Abstract
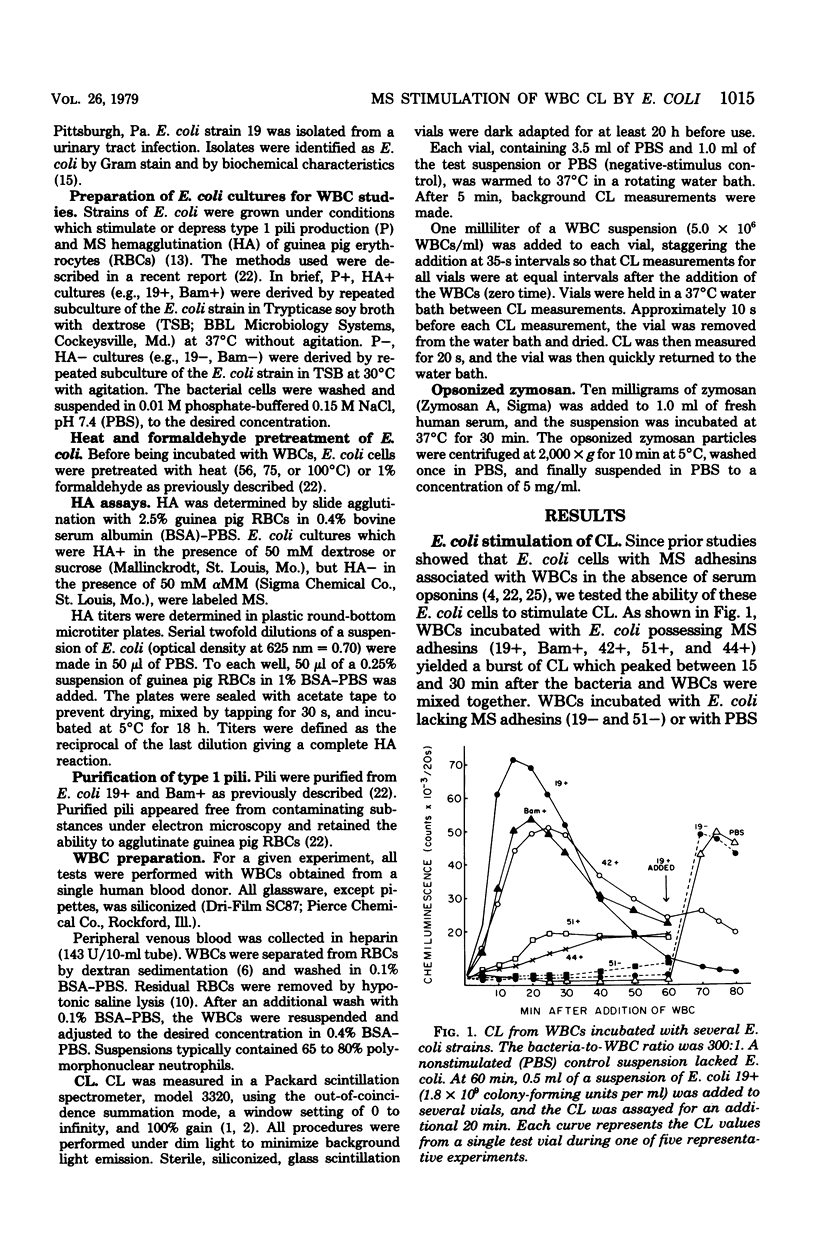
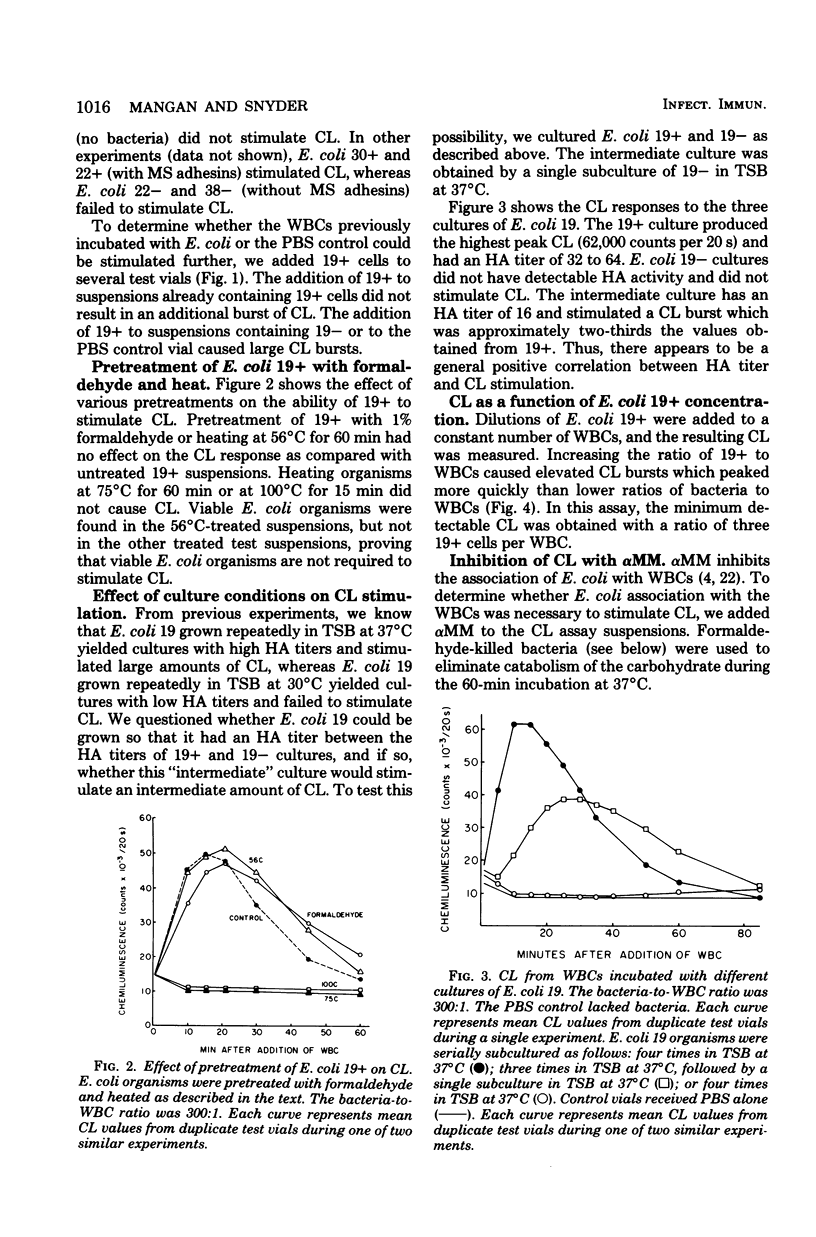
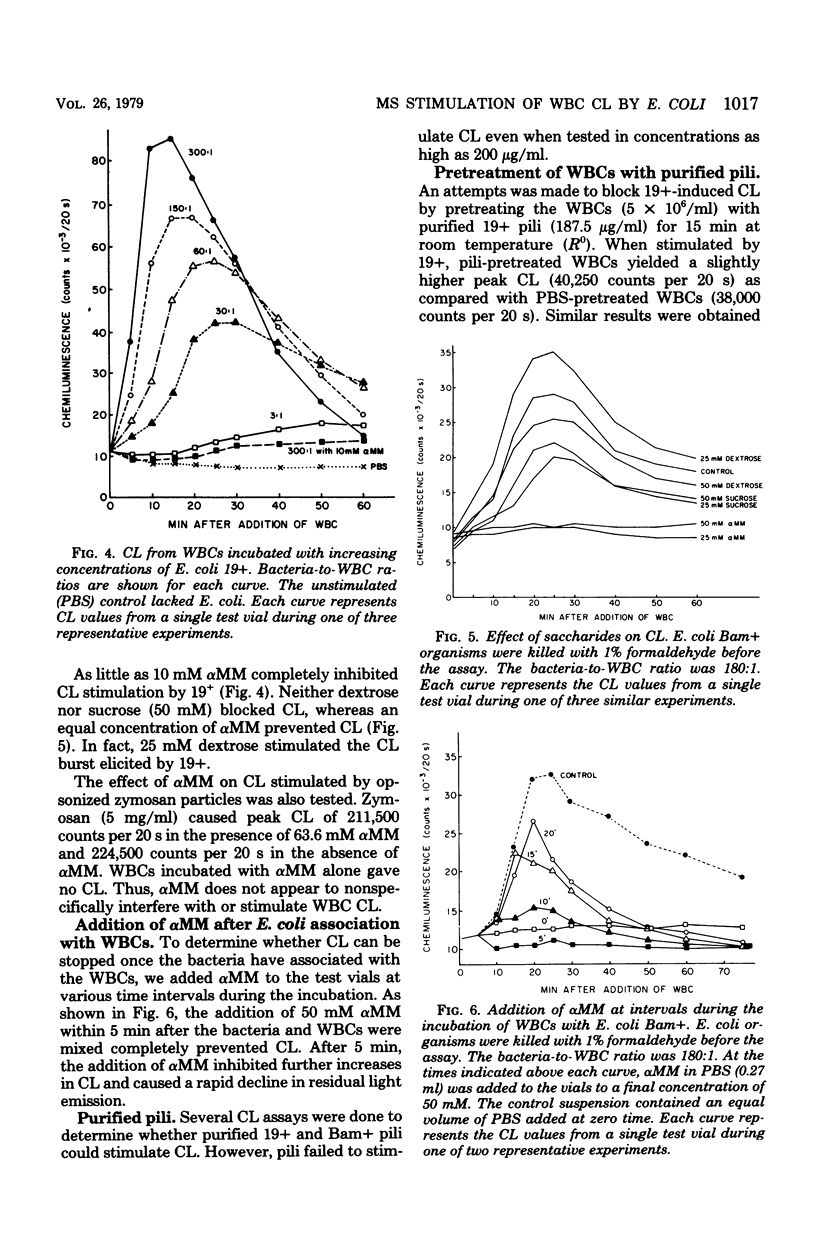
Escherichia coli organisms with mannose-sensitive adherence factors (adhesins) are known to associate with human peripheral leukocytes (WBCs) in vitro in the absence of serum. To determine whether the WBC respiratory burst is activated during the interaction with E. coli, WBC chemiluminescence was measured. E. coli with mannose-sensitive adhesins stimulated a sharp burst of chemiluminescence which peaked 15 to 30 min after the bacteria and WBCs were mixed. Stimulation of chemiluminescence could be abrogated by including 10 mM alpha-methyl-D-mannoside in the test suspension. The addition of alpha-methyl-D-mannoside up to 20 min after the E. coli and WBCs were combined caused a rapid decrease in chemiluminescence. E. coli stimulation of chemiluminescence could not be inhibited by pretreating the WBCs with purified type 1 pili (fimbriae). E. coli lacking mannose-sensitive adhesins failed to stimulate chemiluminescence. The results emphasize the importance of mannose-sensitive adhesins in the association of E. coli with WBCs and suggest that the E. coli-WBC interaction system may be a useful tool for studying the mechanisms involved in the activation of the respiratory burst during phagocytosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Berlin R. D. Effect of concanavalin A on phagocytosis. Nat New Biol. 1972 Jan 12;235(54):44–45. doi: 10.1038/newbio235044a0. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Cheson B. D., Christensen R. L., Sperling R., Kohler B. E., Babior B. M. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976 Oct;58(4):789–796. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Reeke G. N., Jr, Becker J. W., Waxdal M. J., Wang J. L. The covalent and three-dimensional structure of concanavalin A. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2580–2584. doi: 10.1073/pnas.69.9.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- Mangan D. F., Snyder I. S. Mannose-sensitive interaction of Escherichia coli with human peripheral leukocytes in vitro. Infect Immun. 1979 Nov;26(2):520–527. doi: 10.1128/iai.26.2.520-527.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Rossi F. Reversible metabolic stimulation of polymorphonuclear leukocytes and macrophages by concanavalin A. Nat New Biol. 1973 May 23;243(125):111–112. [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


