Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease and the leading cause of dementia. In addition to grey matter pathology, white matter changes are now recognized as an important pathological feature in the emergence of the disease. Despite growing recognition of the importance of white matter abnormalities in the pathogenesis of AD, the causes of white matter degeneration are still unknown. While multiple studies propose Wallerian-like degeneration as the source of white matter change, others suggest that primary white matter pathology may be due, at least in part, to other mechanisms, including local effects of toxic Aβ peptides. In the current study, we investigated levels of soluble amyloid-beta (Aβ) in white matter of AD patients (n=12) compared with controls (n=10). Fresh frozen white matter samples were obtained from anterior (Brodmann area 9) and posterior (Brodmann area 1, 2 and 3) areas of post-mortem AD and control brains. ELISA was used to examine levels of soluble Aβ -42 and Aβ -40. Total cortical neuritic plaque severity rating was derived from individual ratings in the following areas of cortex: mid-frontal, superior temporal, pre-central, inferior parietal, hippocampus (CA1), subiculum, entorhinal cortex, transentorhinal cortex, inferior temporal, amygdala and basal forebrain. Compared with controls, AD samples had higher white matter levels of both soluble Aβ -42 and Aβ -40. While no regional white matter differences were found in Aβ -40, Aβ -42 levels were higher in anterior regions than in posterior regions across both groups. After statistically controlling for total cortical neuritic plaque severity, differences in both soluble Aβ -42 and Aβ -40 between the groups remained, suggesting that white matter Aβ peptides accumulate independent of overall grey matter fibrillar amyloid pathology and are not simply a reflection of overall amyloid burden. These results shed light on one potential mechanism through which white matter degeneration may occur in AD. Given that white matter degeneration may be an early marker of disease, preceding grey matter atrophy, understanding the mechanisms and risk factors that may lead to white matter loss could help to identify those at high risk and to intervene earlier in the pathogenic process.
Keywords: Dementia, Neurodegenerative disease, Pathology, Myelin, Ageing, ELISA
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and the leading cause of dementia [1]. Pathologically, AD is characterized by neuronal loss, amyloid-beta (Aβ) plaques, composed of aggregations of amyloid peptides, and neurofibrillary tangles (NFT), consisting of hyperphosphorylated tau protein. The “amyloid cascade hypothesis”, currently the prevailing hypothesis regarding AD pathogenesis, states that Aβ-42 peptides aggregate to form toxic Aβ-42 oligomers and plaques, which then trigger a cascade of neuropathological events, including neuroinflammation, oxidative stress, tau hyperphosphorylation and NFT formation, and, ultimately, widespread neurodegeneration and dementia [2].
Although AD has traditionally been considered a disease of the grey matter, in recent years, white matter changes have come to be recognized as an important pathological feature [3,4]. Using structural magnetic resonance imaging (MRI), both white matter atrophy [5-8] and white matter lesions that appear as areas of white matter hyperintensity (WMH) on T2-weighted magnetic resonance imaging (MRI) sequences [9-13] have been reported in AD. At the microstructural level, diffusion tensor imaging (DTI) studies found reductions in white matter integrity in AD patients in multiple white matter regions [3,14-22]. In neuropathology studies, white matter abnormalities have been found to occur in more than 50% of AD cases [23], and include axonal defect and loss, demyelination, death of oligodendrocytes, reactive astrocytosis and microglial activation [23-33]. White matter abnormalities are associated with performance across a range of neuropsychological tests [34], and predict AD incidence and rate of cognitive decline in the community [9,35-37]. Although once thought to occur secondary to grey matter degeneration, recent studies demonstrated that some white matter changes in AD may occur independently of and precede grey matter atrophy [3,4].
Despite the growing recognition of the importance of white matter abnormalities in the pathogenesis of AD, the pathological basis of white matter degradation remains to be elucidated. While multiple studies attributed white matter degeneration to concomitant small vessel disease [23,32] or Wallerian-like degeneration [4,32], others suggest that primary white matter pathology may be due, at least in part, to other mechanisms, including toxic Aβ peptides [4]. To investigate this possibility further, we measured levels of soluble Aβ-40 and Aβ-42 peptides in postmortem cerebral white matter from AD patients and non-AD controls. We elected to measure soluble amyloid levels as, in recent years, extensive evidence has accumulated to suggest that it is the levels of soluble Aβ oligomers, rather than insoluble Aβ fibrils, that correlate with the extent of synaptic loss and the severity of cognitive impairment in AD [38-44]. Given the amyloid cascade hypothesis [2] and the known increase in Aβ in AD brains, we hypothesized that AD patients would show increased white matter levels of soluble Aβ-40 and Aβ-42 peptides compared with non-AD controls. We also hypothesized that white matter amyloid beta levels would still be increased in AD brains, even after statistically controlling for cortical plaque severity, suggesting that soluble forms of amyloid in the white matter of AD patients may not merely be a reflection of overall amyloid pathology, but rather could independently contribute to the pathophysiology of the disease. While neuropathological studies have shown that the frontal lobes appear to be the most severely affected by white matter abnormalities [23], we have found using structural MRI that frank white matter damage to more posterior areas is associated with incident AD [9,35]. Given this disparity between pathological and imaging findings, the current study also used tissue sections from both anterior and posterior sections to investigate whether white matter amyloid beta levels vary as a function of region.
Materials and methods
Participants and neuropathological assessment
Twenty-two cases from the New York Brain Bank at Columbia University were included in the current study (see Table 1 for demographic information). Twelve of these cases were pathologically diagnosed as AD and 10 were non-AD controls. Within the control group, 3 cases were pathologically normal, 4 were classified pathologically as ischemic stroke without vascular dementia, 1 was classified with progressive supranuclear palsy, 1 with idiopathic Parkinson’s disease and 1 with lobar atrophy without Picks disease. Neuropathological assessment was performed blind to clinical diagnosis and diagnosis of AD was based on CERAD criteria [45]. Of the twelve clinical AD patients, eight had definite AD and four had probable AD based upon CERAD criteria [46]. Of the ten non-Alzheimer’s controls, nine did not meet CERAD criteria for AD and one was classified as having a low probability of AD [46]. As these are historic cases, they have not been re-evaluated according to the more recent National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria. In addition to CERAD criteria, however, diagnosis was confirmed by also rating each case according to the Braak and Braak neuropathological staging criteria for Alzheimer-related changes [47] and the recommendations of the National Institute on Aging and Reagan Institute (NIA-RI) [48]. Together, these three ratings are largely equivalent to the more contemporary NIA-AA criteria [49]. Within the Alzheimer’s group, the ApoE status of nine of the twelve cases was known; of these, four had an ε4 allele. The ApoE status of nine of the ten cases in the non-Alzheimer’s control group was known; of these, three had an ε4 allele.
Table 1.
Demographic data
| AD (n=12) | Non-AD controls (n=10) | |
|---|---|---|
| Age, years, mean (SD) | 89.00 (10.43) | 81.00 (7.70) |
| Male, % | 25% | 50% |
| Black, % | 8.33% | 20% |
| Hispanic, % | 33.33% | 30% |
This research was reviewed by the Chair of the Columbia University Medical Center Institutional Review Board, who deemed the work exempt from further review (under 45 CFR 46) because there was no interaction with human subjects, no intervention, and private, identifiable information was not collected.
The tissue processing procedure has been described in detail previously [45]. Briefly, the whole fresh brain was first examined grossly, photographed and weighed. The brain was then divided into two halves. One half brain was processed fresh and frozen samples banked for research. The other half brain was formalin fixed processed for thorough neuropathological evaluation.
White matter dissection
For the current study, fresh frozen tissue blocks from Brodmann area (BA) 1, 2, and 3 (primary somatosensory cortex, posterior sections) and BA9 (dorso-lateral prefrontal cortex, anterior sections) were utilized. White matter was manually dissected from each block on dry ice to prevent thawing of the tissue and frozen at −80°C until biochemical analysis.
Analysis of amyloid levels
White matter samples (2 g each) were homogenized via sonication on ice in 880 μL of tissue lysate buffer (20 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM ethyleneglycoltetraacetic acid, 250 mM sucrose) supplemented with protease inhibitors (Roche). The tissue homogenates were treated with diethanolamine to extract soluble Aβ and centrifuged at 100,000 × g for 60 minutes at 4°C. The definition of soluble versus insoluble Aβ was the same as that used in previous studies [40,50]: that is, molecules that remain in the aqueous supernatant after centrifugation for 1 hour are considered soluble Aβ, while those Aβ aggregates that remain in the pellet are considered as insoluble Aβ. The supernatant was collected, total protein concentration was determined by a BCA protein assay (Thermo Scientific) and homogenate concentrations were standardized. Aβ-40 levels were determined using the Aβ-40 Type II ELISA kit from Wako (Catalog Number: 292–64701). Aβ-42 levels were measured using the Aβ-42 High Sensitivity ELISA kit from Wako (Catalog number: 292–64501). These kits have been extensively validated in previous studies (e.g. [51-55]) and are known to show extremely high sensitivity and reproducibility [54]. Both ELISA assays were performed in accordance with the manufacturer’s protocol, and all samples were run in duplicate. Optical density values were measured at 450 nm using a microplate reader, and then converted to concentrations (pmol/L) based on a standard curve. For cortical amyloid plaque ratings, a trained pathologist examined individual tissue sections and the number of Aβ plaques was manually counted. Neuritic plaque severity was rated in one section of each of the following areas from the fixed hemisphere: mid-frontal, superior temporal, pre-central, inferior parietal, hippocampus (CA1), subiculum, entorhinal cortex, transentorhinal cortex, inferior temporal, amygdala and basal forebrain. For each cortical area, a neuropathologist scanned the cortex over the entire slide, picked the most involved area, and then counted neuritic plaques stained with a Bielschowsky stain using the 10x ocular and 10x objective lenses. Each cortical region received a severity rating based on the following: 1, if there were less than 5 neuritic plaques, 2, if the number of neuritic plaques was between 5 and 15 and 3, if there were more than 15 neuritic plaques. We derived the total cortical neuritic plaque severity rating from the individual rating of each cortical region as follows: 1, if the total neuritic plaque rating was mild (i.e. the majority of cortical areas contained fewer than 5 Aβ plaques), 2, if the total neuritic plaque rating was moderate (i.e. the majority of cortical areas contained between 5 and 15 Aβ plaques) and 3, if the total neuritic plaque rating was severe (i.e. the majority of cortical areas contained more than 15 Aβ plaques). White matter tissue immunostained with Aβ antibodies from each case included in the study was also examined for the presence of white matter neuritic plaques, but none were found in any section.
Data analysis
Data were first analyzed with a repeated measures analysis of variance (ANOVA), with Region (2 levels: Anterior, posterior) as a within-subjects variable and Group (2 levels: AD, control) as a between-subjects variable. Two separate ANOVAs were conducted for Aβ-40 levels and Aβ-42 levels. Age at death was included as a covariate. In order to investigate further the effect of region, the nonparametric Wilcoxon signed rank sum test was used. To study the relationship between white matter Aβ levels and cortical plaque burden, Pearson correlations were calculated between white matter Aβ levels and neuritic plaque severity rating in several cortical areas. To investigate whether white matter levels of soluble Aβ were different between AD cases and non-AD controls, independent of cortical plaque pathology, a measure of total cortical neuritic plaque severity was included as a covariate and each ANOVA was re-run to test if the effect of either Group (AD vs. control) or Region (anterior vs. posterior) was still statistically significant.
Results
Aβ-40 levels
AD patients had higher average white matter Aβ-40 levels than non-AD controls (main effect of Group: F(1,15) = 6.338, p = 0.024; Figure 1A). Aβ-40 levels did not differ between anterior and posterior white matter regions in either the overall ANOVA (main effect of Region: F(1,15) = 0.718, p = 0.410; Figure 2A) or when the regional effect was further probed using a Wilcoxon signed-ranks test (Z = −0.675, p = 0.500). There was not an interaction between group and region (Group x Region interaction: F(1,15) = 0.003, p = 0.957). No significant correlations were found between either anterior or posterior white matter levels of Aβ-40 and neuritic plaque severity rating in any cortical region (Table 2). After statistically controlling for total cortical neuritic plaque severity, Alzheimer’s patients still had higher average white matter Aβ-40 levels than non-Alzheimer’s controls (main effect of Group: F(1,14) = 6.312, p = 0.025). After statistically controlling for total cortical neuritic plaque severity, there was still neither a main effect of region (F(1,14) = 0.940, p = 0.349) nor an interaction between group and region (Group x Region interaction: F(1,14) = 0.141, p = 0.713).
Figure 1.
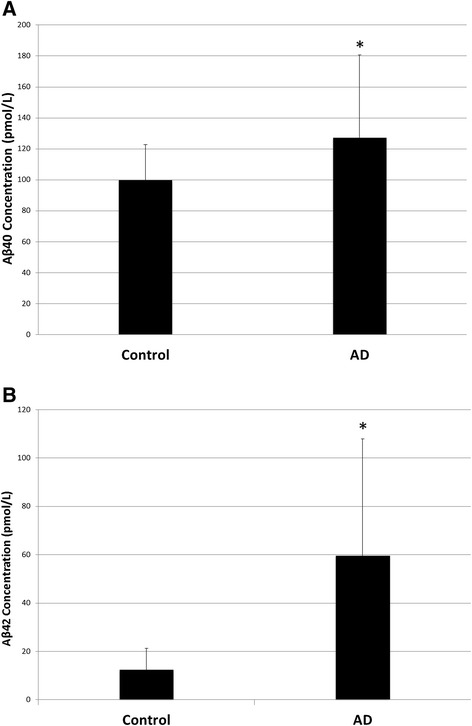
Main effect of group. (A) Average Aβ-40 levels were higher in the white matter of AD patients compared with non-AD controls. (B) Aβ-42 levels were significantly higher in the white matter of AD patients compared with non-AD controls.
Figure 2.
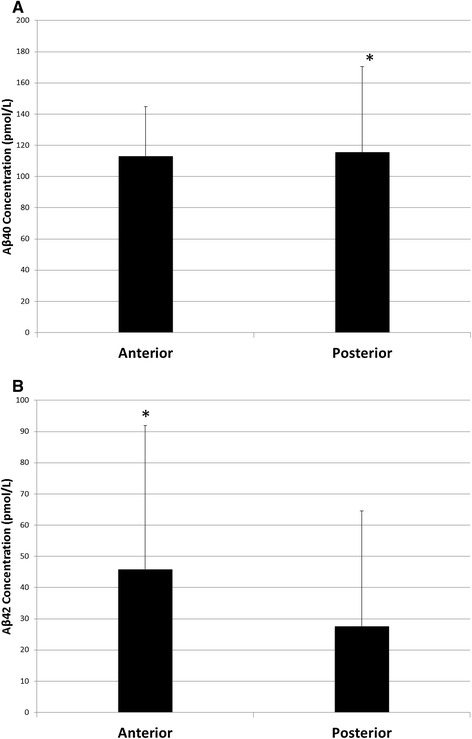
Main effect of region. (A) Aβ-40 levels were not significantly different in anterior or posterior regions of white matter. (B) There was a trend for Aβ-42 levels to be increased in anterior regions of white matter compared to posterior regions.
Table 2.
Correlations between white matter Aβ 40/42 levels (Anterior and posterior) and plaque severity rating, assayed by CERAD criteria, in several CNS regions
| Ant. Aβ-40 | Post. Aβ-40 | Ant. Aβ-42 | Post. Aβ-42 | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Mid-frontal | 0.294 | 0.196 | 0.204 | 0.403 | 0.697 | <0.001 | 0.630 | 0.004 |
| Superior temporal | -0.263 | 0.529 | 0.194 | 0.617 | 0.607 | 0.111 | 0.706 | 0.034 |
| Pre-central | 0.042 | 0.862 | 0.244 | 0.329 | 0.497 | 0.026 | 0.413 | 0.087 |
| Inferior Parietal | 0.351 | 0.118 | 0.228 | 0.347 | 0.744 | <0.001 | 0.633 | 0.004 |
| Hipp-CA1 | 0.019 | 0.937 | 0.082 | 0.746 | 0.618 | 0.004 | 0.596 | 0.009 |
| Subiculum | 0.282 | 0.228 | 0.347 | 0.159 | 0.457 | 0.043 | 0.448 | 0.062 |
| Entorhinal | 0.227 | 0.336 | 0.348 | 0.158 | 0.478 | 0.033 | 0.498 | 0.035 |
| Transentorhinal | 0.220 | 0.352 | 0.349 | 0.155 | 0.565 | 0.009 | 0.584 | 0.011 |
| Inferior Temporal | 0.270 | 0.250 | 0.298 | 0.230 | 0.653 | 0.002 | 0.655 | 0.003 |
| Amygdala | 0.365 | 0.103 | 0.132 | 0.591 | 0.618 | 0.003 | 0.482 | 0.037 |
| Basal forebrain | 0.360 | 0.109 | -.024 | 0.922 | 0.442 | 0.045 | 0.169 | 0.488 |
Note: Significant correlations are indicated by bolded text.
Aβ-42 levels
Compared with non-AD controls, AD patients had higher average levels of Aβ-42 (main effect of Group: F(1,15) = 8.024, p = 0.013; Figure 1B). While Aβ-42 levels were not different in anterior and posterior white matter regions (main effect of Region: F(1,15) = 1.001, p = 0.333; Figure 2B), there was a trend for them to be higher in anterior than in posterior regions (anterior: M = 45.84 pmol/L, SD = 46.06 pmol/L; posterior: M = 27.56 pmol/L, SD = 37.03 pmol/L). This effect was further examined using a Wilcoxon signed-ranks test, which demonstrated that Aβ-42 levels were significantly higher in anterior regions compared with posterior regions (Z = −2.243, p = 0.025). There was not a significant interaction between group and region (Group x Region interaction: F(1,15) = 2.246, p = 0.155). Significant positive correlations were found between anterior white matter levels of Aβ-42 and neuritic plaque severity rating in the mid-frontal cortex, pre-central cortex, inferior parietal cortex, hippocampal CA1, subiculum, entorhinal cortex, transentorhinal cortex, inferior temporal cortex, the amygdala and the basal forebrain (Table 2). Posterior white matter levels of Aβ-42 were positively correlated with neuritic plaque severity rating in the mid-frontal cortex, superior temporal cortex, inferior parietal cortex, hippocampal CA1, entorhinal cortex, transentorhinal cortex, inferior temporal cortex and the amygdala (Table 2). After statistically controlling for total cortical neuritic plaque severity, average white matter Aβ-42 levels were still higher in AD patients than in non-AD controls (main effect of Group: F(1,14) = 5.184, p = 0.039). There was still neither a main effect of region (F(1,14) = 1.513, p = 0.239) nor an interaction between group and region (Group x Region interaction: F(1,14) = 3.237, p = 0.094) after controlling for total cortical neuritic plaque severity.
Discussion
The current study investigated whether levels of soluble Aβ peptide are increased in the white matter of AD patients compared with non-AD controls. Levels of both soluble Aβ-40 and Aβ-42 were higher in the white matter of AD patients, even after statistically controlling for cortical neuritic plaque severity. While no regional white matter differences were found in Aβ -40, Aβ -42 levels were higher in anterior regions than in posterior regions across both subject groups.
The finding that soluble Aβ-40 and Aβ-42 levels are increased in the white matter of AD patients is consistent with the hypothesis that the white matter degeneration seen in AD could be due, in part, to the toxicity of Aβ peptides [4]. A recent study by Horiuchi and colleagues found that soluble Aβ at 10 μM or higher reduced the survival of mature oligodendrocytes and affected myelin sheet formation in vitro [56]. Similarly, Roher and colleagues demonstrated that increased levels of Aβ peptides are associated with reductions in multiple myelin biochemical markers and axonal demyelination [57]. In a triple transgenic mouse model, Aβ-42 led to loss of myelin integrity and increased apoptotic cell death of mouse oligodendrocyte precursor cells in vitro, an effect that was reversed through the use of a viral vector-conjugated Aβ-42 specific intracellular antibody [58]. Amyloid beta may have detrimental effects on white matter via oxidative stress and the activation of transcription factors, such as NF-κB and AP-1, which may cause tissue damage through a pro-inflammatory reaction [59]. Amyloid beta peptide may also activate neutral sphingomyelinase, leading to increases in the apoptogenic mediator ceramide [60].
The current study found significant correlations between Aβ42 levels and neuritic plaque severity ratings in several cortical regions. Given the amyloid cascade hypothesis and the large increases in Aβ42 known to occur in AD [2,38], this relationship is not surprising. Aβ42 is the predominant form of Aβ in parenchymal plaques [53,54] and Aβ42 displays enhanced neurotoxicity compared to Aβ40 [61-63]. Additionally, Aβ42 is known to form fibrils faster than Aβ40 [64]. While Aβ40 exists as monomers, dimers, trimers and tetramers in rapid equilibrium, Aβ42 preferentially forms pentamer and hexamer units, which assemble into higher-order oligomers and early protofibrils [65,66]. Hence, it was not unexpected that white matter Aβ42 levels would be related to the severity of cortical plaque pathology. It is noteworthy, however, that levels of Aβ-40 and Aβ-42 were increased in AD patients compared with non-AD controls, even after statistically controlling for cortical neuritic plaque severity, indicating that white matter levels of Aβ are higher in AD patients than non-AD controls, above and beyond cortical plaque pathology. These observations suggest that white matter Aβ peptides contribute to disease pathophysiology, at least in part, independent of grey matter pathology. This observation is consistent with several recent studies that suggest that, rather than being secondary to grey matter degeneration (i.e. Wallerian-like degeneration), white matter structural changes in AD might precede the emergence of grey matter pathology [4]. Several recent neuroimaging studies showed the presence of widespread white matter deficits in patients with amnestic mild cognitive impairment (aMCI) [67], subjective cognitive impairment [68] and cognitively normal individuals who go on to develop aMCI [21], despite a lack of grey matter cortical atrophy. Neuropathological studies also indicated pathological alterations in white matter preceding grey matter atrophy [23,33]. In one neuropathological study, while widespread white matter degeneration was observed in the brains of preclinical AD patients, the grey matter structure was relatively unaltered; conversely, the brains of patients with end-stage AD showed both grey and white matter changes [27]. Taken together, these findings would suggest that white matter disease might be an early event in the pathogenesis of AD. In fact, Castano and colleagues recently hypothesized that AD pathogenesis may actually begin in the white matter with oligodendrocyte dysfunction and associated pathological changes precipitating AD dementia and its progression [26]. The investigation of the pathophysiological mechanisms of white matter structural impairments could thus be critical for the identification and treatment of individuals at a high risk for developing AD.
In addition to the current findings, several other studies highlighted the importance of frontal lobe Aβ levels in disease pathology. In a postmortem study of patients with AD, Chalmers and colleagues reported that parenchymal amyloid load was more predictive of white matter degeneration in the frontal lobe than degenerative vascular disease, cerebral amyloid angiopathy or APOE ε4 genotype [69]. Similarly, postmortem studies using antibodies to Aβ identified the frontal cortex as a location for high amounts of amyloid deposition [70]. This observation is consistent with [11C]-PIB PET scan studies, where the highest amount of uptake has been observed in the frontal cortex, indicating more severe amyloid deposition in the grey matter in this area [71]. It is important to note, however, that PIB scans can only reliably measure fibrillar forms of amyloid in grey matter regions. In the current study, soluble Aβ levels were studied exclusively in white matter tissue. Neuroimaging studies from our group reported that it is WMH burden in posterior regions, not the frontal lobe, that is most predictive of incident clinical AD in the community and of the rate of cognitive decline in AD [9,10,35]. Thus, it is possible that, while amyloid may have a propensity to deposit in frontal regions, it is macrostructural changes in posterior areas that may drive the clinical presentation of the disease.
One potential limitation of the current study is the relatively heterogeneous control group. In future studies, it would be interesting to include a larger number of neuropathologically normal controls, in order to investigate more thoroughly how white matter pathology differs between normal, healthy aging and AD. Another potential limitation of the study is that the concentration of soluble Aβ in the cortex was not measured. While it is possible that cortical levels of soluble Aβ may be more appropriate for direct comparison with white matter levels of soluble Aβ, previous studies have suggested that there is a reliable correlation between cortical levels of soluble Aβ and both diffuse and neuritic plaques in the cortex in AD [72,73]. Hence, in the current study, we chose to use a rating of neuritic plaque severity as our marker of cortical amyloid pathology. Given the growing body of evidence that it is the levels of soluble Aβ oligomers, rather than insoluble Aβ fibrils, that correlate with the extent of synaptic loss and the severity of cognitive impairment in AD [38,39,43], however, future studies should also investigate the correlation between cortical and white matter levels of soluble Aβ oligomers.
Conclusion
The current study found that both Aβ -42 and Aβ -40 levels were higher in the white matter of AD brains compared with non-AD controls, even after statistically controlling for cortical neuritic plaque severity. These findings suggest that Aβ peptides in white matter tissue accumulate independent of overall grey matter fibrillar amyloid pathology and are not simply a reflection of overall amyloid burden. Thus, white matter Aβ peptides may contribute to disease pathophysiology, at least in part, independent of grey matter pathology. Additionally, while no regional white matter differences were found in Aβ -40, Aβ -42 levels were higher in anterior regions than in posterior regions across both subject groups. This observation may suggest that Aβ -42 has a propensity to deposit in frontal regions, although it is not yet clear if this is related to cognitive change, or if macrostructural changes in posterior areas, likely related to cerebrovascular or other factors, may be more important for the clinical presentation of the disease.
In conclusion, the results of the current study help to shed light on a potential pathological mechanism that may contribute to white matter abnormalities in AD. Given that white matter change may be an early marker of disease, preceding grey matter atrophy, understanding the mechanisms and risk factors that may lead to white matter damage could provide a novel mechanism by which to identify those at high risk, allowing us to intervene earlier in the pathogenic process.
Acknowledgments
This work was supported by grants from the Alzheimer’s Association, the Taub Institute at Columbia University, the Mary E. Groff Surgical Medical Research and Education Charitable Trust Groff Foundation, and NIH (P50 AG008702, R01 AG034189). We are grateful to the New York Brain Bank for material support.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid beta
- NFT
Neurofibrillary tangle
- MRI
Magnetic resonance imaging
- WMH
White matter hyperintensity
- DTI
Diffusion tensor imaging
- ANOVA
Analysis of variance
- aMCI
Amnestic mild cognitive impairment
- NIA-RI
National Institute on Aging and Reagan Institute
- NIA-AA
National Institute of Health-Alzheimer’s Association
Footnotes
Competing interests
The authors declare that there are no any sources of financial support or relationship(s) that may pose a competing interest for the current work.
Authors’ contributions
All authors 1) have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) have been involved in drafting the manuscript or revising it critically for important intellectual content; 3) have given final approval of the version to be published; and 4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Additional specific contributions are as follows: LEC-P conceptualized the study, carried out assays, conducted analyses, and drafted the manuscript. YF supervised and carried out assays. E.Y.G. coordinated the study, assisted with tissue preparation, and helped carry out assays. AW conducted tissue dissection and assisted with carrying out assays. AL coordinated the autopsy study for clinicopathological analysis. LSH oversaw clinical and autopsy studies. EC conducted pathological examination of tissue and gross dissection. JPV oversaw and conducted pathological examination, gross dissection, and diagnostics. PC supervised the study. JEG supervised the study. AMB conceptualized the study and drafted the manuscript.
Contributor Information
Lyndsey E Collins-Praino, Email: lyndsey.collins-praino@adelaide.edu.au.
Yitshak I Francis, Email: Itsikfrancis@hotmail.com.
Erica Y Griffith, Email: eyg2102@columbia.edu.
Anne F Wiegman, Email: afwiegman@gmail.com.
Jonathan Urbach, Email: jurbach@bu.edu.
Arlene Lawton, Email: asl1@columbia.edu.
Lawrence S Honig, Email: lh456@cumc.columbia.edu.
Etty Cortes, Email: epc2107@columbia.edu.
Jean Paul G Vonsattel, Email: jgv2001@columbia.edu.
Peter D Canoll, Email: pc561@columbia.edu.
James E Goldman, Email: jeg5@columbia.edu.
Adam M Brickman, Email: amb2139@columbia.edu.
References
- 1.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Amlien IK, Fjell AM (2014) Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. doi:10.1016/j.neuroscience.2014.02.017 [DOI] [PubMed]
- 4.Sachdev PS, Zhuang L, Braidy N, Wen W. Is Alzheimer’s a disease of the white matter? Curr Opin Psychiatry. 2013;26(3):244–251. doi: 10.1097/YCO.0b013e32835ed6e8. [DOI] [PubMed] [Google Scholar]
- 5.Balthazar ML, Yasuda CL, Pereira FR, Pedro T, Damasceno BP, Cendes F. Differences in grey and white matter atrophy in amnestic mild cognitive impairment and mild Alzheimer’s disease. Eur J Neurol. 2009;16(4):468–474. doi: 10.1111/j.1468-1331.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Wang Z, Li K, Li Z, Qi Z, Jin Z, Yao L, Chen K. Voxel-based assessment of gray and white matter volumes in Alzheimer’s disease. Neurosci Lett. 2010;468(2):146–150. doi: 10.1016/j.neulet.2009.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Pu F, Shi F, Xie S, Wang Y, Jiang T. Regional white matter decreases in Alzheimer’s disease using optimized voxel-based morphometry. Acta Radiol. 2008;49(1):84–90. doi: 10.1080/02841850701627181. [DOI] [PubMed] [Google Scholar]
- 8.Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer’s disease. Neuroimage. 2009;44(4):1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, Brandt J, Blacker D, Stern Y. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65(9):1202–1208. doi: 10.1001/archneur.65.9.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Altheimer’s disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11(2):181–190. doi: 10.31887/DCNS.2009.11.2/ambrickman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YF, Wang H, Chu Y, Huang YC, Su MY. Regional quantification of white matter hyperintensity in normal aging, mild cognitive impairment, and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22(2):177–184. doi: 10.1159/000094785. [DOI] [PubMed] [Google Scholar]
- 12.Scheltens P, Barkhof F, Valk J, Algra PR, van der Hoop RG, Nauta J, Wolters EC. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain. 1992;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 13.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010;133(Pt 2):529–539. doi: 10.1093/brain/awp257. [DOI] [PubMed] [Google Scholar]
- 15.Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33(1):61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Damoiseaux JS, Smith SM, Witter MP, Sanz-Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA. White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp. 2009;30(4):1051–1059. doi: 10.1002/hbm.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, Norimoto K, Hashimoto K, Kosaka J, Inoue Y, Inoue M, Kichikawa K, Kishimoto T. Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer’s disease: a diffusion tensor tractography study. Brain Res. 2009;1287:184–191. doi: 10.1016/j.brainres.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 18.Serra L, Cercignani M, Basile B, Spano B, Perri R, Fadda L, Marra C, Giubilei F, Caltagirone C, Bozzali M. White matter damage along the uncinate fasciculus contributes to cognitive decline in AD and DLB. Curr Alzheimer Res. 2012;9(3):326–333. doi: 10.2174/156720512800107555. [DOI] [PubMed] [Google Scholar]
- 19.Sexton CE, Kalu UG, Filippini N, Mackay CE, Ebmeier KP. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2011;32(12):2322. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain. 2009;132(Pt 9):2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang L, Sachdev PS, Trollor JN, Reppermund S, Kochan NA, Brodaty H, Wen W. Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One. 2013;8(3):e58887. doi: 10.1371/journal.pone.0058887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang L, Wen W, Trollor JN, Kochan NA, Reppermund S, Brodaty H, Sachdev P. Abnormalities of the fornix in mild cognitive impairment are related to episodic memory loss. J Alzheimers Dis. 2012;29(3):629–639. doi: 10.3233/JAD-2012-111766. [DOI] [PubMed] [Google Scholar]
- 23.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19(3):253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 24.Beach TG, Walker R, McGeer EG. Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia. 1989;2(6):420–436. doi: 10.1002/glia.440020605. [DOI] [PubMed] [Google Scholar]
- 25.Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord. 2002;13(4):205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- 26.Castano EM, Maarouf CL, Wu T, Leal MC, Whiteside CM, Lue LF, Kokjohn TA, Sabbagh MN, Beach TG, Roher AE. Alzheimer disease periventricular white matter lesions exhibit specific proteomic profile alterations. Neurochem Int. 2013;62(2):145–156. doi: 10.1016/j.neuint.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer’s disease. Ann Neurol. 1989;25(5):450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- 28.Englund E. Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9(Suppl 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- 29.Englund E, Brun A. White matter changes in dementia of Alzheimer’s type: the difference in vulnerability between cell compartments. Histopathology. 1990;16(5):433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- 30.Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9(3–4):112–122. [PMC free article] [PubMed] [Google Scholar]
- 31.Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer’s disease and normal aging. Neurology. 1995;45(5):883–888. doi: 10.1212/WNL.45.5.883. [DOI] [PubMed] [Google Scholar]
- 32.Sjobeck M, Haglund M, Englund E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer’s disease–a neuropathological study. Int J Geriatr Psychiatry. 2005;20(10):919–926. doi: 10.1002/gps.1384. [DOI] [PubMed] [Google Scholar]
- 33.Sjobeck M, Haglund M, Englund E. White matter mapping in Alzheimer’s disease: A neuropathological study. Neurobiol Aging. 2006;27(5):673–680. doi: 10.1016/j.neurobiolaging.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69(12):1621–1627. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Defrancesco M, Marksteiner J, Deisenhammer E, Kemmler G, Djurdjevic T, Schocke M. Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer’s disease. J Alzheimers Dis. 2013;34(3):665–672. doi: 10.3233/JAD-122095. [DOI] [PubMed] [Google Scholar]
- 37.Solodkin A, Chen EE, Van Hoesen GW, Heimer L, Shereen A, Kruggel F, Mastrianni J. In vivo parahippocampal white matter pathology as a biomarker of disease progression to Alzheimer’s disease. J Comp Neurol. 2013;521(18):4300–4317. doi: 10.1002/cne.23418. [DOI] [PubMed] [Google Scholar]
- 38.Cavallucci V, D’Amelio M, Cecconi F. Abeta toxicity in Alzheimer’s disease. Mol Neurobiol. 2012;45(2):366–378. doi: 10.1007/s12035-012-8251-3. [DOI] [PubMed] [Google Scholar]
- 39.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 40.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155(3):853–862. doi: 10.1016/S0002-9440(10)65184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::AID-ANA8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 43.Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer’s disease from normal and pathologic aging. Exp Neurol. 1999;158(2):328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 45.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol. 2008;115(5):509–532. doi: 10.1007/s00401-007-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/WNL.41.4.479. [DOI] [PubMed] [Google Scholar]
- 47.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 48.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease The National Institute on Aging, and Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer’s disease. Neurobiol Aging. 1997;18(4 Suppl):S1–S2. [PubMed] [Google Scholar]
- 49.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Montine TJ. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. ᅟ;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271(8):4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 51.Asami-Odaka A, Ishibashi Y, Kikuchi T, Kitada C, Suzuki N. Long amyloid beta-protein secreted from wild-type human neuroblastoma IMR-32 cells. Biochemistry. 1995;34(32):10272–10278. doi: 10.1021/bi00032a022. [DOI] [PubMed] [Google Scholar]
- 52.Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010;24(8):2716–2726. doi: 10.1096/fj.09-150359. [DOI] [PubMed] [Google Scholar]
- 53.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L, Jr, Eckman C, Golde TE, Younkin SG. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Browne A, Divito JR, Stevenson JA, Romano D, Dong Y, Xie Z, Tanzi RE. Amyloid-beta production via cleavage of amyloid-beta protein precursor is modulated by cell density. J Alzheimers Dis. 2010;22(2):683–984. doi: 10.3233/JAD-2010-100816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horiuchi M, Maezawa I, Itoh A, Wakayama K, Jin LW, Itoh T, Decarli C. Amyloid beta1-42 oligomer inhibits myelin sheet formation in vitro. Neurobiol Aging. 2012;33(3):499–509. doi: 10.1016/j.neurobiolaging.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer’s disease. Biochemistry. 2002;41(37):11080–11090. doi: 10.1021/bi026173d. [DOI] [PubMed] [Google Scholar]
- 58.Desai MK, Mastrangelo MA, Ryan DA, Sudol KL, Narrow WC, Bowers WJ. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am J Pathol. 2010;177(3):1422–1435. doi: 10.2353/ajpath.2010.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY. Amyloid-beta peptides are cytotoxic to oligodendrocytes. J Neurosci. 2001;21(1):RC118. doi: 10.1523/JNEUROSCI.21-01-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, Chen S, Hsu CY. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. J Cell Biol. 2004;164(1):123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277(35):32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 62.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399(6738 Suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 63.Younkin SG. Evidence that A beta 42 is the real culprit in Alzheimer’s disease. Ann Neurol. 1995;37(3):287–288. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- 64.Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 65.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci U S A. 2003;100(1):330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urbanc B, Betnel M, Cruz L, Bitan G, Teplow DB. Elucidation of amyloid beta-protein oligomerization mechanisms: discrete molecular dynamics study. J Am Chem Soc. 2010;132(12):4266–4280. doi: 10.1021/ja9096303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agosta F, Pievani M, Sala S, Geroldi C, Galluzzi S, Frisoni GB, Filippi M. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology. 2011;258(3):853–863. doi: 10.1148/radiol.10101284. [DOI] [PubMed] [Google Scholar]
- 68.Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Grambaite R, Stenset V, Fladby T. White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement. 2012;8(5 Suppl):S112–S121. doi: 10.1016/j.jalz.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Chalmers K, Wilcock G, Love S. Contributors to white matter damage in the frontal lobe in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2005;31(6):623–631. doi: 10.1111/j.1365-2990.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 70.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 71.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 72.van Helmond Z, Miners JS, Kehoe PG, Love S. Oligomeric Abeta in Alzheimer’s disease: relationship to plaque and tangle pathology, APOE genotype and cerebral amyloid angiopathy. Brain Pathol. 2010;20(2):468–480. doi: 10.1111/j.1750-3639.2009.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia W, Yang T, Shankar G, Smith IM, Shen Y, Walsh DM, Selkoe DJ. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol. 2009;66(2):190–199. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


