.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebl FH, Bataille F, Hofstädter F, Herfarth H, Schölmerich J, Rogler G. Optimising the diagnostic value of anti-Saccharomyces cerevisiae-antibodies (ASCA) in Crohn's disease. Int J Colorectal Dis. 2004;19:319–324. doi: 10.1007/s00384-003-0557-1. [DOI] [PubMed] [Google Scholar]
- 15.Török HP, Glas J, Gruber R, Brumberger V, Strasser C, Kellner H, Märker-Hermann E, Folwaczny C. Inflammatory bowel disease-specific autoantibodies in HLA-B27-associated spondyloarthropathies: increased prevalence of ASCA and pANCA. Digestion. 2004;70:49–54. doi: 10.1159/000080081. [DOI] [PubMed] [Google Scholar]
- 16.Muratori P, Muratori L, Guidi M, Maccariello S, Pappas G, Ferrari R, Gionchetti P, Campieri M, Bianchi FB. Anti-Saccharomyces cerevisiae antibodies (ASCA) and autoimmune liver diseases. Clin Exp Immunol. 2003;132:473–476. doi: 10.1046/j.1365-2249.2003.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause I, Monselise Y, Milo G, Weinberger A. Anti-Saccharomyces cerevisiae antibodies--a novel serologic marker for Behçet's disease. Clin Exp Rheumatol. 2002;20:S21–S24. [PubMed] [Google Scholar]
- 18.Condino AA, Hoffenberg EJ, Accurso F, Penvari C, Anthony M, Gralla J, O'Connor JA. Frequency of ASCA seropositivity in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:23–26. doi: 10.1097/01.mpg.0000166801.61708.60. [DOI] [PubMed] [Google Scholar]
- 19.Fresko I, Ugurlu S, Ozbakir F, Celik A, Yurdakul S, Hamuryudan V, Yazici H. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Behçet's syndrome. Clin Exp Rheumatol. 2005;23:S67–S70. [PubMed] [Google Scholar]
- 20.Vermeire S, Peeters M, Vlietinck R, Joossens S, Den Hond E, Bulteel V, Bossuyt X, Geypens B, Rutgeerts P. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: a study in IBD families. Inflamm Bowel Dis. 2001;7:8–15. doi: 10.1097/00054725-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Harrer M, Reinisch W, Dejaco C, Kratzer V, Gmeiner M, Miehsler W, Norman GL, Gangl A, Vogelsang H. Do high serum levels of anti-Saccharomyces cerevisiae antibodies result from a leakiness of the gut barrier in Crohn's disease? Eur J Gastroenterol Hepatol. 2003;15:1281–1285. doi: 10.1097/00042737-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Lawrance IC, Murray K, Hall A, Sung JJ, Leong R. A prospective comparative study of ASCA and pANCA in Chinese and Caucasian IBD patients. Am J Gastroenterol. 2004;99:2186–2194. doi: 10.1111/j.1572-0241.2004.40486.x. [DOI] [PubMed] [Google Scholar]
- 23.Walker LJ, Aldhous MC, Drummond HE, Smith BR, Nimmo ER, Arnott ID, Satsangi J. Anti-Saccharomyces cerevisiae antibodies (ASCA) in Crohn's disease are associated with disease severity but not NOD2/CARD15 mutations. Clin Exp Immunol. 2004;135:490–496. doi: 10.1111/j.1365-2249.2003.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canani RB, Romano MT, Greco L, Terrin G, Sferlazzas C, Barabino A, Fontana M, Roggero P, Guariso G, De Angelis G, et al. Effects of disease activity on anti-Saccharomyces cerevisiae antibodies: implications for diagnosis and follow-up of children with Crohn's disease. Inflamm Bowel Dis. 2004;10:234–239. doi: 10.1097/00054725-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Arnott ID, Landers CJ, Nimmo EJ, Drummond HE, Smith BK, Targan SR, Satsangi J. Sero-reactivity to microbial components in Crohn's disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–2384. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 26.Amre DK, Lambrette P, Law L, Krupoves A, Chotard V, Costea F, Grimard G, Israel D, Mack D, Seidman EG. Investigating the hygiene hypothesis as a risk factor in pediatric onset Crohn's disease: a case-control study. Am J Gastroenterol. 2006;101:1005–1011. doi: 10.1111/j.1572-0241.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 27.Dassopoulos T, Frangakis C, Cruz-Correa M, Talor MV, Burek CL, Datta L, Nouvet F, Bayless TM, Brant SR. Antibodies to Saccharomyces cerevisiae in Crohn's disease: Higher titers are associated with a greater frequency of mutant NOD2/CARD15 alleles and with a higher probability of complicated disease. Inflamm Bowel Dis. 2007;13:143–151. doi: 10.1002/ibd.20031. [DOI] [PubMed] [Google Scholar]
- 28.Russell RK, Drummond HE, Nimmo EE, Anderson N, Smith L, Wilson DC, Gillett PM, McGrogan P, Hassan K, Weaver LT, et al. Genotype-phenotype analysis in childhood-onset Crohn's disease: NOD2/CARD15 variants consistently predict phenotypic characteristics of severe disease. Inflamm Bowel Dis. 2005;11:955–964. doi: 10.1097/01.mib.0000183423.38037.f3. [DOI] [PubMed] [Google Scholar]
- 29.Vasiliauskas EA, Kam LY, Karp LC, Gaiennie J, Yang H, Targan SR. Marker antibody expression stratifies Crohn's disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zholudev A, Zurakowski D, Young W, Leichtner A, Bousvaros A. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn's disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99:2235–2241. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 31.Saibeni S, Folli C, de Franchis R, Borsi G, Vecchi M. Diagnostic role and clinical correlates of anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (p-ANCA) in Italian patients with inflammatory bowel diseases. Dig Liver Dis. 2003;35:862–868. doi: 10.1016/j.dld.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Stöcker W, Otte M, Ulrich S, Normann D, Finkbeiner H, Stöcker K, Jantschek G, Scriba PC. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41–52. doi: 10.3109/00365528709089774. [DOI] [PubMed] [Google Scholar]
- 33.Fricke H, Birkhofer A, Folwaczny C, Meister W, Scriba PC. Characterization of antigens from the human exocrine pancreatic tissue (Pag) relevant as target antigens for autoantibodies in Crohn's disease. Eur J Clin Invest. 1999;29:41–45. doi: 10.1046/j.1365-2362.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- 34.Lawrance IC, Hall A, Leong R, Pearce C, Murray K. A comparative study of goblet cell and pancreatic exocine autoantibodies combined with ASCA and pANCA in Chinese and Caucasian patients with IBD. Inflamm Bowel Dis. 2005;11:890–897. doi: 10.1097/01.mib.0000182872.76434.8c. [DOI] [PubMed] [Google Scholar]


