Abstract
Background
Chronic obstructive pulmonary disease (COPD) is an important cause of occupational mortality in miners exposed to coal mine dust. Although the inflammatory mediators involved in COPD have not been defined, many studies have shown that inflammatory mediators such as reactive oxygen and nitrogen species are involved in orchestrating the complex inflammatory process in COPD.
Methods
To investigate the relevance of exhaled biomarkers of oxidative and nitrosative stress in participants with COPD, we determined the levels of hydrogen peroxide, malondialdehyde (MDA), and 3-nitrotyrosine (3-NT) in exhaled breath condensate (EBC) in 90 retired elderly coal miners (53 non-COPD and 37 COPD participants).
Results
Mean levels of MDA (4.64 nM vs. 6.46 nM, p = 0.005) and 3-NT (3.51 nM vs. 5.50 nM, p = 0.039) in EBC were significantly higher in participants with COPD. The median level of MDA did show statistical difference among the COPD severities (p = 0.017), and the area under the receiver operating characteristic curve for MDA (0.67) for the diagnostic discrimination of COPD indicated the biomarker. The optimal cutoff values were 5.34 nM (64.9% sensitivity and 64.2% specificity) and 5.58 nM (62.2% sensitivity and 62.3% specificity) for MDA and 3-NT, respectively. The results suggest that high levels of MDA and 3-NT in EBC are associated with COPD in retired elderly miners.
Conclusion
These results showed that the elevated levels of EBC MDA and EBC 3-NT in individuals with COPD are biomarkers of oxidative or nitrosative stress.
Keywords: 3-nitrotyrosine, chronic obstructive pulmonary disease, exhaled breath condensate, hydrogen peroxide, malondialdehyde
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs [1]. Among occupational lung diseases, the most prevalent diseases are induced by inhalation of dust, including asbestos, crystalline silica, and coal. Exposure to silica and coal mine dust may result in pulmonary scarring in a pattern that mimics idiopathic pulmonary fibrosis, and in COPD that appears indistinguishable from the obstructive lung disease caused by exposure to tobacco smoke [2]. Coal workers' pneumoconiosis and COPD are important causes of occupational mortality in miners with extensive exposure to coal mine dust [3].
The role of inflammatory mediators in the development of COPD has not been clearly defined, but it is now apparent that many of them, such as reactive oxygen and nitrogen species, cytokines, chemokines, and growth factors, are involved in orchestrating the complex inflammatory process in COPD [4]. One of the pathogenic processes of COPD is oxidative stress, which may be a mechanism that enhances the inflammatory response [5].
Detection of inflammatory biomarkers of pulmonary diseases involves invasive techniques, such as obtaining samples of blood, bronchoalveolar lavage fluid, and induced sputum. Because invasive sampling procedures may induce an inflammatory response, noninvasive techniques, such as sampling exhaled breath condensate (EBC) and exhaled air, have been developed. Analysis of various biomarkers in exhaled breath has allowed noninvasive monitoring of inflammatory and oxidative stress in the respiratory tract in inflammatory lung diseases [6–9]. Malondialdehyde (MDA) has been widely studied as a product of polyunsaturated fatty acid peroxidation resulting from oxidative stress. Increased MDA levels have been observed in several biological fluids obtained from patients with different airway diseases including asthma and COPD [10]. Exhaled nitric oxide (NO) released from lung cells, including inflammatory cells, epithelial cells, and endothelial cells in the respiratory tract, has been known to be an effective biomarker for diagnosis and treatment of lung disorders including COPD [11–13]. The main form of NO degradation is the rapid reaction with superoxide anion to form the more reactive product peroxynitrite. Peroxynitrite reacts with tyrosine to form 3-nitrotyrosine (3-NT) [14]. Thus, NO and protein-associated NT are considered as characteristic markers of nitrosative stress [15]. There have been relatively few reports analyzing EBC biomarkers in elderly retired coal miners with COPD.
In this study, we hypothesized that the levels of biomarkers of oxidative and nitrosative stress in the EBC of patients with COPD are elevated. To test this hypothesis, we evaluated the hydrogen peroxide (H2O2), MDA, and 3-NT levels in the EBC.
2. Materials and methods
2.1. Participants
A group of 90 retired male miners were recruited and examined for pneumoconiosis at Ansan Workers’z Compensation Hospital, Korea Workers' Compensation and Welfare Service (KCOMWEL). The data collected included the forced vital capacity (FVC) and the forced expiratory volume in 1 second (FEV1). Personal information, including age, body mass index (BMI), and demographic information such as job and smoking status were obtained using a structured questionnaire. Pulmonary function testing was performed in accordance with international guidelines using a Vmax22 spirometer (SensorMedics, San Diego, CA, USA) [16]. FVC, which is the volume delivered during an expiration made as forcefully and completely as possible starting from full inspiration, and FEV1 were measured. A clinical diagnosis of COPD was considered in participants who showed an FEV1/FVC ratio < 70% and without FEV1 increasing higher than 200 mL and 12% after β2-bronchodilator inhalation.
Chest radiographs for the diagnosis of pneumoconiosis were obtained and scored according to the International Labor Office classification rules [17] by an experienced panel of physicians from the Pneumoconiosis Review Committee associated with KCOMWEL.
2.2. EBC collection
Collection of EBC was performed in accordance with the guidelines of the American Thoracic Society [18]. In brief, all participants rinsed their mouths with purified saline prior to collection. Between 1 mL and 3 mL of EBC was collected during 10 minutes of oral tidal breathing using an EBC collection system (ECoscreen 2; FILT GmBH, Berlin, Germany). The participants wore nose clips while collecting the condensate to prevent air from escaping. The collected EBC samples were immediately stored at −80°C and analysis was performed within 1 month of collection.
2.3. Analysis of biomarkers in EBC
The H2O2 levels in EBC were measured by automated flow injection analysis with fluorescence detection (high-performance liquid chromatography system; Agilent 1200 Series; Agilent technologies, Waldbronn, Germany), as previously described [19]. In brief, 150 μL of EBC samples or standard was added to 15 μL of a mixture of 2.5 U/L horseradish peroxidase and 2.5 mM p-hydroxyphenylacetic acid. The injection volume was 20 μL and the excitation and emission wavelengths were 285 nm and 400 nm, respectively.
Analysis of MDA levels in EBC samples was performed by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (LC-APCI-MS/MS, 3200QTRAP, AB SCIEX, CA, USA; Fig. 1), as previously described [20]. In brief, samples were derivatized with 2,4-dinitrophenylhydrazine solution (12 mM in acetonitrile and 2% formic acid) at room temperature for 60 minutes, after which 20 μL of the derivatized samples was injected onto the LC-APCI-MS/MS system (3200QTRAP). Chromatography of derivatives was performed on a C18 reverse-phase column (Triart-C18; 2.0 mm × 100 mm, 3 μm, YMC Co. Ltd, Shimogyo-ku, Kyoto, Japan) using 20 mM aqueous acetic acid and methanol under gradient elution condition at a flow rate of 0.2 mL min−1. The gradient program was as follows: from 45% to 98% methanol in 4 minutes, linear gradient, and then hold for 4.5 minutes. The target ion of derivatives was m/z 235/159 in the positive mode.
Fig. 1.
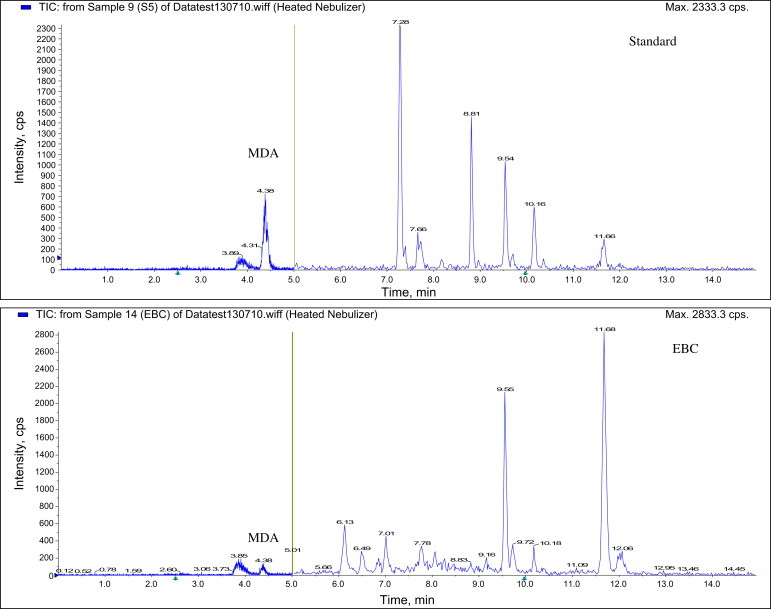
Chromatogram of malondialdehyde (MDA) using liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. EBC, exhaled breath condensate.
A sandwich enzyme immunoassay was used to measure 3-NT (NWLSS Nitrosine ELISA Kit, NWK-NTR01, Northwest Life Science Specialties, Vancouver, Canada) levels in accordance with the manufacturer's instructions.
2.4. Statistical analyses
Levels of H2O2 and 3-NT in EBC showed a log-normal distribution. The values were log-transformed for parametric statistical tests, and the parametric unpaired Student t test and analysis of variance test were used to determine the magnitudes of between-group differences. If the sample sizes among the study groups were also different or the number of cases for each group were incompatible for parametric statistical tests, the nonparametric Mann–Whitney U test and Kruskal–Wallis one-way analysis based on rank sums were used to determine differences among the study groups. A multiple regression model was constructed to compare non-COPD participants with those who had COPD, which was then adjusted for age, BMI, exposure period, smoking status, and pneumoconiosis. Receiver operating characteristic (ROC) curve analysis was used to assess the potential of each exhaled biomarker for the discrimination of COPD. Statistical cutoff values were calculated by minimizing the distance between the point with specificity = 1 and sensitivity = 1 and various points on the ROC curve. For ROC analysis, an area under the curve (AUC) of 1.0 indicates perfect discrimination, whereas an area of 0.5 indicates that the test discriminates no better than chance. The relationship between biomarkers and effective variables was evaluated using stepwise multiple regression analysis. Values of p < 0.05 were considered statistically significant. All statistical evaluations were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
2.5. Ethics statement
The study protocol was approved by our institute's Research Ethics Committee (Approval number 2010-03-04). All participants signed an informed consent form that conformed to the recommendations of the committee.
3. Results
3.1. General characteristics
The general characteristics of the study participants are shown in Table 1. Of the 90 study participants, 37 were diagnosed with COPD, whereas the remaining 53 were classified as the non-COPD group. General characteristics, including median age, BMI, exposure period, %FVC predicted, and smoking status were similar in both study groups, whereas %FEV1 predicted and chest radiographs of pneumoconiosis were different.
Table 1.
General characteristics of the study participants
| Non-COPD (n = 53) | COPD∗ (n = 37) | p | |
|---|---|---|---|
| Age (y) | 62.1 ± 8.0 | 65.1 ± 7.4 | 0.072† |
| BMI (kg/m2) | 23.0 ± 3.2 | 23.4 ± 2.4 | 0.338† |
| Exposure period (y) | 18.7 ± 5.9 | 16.6 ± 8.2 | 0.645† |
| %FVC predicted | 94.7 ± 15.4 | 94.4 ± 11.6 | 0.904† |
| %FEV1 predicted | 104.6 ± 18.4 | 85.4 ± 13.4 | <0.001† |
| %FEV1/FVC ratio | 77.9 ± 4.6 | 62.7 ± 7.4 | <0.001† |
| Smoking, N (%) | |||
| Never | 8 (15.1) | 2 (5.4) | 0.343‡ |
| Past | 27 (50.9) | 20 (54.1) | |
| Current | 18 (34.0) | 15 (40.5) | |
| Cumulative smoking (pack-years) | 17.1 ± 15.6 | 20.5 ± 13.2 | 0.291† |
| Pneumoconiosis, N (%) | |||
| No | 29 (54.7) | 8 (21.6) | 0.001‡ |
| Yes | 24 (45.3) | 29 (78.4) | |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Individuals with COPD are those with FEV1/FVC ratio < 70% and without FEV1 increasing higher than 200 mL and 12% after β2-bronchodilator inhalation.
Calculated by t test, arithmetic mean ± arithmetic standard deviation.
Calculated by χ2 test.
3.2. Exhaled biomarker concentrations
Exhaled biomarker concentrations in the COPD and non-COPD groups are shown in Tables 2 and 3. The mean EBC MDA level was significantly elevated in participants with COPD (4.64 nM vs. 6.46 nM, p = 0.005). This effect held true in the adjusted multiple regression model (p = 0.002 for all comparisons). The mean EBC 3-NT level was also significantly higher in participants with COPD (3.51 nM vs. 5.50 nM, p = 0.039), and the significance did show in the adjusted multiple regression model (p = 0.042). We did not find statistically significant intergroup differences in the mean value of EBC H2O2 levels. The median level of EBC MDA tends to increase with regard to COPD severities, non-COPD (4.70 nM), mild COPD (5.93 nM), and moderate COPD (7.20 nM), and there was statistical significance related to COPD severities (p = 0.017; Table 4). The median level of EBC 3-NT tends to increase with regard to COPD severities, non-COPD (3.31 nM), mild COPD (6.81 nM), and moderate COPD (7.71 nM); however, the median level did not show any statistical significance related to COPD severities.
Table 2.
Associations between mean levels of EBC biomarkers related to general characteristics
| N | H2O2 (μM) |
MDA (nM) |
3-NT (nM) |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD)∗ | p | Mean (SD)† | p | Mean (SD)∗ | p | |||
| Age (y)‡ | <59 | 30 | 0.37 (1.97) | 0.654 | 4.97 (3.29) | 0.654 | 3.76 (2.28) | 0.697 |
| 60–69 | 44 | 0.38 (2.29) | 5.56 (2.70) | 4.62 (2.76) | ||||
| >70 | 16 | 0.45 (1.82) | 5.72 (3.83) | 4.11 (2.64) | ||||
| BMI (kg/m2)§ | <25 | 62 | 0.40 (2.26) | 0.340 | 5.59 (3.01) | 0.358 | 4.66 (2.71) | 0.173 |
| ≥25 | 28 | 0.34 (1.70) | 4.95 (3.30) | 3.40 (2.84) | ||||
| Exposure period (y)‡ | >9 | 15 | 0.37 (1.79) | 0.320 | 4.10 (2.36) | 0.017 | 4.88 (2.93) | 0.797 |
| 10–19 | 47 | 0.43 (2.16) | 6.26 (3.32) | 3.99 (2.72) | ||||
| >20 | 28 | 0.33 (2.12) | 4.63 (2.68) | 4.31 (2.83) | ||||
| Smoking‡ | Never | 10 | 0.39 (1.95) | 0.927 | 6.06 (2.16) | 0.273 | 4.57 (3.16) | 0.617 |
| Past | 47 | 0.37 (1.74) | 4.89 (2.74) | 4.58 (2.69) | ||||
| Current | 33 | 0.40 (2.65) | 5.91 (3.72) | 3.67 (2.80) | ||||
| Cumulative smoking | 0 | 10 | 0.39 (1.95) | 0.980 | 6.06 (2.16) | 0.339 | 4.57 (3.16) | 0.820 |
| (pack-y)‡ | 1–9 | 18 | 0.38 (1.69) | 5.58 (3.84) | 3.47 (2.99) | |||
| 10–19 | 23 | 0.40 (1.98) | 6.07 (3.36) | 4.19 (3.24) | ||||
| >20 | 39 | 0.37 (2.41) | 4.73 (2.73) | 4.55 (2.36) | ||||
| COPD§ | No | 53 | 0.38 (1.96) | 0.842 | 4.64 (2.66) | 0.005 | 3.51 (2.91) | 0.039 |
| Yes | 37 | 0.39 (2.30) | 6.46 (3.40) | 5.50 (2.41) | ||||
| Pneumoconiosis | No | 37 | 0.38 (1.75) | 0.828 | 5.35 (2.98) | 0.912 | 4.15 (2.81) | 0.893 |
| Yes | 53 | 0.39 (2.33) | 5.42 (3.21) | 4.28 (2.75) | ||||
3-NT, 3-nitrotyrosine; BMI, body mass index; COPD, chronic obstructive pulmonary disease; EBC, exhaled breath condensate; MDA, malondialdehyde; SD, standard deviation.
Geometric mean (geometric standard deviation).
Arithmetic mean (standard deviation).
Calculated by analysis of variance test.
Calculated by t test.
Table 3.
Mean concentration of biomarkers in exhaled breath condensate related to COPD
| Non-COPD (n = 53) | COPD (n = 37) |
p |
||||
|---|---|---|---|---|---|---|
| Univariate∗ | Multivariate† | |||||
| H2O2 (μM)‡ | 0.38 | (1.96) | 0.39 | (2.30) | 0.842 | 0.425 |
| MDA (nM)§ | 4.64 | (2.66) | 6.46 | (3.40) | 0.005 | 0.002 |
| 3-NT (nM)‡ | 3.51 | (2.91) | 5.50 | (2.41) | 0.039 | 0.042 |
3-NT, 3-nitrotyrosine; COPD, chronic obstructive pulmonary disease; MDA, malondialdehyde.
Calculated by t test.
Calculated using a multiple regression model to adjust for age, body mass index, exposure period, smoking status (no/yes), cumulative smoking, pneumoconiosis (no/yes), and each biomarkers in exhaled breath condensate (log H2O2, MDA, log 3-NT).
Geometric mean (geometric standard deviation).
Arithmetic mean (standard deviation).
Table 4.
Median levels of biomarkers in exhaled breath condensate related to COPD severities∗
| GOLD 0 (n = 53) | GOLD I (n = 27) | GOLD II (n = 10) | p† | |
|---|---|---|---|---|
| H2O2 (μM) | 0.36 (44.2) | 0.42 (47.3) | 0.39 (47.7) | 0.844 |
| MDA (nM) | 4.70 (39.2) | 5.93 (52.6) | 7.20 (59.9) | 0.017 |
| 3-NT (nM) | 3.31 (41.1) | 6.81 (50.5) | 7.77 (55.1) | 0.146 |
3-NT, 3-nitrotyrosine; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; MDA, malondialdehyde.
COPD severity was categorized based on global initiative for chronic obstructive lung disease (GOLD) classification as follows: GOLD 0: FEV1/FVC ratio ≥ 70% and FEV1 ≥ 80% predicted. GOLD I (mild): FEV1/FVC ratio < 70% and FEV1 ≥ 80% predicted. GOLD II (moderate): FEV1/FVC ratio < 70% and 50% ≤ FEV1 < 80% predicted.
Calculated by Kruskal–Wallis H test, median (mean rank).
The result of ROC curve of MDA and 3-NT in EBC for the diagnostic discrimination of COPD is shown in Fig. 2. The area under the ROC curve for EBC MDA and 3-NT were 0.67 [95% confidence interval (CI): 0.56–0.79, p = 0.004] and 0.62 (95% CI: 0.50–0.74, p = 0.058), respectively. The optimal cutoff values were 5.34 nM (64.9% sensitivity and 64.2% specificity) and 5.58 nM (62.2% sensitivity and 62.3% specificity), respectively.
Fig. 2.
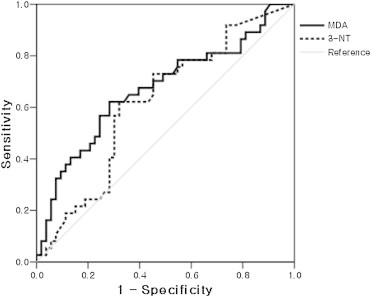
Receiver operating characteristics (ROC) curve of malondialdehyde (MDA) and 3-nitrotyrosine (3-NT) in exhaled breath condensate (EBC) for the diagnostic discrimination of chronic obstructive pulmonary disease. Area under the curve of the ROC curve of EBC MDA and 3-NT were 0.67 [95% confidence interval (CI): 0.56–0.79; p = 0.004] and 0.62 (95% CI: 0.50–0.74, p = 0.058), respectively. The optimal cutoff values were 5.34 nM (64.9% sensitivity and 64.2% specificity) and 5.58 nM (62.2% sensitivity and 62.3% specificity), respectively.
4. Discussion
The toxicity of crystalline silica and coal dust is mediated by the activation of macrophages and induction of lung inflammation. Many researchers expressed concern over the induction of crucial mediators of pulmonary disorders by these mineral dusts [2]. Inhaled dusts or other transitional metals, including iron, copper, and vanadium, are known to induce the generation of reactive oxygen species (ROS) from activated phagocytes in the lung [21–23]. Pneumoconiosis is the most prevalent lung disease showing a decrease of pulmonary function. Exposure to coal mine dust can cause both pneumoconiosis and chronic airflow limitation, especially small airway dysfunction [2,24].
Similar to the use of biomarkers found in blood and urine, analysis of biomarkers in breath samples using noninvasive techniques such as EBC collection could be useful for detection of respiratory diseases as well as systemic diseases. EBC has been studied in a variety of diseases, including asthma, COPD, cystic fibrosis, allergic rhinitis, and lung cancer [6]. EBC can be collected by cooling or freezing exhaled air and thereby condensing vapor and aerosolized droplets emerging with the breath [25]. The composition of EBC is thought to closely reflect the composition of the alveolar lining fluid because of the substantial surface area from which the generation of aerosols may occur [26].
Oxidative stress is an important feature of COPD, and there is increasing evidence that it is involved in the pathophysiology of COPD. Oxidative stress may be induced by cigarette smoke that activates inflammatory cells, such as macrophages and neutrophils [4]. In previous studies, the levels of EBC H2O2, a type of ROS, were increased in patients with COPD, particularly during exacerbations [27,28]. However, in this study, there were no significant correlations between EBC H2O2 levels and COPD. This discrepancy might be due to the fact that previous reports compared the general population and patients with respiratory disease, whereas our study was conducted in retired elderly coal miners. van Beurden et al [29] reported that the concentrations of EBC H2O2 reduced after inhaled corticosteroid therapy in patients with COPD. Therefore, future studies should aim to ascertain the correlation between EBC H2O2 levels and COPD adjusted for age and pharmacological therapy.
There is considerable evidence that oxidative stress is increased in patients with COPD, and ROS contributes to the pathophysiology of this disease entity [28,30]. Oxidative stress leads to the direct oxidation of arachidonic acid [31]. MDA is generated by arachidonic and docosahexenoic acid resulting from lipid peroxidation [32]. Among the mechanisms of ROS damage, lipid peroxidation is probably the most extensively investigated process. Oxidation of polyunsaturated fatty acid results in the formation of unstable lipid hydroperoxides and secondary carbonyl compounds such as aldehydes. Among these, EBC MDA has been most frequently demonstrated as a biomarker of lipid peroxidation in patients with several respiratory diseases, such as COPD [32] and asthma [33]. In this study, EBC MDA concentration was higher in the COPD group, confirming similar observations in previous reports [28,34]. Our results show that the EBC MDA concentration in the COPD group was higher than that in the non-COPD group and tends to increase in relation to COPD severities. Applying ROC curve analyses, AUC for EBC MDA value (0.67; p = 0.004) for the identification of COPD indicated the reasonable potential of EBC MDA as biomarkers. The cutoff value of EBC MDA was 5.34 nM (sensitivity 64.9; specificity 64.2%). These results suggest that the level of EBC MDA might serve as a biomarker for oxidative stress in COPD.
In patients with COPD, NO is generated by an enzyme called inducible NO synthase that is expressed in macrophages and the lung parenchyma, particularly in patients with severe stage of COPD [4]. NO can react in vitro with the superoxide ion to produce peroxynitrite, a potent oxidant with a short half-life. Peroxynitrite is considered to be the major reactive form of NO in vivo, especially under conditions of inflammation in which the production of oxygen-free radicals is prominent [35]. Peroxynitrite reacts with free or protein-bound tyrosine residues producing 3-NT, a stable end product [14]. In this study, the mean EBC 3-NT level was significantly higher in participants with COPD (3.51 nM vs. 5.50 nM, p = 0.039), and this effect held true in the adjusted multiple regression model (p = 0.002 for all comparisons). The median level of EBC 3-NT tends to increase in relation to COPD severities, non-COPD (3.31 nM), mild COPD (6.81 nM), and moderate COPD (7.71 nM); however, the median level did not show any statistical significance related to COPD severities. Applying ROC curve analyses, AUC for the EBC 3-NT value for the identification of COPD was 0.62 (p = 0.058). Therefore, future studies should aim to ascertain the correlation between EBC 3-NT levels and COPD from a large number of study participants.
Toxicities of crystalline silica and coal dust are based on the activation of macrophages and lung inflammation. Many researchers have shown concern with regard to crucial mediators of the pulmonary disorder resulting from these mineral dusts [2]. Inhaled dusts or other transitional metals, including iron, copper, and vanadium have been known to induce ROS generated from activated phagocytes in the lung [22]. Exposure to coal mine dust may result in pneumoconiosis and COPD, including emphysema and chronic bronchitis that appears indistinguishable from obstructive lung disease. Coal mine and silica dust may therefore result in restrictive, obstructive, or mixed patterns of impairment in pulmonary function testing [36]. Increased ROS levels were reported in several biological conditions, including smoking [5], pneumoconiosis [2], asthma [33], and COPD. In this study, the mean EBC MDA level was significantly elevated in participants with COPD (p = 0.005) and this effect held true in the adjusted multiple regression model to adjust for age, BMI, exposure period, smoking status (no/yes), cumulative smoking, pneumoconiosis (no/yes), and each biomarker in EBC (p = 0.002). For excluding asthma, we considered data only from participants who showed an FEV1/FVC ratio < 70% and without FEV1 increasing higher than 200 mL and 12% after β2-bronchodilator inhalation.
The study has several limitations. One is that 90 participants (53 non-COPD and 37 COPD participants) were insufficient to draw conclusions about the effects on COPD. Another limitation is with regard to the lack of data on female participants. In this study, no significant correlations were observed between the incidence of pneumoconiosis and EBC H2O2, MDA, and 3-NT levels. The explanation for these results may be that the radiological findings for pneumoconiosis are inflammation or fibrosis in the lung, whereas the analyzed biomarkers result from the current oxidative stress response to inflammation or exposure to hazardous materials.
In summary, this study showed that the elevated levels of EBC MDA and EBC 3-NT of COPD patients are biomarkers of oxidative or nitrosative stress in retired elderly coal miners. Further longitudinal follow-up studies are needed to investigate the potential of using exhaled mediators as biomarkers of COPD exacerbation or progressive pneumoconiosis.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
The authors thank all the retired miners who participated in this study. This study was supported by the KCOMWEL research grant.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Vestbo J., Hurd S.S., Agustí A.G., Jones P.W., Vogelmeier C., Anzueto A., Barnes P.J., Fabbri L.M., Martinez F.J., Nishimura M., Stockley R.A., Sin D.D., Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Schins R.P., Borm P.J. Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg. 1999;43:7–33. doi: 10.1016/s0003-4878(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 3.Meijers J.M., Swaen G.M., Slangen J.J. Mortality of Dutch coal miners in relation to pneumoconiosis, chronic obstructive pulmonary disease, and lung function. Occup Environ Med. 1997;54:708–713. doi: 10.1136/oem.54.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes P.J. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 5.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:258–266. doi: 10.1513/pats.200504-045SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grob N.M., Aytekin M., Dweik R.A. Biomarkers in exhaled breath condensate: a review of collection, processing and analysis. J Breath Res. 2008;2:037004. doi: 10.1088/1752-7155/2/3/037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montuschi P. Analysis of exhaled breath condensate in respiratory medicine: methodological aspects and potential clinical applications. Ther Adv Respir Dis. 2007;1:5–23. doi: 10.1177/1753465807082373. [DOI] [PubMed] [Google Scholar]
- 8.Montuschi P. Exhaled breath condensate analysis in patients with COPD. Clin Chim Acta. 2005;356:22–34. doi: 10.1016/j.cccn.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Kharitonov S.A., Barnes P.J. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers. 2002;7:1–32. doi: 10.1080/13547500110104233. [DOI] [PubMed] [Google Scholar]
- 10.Corradi M., Pignatti P., Manini P., Andreoli R., Goldoni M., Poppa M., Moscato G., Balbi B., Mutti A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur Respir J. 2004;24:1011–1017. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gelb A.F., Barnes P.J., George S.C., Ricciardolo F.L., DiMaria G., Zamel N. Review of exhaled nitric oxide in chronic obstructive pulmonary disease. J Breath Res. 2012;6:047101. doi: 10.1088/1752-7155/6/4/047101. [DOI] [PubMed] [Google Scholar]
- 12.Brindicci C., Ito K., Resta O., Pride N.B., Barnes P.J., Kharitonov S.A. Exhaled nitric oxide from lung periphery is increased in COPD. Eur Respir J. 2005;26:52–59. doi: 10.1183/09031936.04.00125304. [DOI] [PubMed] [Google Scholar]
- 13.Maziak W., Loukides S., Culpitt S., Sullivan P., Kharitonov S.A., Barnes P.J. Exhaled nitric oxide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:998–1002. doi: 10.1164/ajrccm.157.3.97-05009. [DOI] [PubMed] [Google Scholar]
- 14.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 15.Kharitonov S.A., Barnes P.J. Nitric oxide, nitrotyrosine, and nitric oxide modulators in asthma and chronic obstructive pulmonary disease. Curr Allergy Asthma Rep. 2003;3:121–129. doi: 10.1007/s11882-003-0024-7. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., Crapo R., Enright P., van der Grinten C.P., Gustafsson P., Jensen R., Johnson D.C., MacIntyre N., McKay R., Navajas D., Pedersen O.F., Pellegrino R., Viegi G., Wanger J. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.International Labour Office (ILO) International Labor Organization; Geneva (Switzerland): 2002. Guidelines for the use of the ILO international classification of radiographs of pneumoconiosis. Revised ed. [Google Scholar]
- 18.Horváth I., Hunt J., Barnes P.J., Alving K., Antczak A., Baraldi E., Becher G., van Beurden W.J., Corradi M., Dekhuijzen R., Dweik R.A., Dwyer T., Effros R., Erzurum S., Gaston B., Gessner C., Greening A., Ho L.P., Hohlfeld J., Jöbsis Q., Laskowski D., Loukides S., Marlin D., Montuschi P., Olin A.C., Redington A.E., Reinhold P., van Rensen E.L., Rubinstein I., Silkoff P., Toren K., Vass G., Vogelberg C., Wirtz H. ATS/ERS Task Force on Exhaled Breath Condensate. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 19.Svensson S., Olin A.C., Lärstad M., Ljungkvist G., Torén K. Determination of hydrogen peroxide in exhaled breath condensate by flow injection analysis with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;809:199–203. doi: 10.1016/j.jchromb.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Andreoli R., Manini P., Corradi M., Mutti A., Niessen W.M. Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:637–645. doi: 10.1002/rcm.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker S., Soukup J.M., Gilmour M.I., Devlin R.B. Stimulation of human and rat alveolar macrophages by urban air particulates: effects on oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- 22.Castranova V., Vallyathan V., Ramsey D.M., McLaurin J.L., Pack D., Leonard S., Barger M.W., Ma J.Y., Dalal N.S., Teass A. Augmentation of pulmonary reactions to quartz inhalation by trace amounts of iron-containing particles. Environ Health Perspect. 1997;105:1319–1324. doi: 10.1289/ehp.97105s51319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalal N.S., Jafari B., Peterson M., Green F.H., Vallyathan V. Presence of stable coal radicals in autopsied coal miners’z lungs and its possible correlation to coal workers' pneumoconiosis. Arch Environ Health. 1991;46:366–372. doi: 10.1080/00039896.1991.9934404. [DOI] [PubMed] [Google Scholar]
- 24.Stansbury R.C., Beeckman-Wagner L.A., Wang M.L., Hogg J.P., Petsonk E.L. Rapid decline in lung function in coal miners: evidence of disease in small airways. Am J Ind Med. 2013;56:1107–1112. doi: 10.1002/ajim.22211. [DOI] [PubMed] [Google Scholar]
- 25.American Thoracic Society Workshop ATS Workshop Proceedings. Exhaled nitric oxide and nitric oxide oxidative metabolism in exhaled breath condensate: executive summary. Am J Respir Crit Care Med. 2006;173:811–813. doi: 10.1164/rccm.2601014. [DOI] [PubMed] [Google Scholar]
- 26.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110:28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 27.Inonu H., Doruk S., Sahin S., Erkorkmaz U., Celik D., Celikel S., Seyfikli Z. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respir Care. 2012;57:413–419. doi: 10.4187/respcare.01302. [DOI] [PubMed] [Google Scholar]
- 28.Dekhuijzen P.N., Aben K.K., Dekker I., Aarts L.P., Wielders P.L., van Herwaarden C.L., Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 29.van Beurden W.J., Harff G.A., Dekhuijzen P.N., van der Poel-Smet S.M., Smeenk F.W. Effects of inhaled corticosteroids with different lung deposition on exhaled hydrogen peroxide in stable COPD patients. Respiration. 2003;70:242–248. doi: 10.1159/000072004. [DOI] [PubMed] [Google Scholar]
- 30.Repine J.E., Bast A., Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med. 1997;156:341–357. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 31.Gutteridge J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 32.Corradi M., Rubinstein I., Andreoli R., Manini P., Caglieri A., Poli D., Alinovi R., Mutti A. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1380–1386. doi: 10.1164/rccm.200210-1253OC. [DOI] [PubMed] [Google Scholar]
- 33.Celik M., Tuncer A., Soyer O.U., Saçkesen C., Tanju Besler H., Kalayci O. Oxidative stress in the airways of children with asthma and allergic rhinitis. Pediatr Allergy Immunol. 2012;23:556–561. doi: 10.1111/j.1399-3038.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- 34.Bartoli M.L., Novelli F., Costa F., Malagrinò L., Melosini L., Bacci E., Cianchetti S., Dente F.L., Di Franco A., Vagaggini B., Paggiaro P.L. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators Inflamm. 2011;2011:891752. doi: 10.1155/2011/891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu R.H., Hotchkiss J.H. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutat Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 36.Cohen R.A., Patel A., Green F.H. Lung disease caused by exposure to coal mine and silica dust. Semin Respir Crit Care Med. 2008;29:651–661. doi: 10.1055/s-0028-1101275. [DOI] [PubMed] [Google Scholar]


