Abstract
Objectives
Brucellosis remains one of the most common zoonotic diseases worldwide. In humans, brucellosis can be a serious, debilitating, and sometimes chronic disease. Different mechanisms can be postulated as to the basis for the induction of the chronic status of infectious diseases that T regulatory cells are one of the most important related mechanisms. The current study was designed to determine whether percentage of CD4+Treg cells and their CD25high and FoxP3high subpopulations in peripheral blood are changed in human brucellosis samples in comparison to a control group.
Methods
In total, 68 brucellosis patients (acute form: n = 43, chronic form: n = 25) and 36 healthy volunteers entered our study. After isolating of peripheral blood mononuclear cells, heparinized venous blood samples were obtained from both patients and healthy donors, CD4, CD25, and FoxP3 molecules were evaluated by two- and three-color flow cytometric methods.
Results
The results revealed a new finding in relation to Treg cells and human brucellosis. The numbers of CD4+Treg cells and their CD25high and FoxP3high subsets increase significantly in the peripheral blood of acute and chronic forms of brucellosis samples compared with healthy groups, with this increase being greater in the chronic group.
Conclusion
There seems to be a correlation between increase of CD4+Treg cells and their subsets and the disease progress from healthy state to acute and chronic brucellosis.
Keywords: brucellosis, CD4+ T regulatory cells, CD25high subsets, FoxP3high subsets
1. Introduction
Brucellosis is an intracellular bacterial infection with worldwide distribution with > 500,000 human cases reported annually [1,2]. The disease is caused by organisms of the genus Brucella, especially by Brucella melitensis, and is known as the most common zoonotic disease [3]. In humans, the disease culminates in high clinical morbidity and diverse clinical manifestations, as any organ can be affected [4]. Despite early diagnosis and treatment, approximately 10–30% of patients develop chronic disease [5,6].
Host protection against intracellular pathogens such as Brucella spp. depends on cell-mediated immunity (CMI) [7,8]. Brucella can survive within macrophages of the host and invades the normal mechanisms of bacterial killing, causing chronic disease [6,7]. Th1 related cytokines [interleukin (IL)-12 and interferon-γ], which are fundamental for the clearance of brucellosis agent, and percentages of ex vivo and phytohemagglutinin (PHA)-cultured CD4+ T lymphocytes are found to be diminished in chronic patients [9–11].
In recent years, regulatory T cells (Tregs) have become a popular subject for immunological research. Numerous reports shed light on the major aspects of Treg biology in humans, with the characterization of different T-cell subpopulations, including naturally occurring CD4+ CD25+Tregs, induced Tregs [IL-10 producing CD4+ type I regulatory T cells (Tr1) and T helper type 3 (Th3) cells], and CD4+ CD25+ T cells that develop in the periphery by conversion of CD4+ CD25– T cells. All these different T-cell populations with regulatory function coexist and contribute to immune suppression [12–14].
There is now considerable evidence that CD4+ CD25+ Tregs represent a stable population of peripheral lymphocytes. These CD4+ CD25+ Tregs represent about 5–10% of human CD4+ T cells, and are commonly identified by the constitutive expression of IL-2 receptor α (IL-2Ra; CD25), as well as the transcription factor scurfin, encoded by the forkhead family transcription factor 3 (Foxp3) gene [15], and are also characterized by their very low levels of proliferation on T-cell receptor (TCR) stimulation in vitro. The CD4+ CD25high subset (which has high levels of CD25 expression) in normal individuals comprises about 1–3% of human circulating CD4+ T cells. Unlike the total population of CD4+ CD25+ T cells, these CD4+ CD25high cells constitutively expressing FoxP3 molecules at high levels can significantly inhibit the proliferation and cytokine secretion induced by TCR cross-linking of CD4+ CD25− responder T cells, CD8+ T cells, dendritic cells, natural killer cells, and B cells [16–20]. However, FoxP3 is emerged transiently in CD25low CD4+T lymphocytes, which may be without suppressive activities; but they contain effector functions [21].
The role of CD4+CD25hiT and CD4+FoxP3hiT cells in immunopathology of Brucella infection has not yet been elucidated [22]. So, in our study these cells were evaluated to determine two substantial questions: is the frequency of CD4+CD25highT, CD4+FoxP3highT, CD4+CD25highFoxP3+, and CD4+CD25+FoxP3high cells increased in the peripheral blood (PB) of patients with acute or chronic brucellosis as a direct result and, if so, can these Treg cells impinge on antibacterial immune responses as an inductive result, like other infectious diseases.
2. Materials and methods
2.1. Participants
There were 104 unrelated participants enrolled in the study: 68 brucellosis patients; and 36 healthy age- and gender-matched volunteers who were used as controls. According to disease history, clinical picture, and laboratory findings, patients were divided into acute (AB) and chronic (CB) brucellosis groups (Table 1).
Table 1.
Demographic data of the groups (acute, chronic, and control) studied
| AB | CB | Healthy control | |
|---|---|---|---|
| N | 43 | 25 | 36 |
| Female | 14 | 8 | 13 |
| Male | 29 | 17 | 23 |
| Age (mean ± standard deviation, y) | 43.5 ± 19.2 | 48.7 ± 14.3 | 42.6 ± 15.2 |
AB = acute brucellosis; CB = chronic brucellosis.
The AB group included 43 consecutive patients. All AB patients had a disease duration ≤8 weeks (mean ± standard deviation, 3.5 ± 2.3 weeks). The diagnosis was based on compatible clinical picture (Table 2) in combination to high serum titers of anti-brucellar antibodies or fourfold increase of the initial titers in two paired samples drawn 2 weeks apart. In addition, in 19 out of 43 AB patients, Brucella melitensis was isolated in blood culture. In 33 patients brucellar DNA was detected by PCR analysis in the blood and the serum.
Table 2.
Clinical characteristic of the patient groups studied
| AB (n = 43) | CB (n = 25) | |
|---|---|---|
| Symptoms | ||
| Fever | 43 | 6 |
| Sweating | 27 | 3 |
| Chills | 30 | 5 |
| Malaise/fatigue | 24 | 17 |
| Arthalgias | 19 | 10 |
| Lumbar pain | 16 | 6 |
| Headache | 13 | |
| Myalgias | 11 | 10 |
| Focal disease | ||
| Spondylitis | 5 | 3 |
| Sacroiliitis | 3 | |
| Epididymoorchitis | 3 | |
| Meningoencephalitis | 6 | |
AB = acute brucellosis; CB = chronic brucellosis.
The CB group was composed of 25 patients. All CB patients had disease duration ≥6 months [23]. Thirteen of 22 CB patients had also positive PCR analysis in the blood.
High specific titers were defined by anti-brucellar antibodies, which were considered for the Wright agglutination test ≥1:320, for the Coombs' agglutination test ≥1:320, and for the complement fixation test ≥32. Healthy volunteers were tested serologically for brucellosis and found to be negative.
2.2. Flow cytometry
Surface CD4 and CD25, and intracellular FoxP3 expression were assessed by flow cytometry using the eBioscience human regulatory T cell staining kit Number 3 (eBioscience, San Diego, CA, USA). In brief, PB mononuclear cells (PBMCs) were obtained by Ficoll–Hypaque density gradient centrifugation. Cells were incubated with FITC anti-human CD4 (RPA-T4) and PE anti-human CD25 (BC96) for 30 minutes at 4°C.Then, after twice washing and fixing with fixation/permeabilization solutions, the cells were stained with PE-Cy5 anti-human FoxP3 (PH101), and incubated similarly. Finally, two- and three-color fluorescence flow cytometric analysis by a BD FACS Calibur system were used to demonstrate the frequency of CD4+CD25+, CD4+ FoxP3+ and CD4+CD25+FoxP3+ cells in PB samples.
2.3. Statistical analysis
Parametric statistical tests were applied as the variables were distributed normally (Kolmogorov–Smirnov test). Data were analyzed by one-way analysis of variance using SPSS version 21 (SPSS Inc., Chicago, IL, USA) and were represented as mean ± SD. A p value <0·05 was considered to be statistically significant.
2.4. Ethics statement
Our study was confirmed by the ethical committee of Tehran University of Medical Sciences, Tehran, Iran. The participants were informed about the study and they chose whether or not to participate in this study.
3. Results
3.1. CD4+Treg cells and their subsets
The percentages of Treg cells were calculated as the frequencies of CD4+CD25+, CD4+CD25high, CD4+FoxP3+, CD4+FoxP3high, CD4+CD25+FoxP3+, CD4+CD25highFoxP3+, and CD4+CD25+FoxP3high cells in the CD4+ population of PBMCs, and their frequencies were 6.7%, 2.0%, 1.5%, 0.4%, 1.4%, 0.41%, and 0.4% of CD4+ cells, respectively, in PBMCs of healthy age-matched controls (Table 3, Figures 1 and 2). FoxP3+ cells, similar to CD25+cells, were classified into two subsets (FoxP3high and FoxP3low) based on the intensity of FoxP3 expression (Figure 3).
Table 3.
Frequencies of Treg cell subsets determined by surface markers (CD4 and CD25) and intracellular FoxP3 levels (p < 0.01)
| Control | Acute | Chronic | |
|---|---|---|---|
| Expression of surface markers (%) | |||
| CD4+CD25+ cells | 6.7 ± 1.8 | 16.6 ± 4.2 | 27.6 ± 6.4 |
| CD4+CD25low cells | 4.7 ± 1.3 | 12.6 ± 3.4 | 21.2 ± 4.6 |
| CD4+CD25high cells | 2.0 ± 0.5 | 3.9 ± 1.1 | 6.8 ± 2.1 |
| Expression of intracellular Foxp3 molecule (%) | |||
| CD4+Foxp3+cells | 1.5 ± 0.6 | 5.7 ± 1.8 | 10.6 ± 2.5 |
| CD4+Foxp3low cells | 1.2 ± 0.5 | 4.0 ± 1.2 | 7.7 ± 1.5 |
| CD4+Foxp3high cells | 0.4 ± 0.2 | 1.6 ± 0.4 | 2.9 ± 0.60 |
| CD4+CD25+FoxP3+cells | 1.4 ± 0.48 | 5.06 ± 1.37 | 9.5 ± 2.06 |
| CD4+CD25lowFoxP3+cells | 1.11 ± 0.5 | 3.76 ± 0.94 | 7.09 ± 1.25 |
| CD4+CD25highFoxP3+cells | 0.41 ± 0.18 | 1.28 ± 0.47 | 2.45 ± 0.57 |
| CD4+CD25+FoxP3lowcells | 1.18 ± 0.48 | 3.57 ± 1.11 | 5.28 ± 1.32 |
| CD4+CD25+FoxP3highcells | 0.4 ± 0.2 | 1.41 ± 0.38 | 2.03 ± 0.4 |
Data are presented as mean ± SD.
Figure 1.
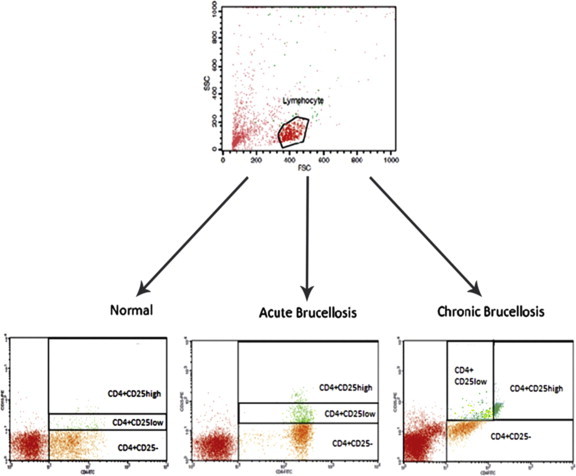
Two-color flow cytometric analysis of CD4+CD25+ cells and subdivision of these cells tool into CD4+CD25high Treg cells and CD4+CD25low cell subsets. First, lymphocytes gated in FSC (forward light scatter)–SCC (side light scatter) dot plot and then CD4+CD25- and CD4+CD25+ (high and low) gated in CD4-FITC intensity versus CD25-PE intensity.
Figure 2.
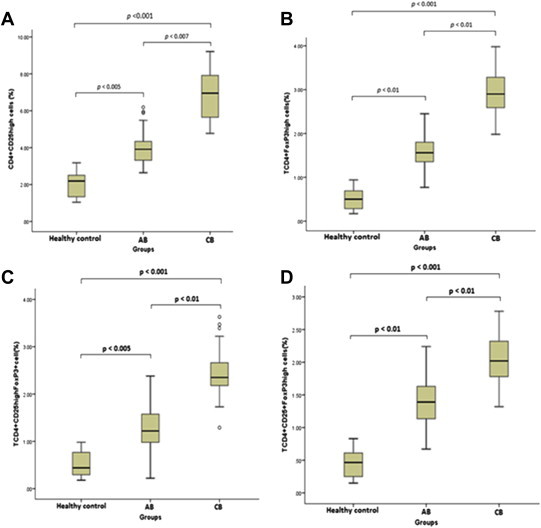
Percentages of (A) CD4+CD25high, (B) CD4+FoxP3high, (C) CD4+CD25highFoxP3+, and (D) CD4+CD25+FoxP3high. Treg cells within lymphocytes in the peripheral blood of healthy control (n = 36), AB (n = 43), and CB (n = 25) groups. Difference among groups was analyzed through the one-way analysis of variance test; and p values are shown on the figure; ° represents extreme values.
Figure 3.
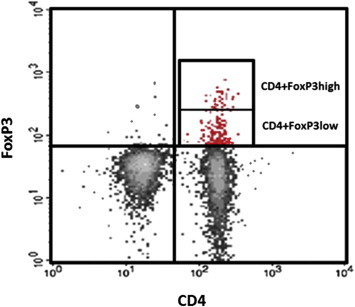
Classification of CD4+FoxP3+ cells based on levels of intracellular FoxP3 expression (FoxP3high and FoxP3low) via flow cytometric two-color method.
The proportions of CD4+CD25+ cells included 16.6% in AB and 27.6% in CB patients. Also, the CD25high subset of these cells contained 3.9% in AB and 6.8% in CB patients (Table 3, Figures 1 and 2).
CD4+FoxP3+ cells accounted for 5.7% of CD4+T-cells in AB and 10.6% of CD4+T cells in CB patients. In total CD4+lymphocytes, FoxP3high subpopulation consisted of 1.6% and 2.9% in AB and CB, respectively.
In patients with AB, CD4+CD25+FoxP3+cells comprised 5.06% of total CD4+ lymphocytes; in CB patients, percentage of these cells was 9.5% of total CD4+ lymphocytes (Table 3).
CD4+CD25highFoxP3+cells subtended 1.28% of CD4+ lymphocytes in AB and 2.45% in CB patients. Moreover, frequencies of CD4+CD25+FoxP3high cells encompassed 1.41% and 2.03% of CD4+ lymphocytes in attribution of AB and CB patients, respectively (Table 3, Figure 3).
3.2. Correlation between the level of CD25 expression in CD4+ lymphocytes and CD4+FoxP3+cells
Correlation coefficients of CD4+CD25high cells and CD4+FoxP3+ cells in the three groups consisted of R = 0.792, p < 0.001 (healthy control), R = 0.817, p < 0.001 (AB), and R = 0.661, p < 0.001 (CB) versus correlation between CD4+CD25low cells and CD4+FoxP3+ cells with coefficients including R = 0.680, p < 0.001 (healthy control), R = 0.586, p < 0.001 (AB), and R = 0.483, p = 0.014 (CB; Figure 4).
Figure 4.
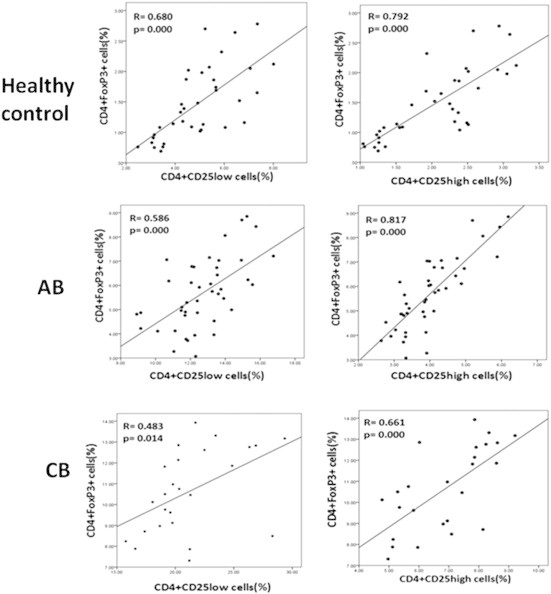
Correlation of CD4+FoxP3+ cells with CD4+CD25+ cells. CD4+CD25high cells exhibited more vigorous power of coefficient to CD4+FoxP3+ cells than to CD4+CD25low T cells in all three groups (control, acute brucellosis, chronic brucellosis). Each row was assigned to the one group, in which the left and the right diagrams depict the correlation among frequencies of CD4+FoxP3+ cells with CD4+CD25low or CD4+CD25high cells, respectively.
4. Discussion
Regulatory T cells (Treg cells) were originally identified through their function in controlling autoimmunity, where they mediate immunological tolerance to self-antigens [24]. However, it later became apparent that Treg cells also control immune responses to foreign antigens on pathogens [25]. The function of Treg cells during infection appears to be primarily to prevent collateral damage from unrestrained immune responses to the pathogen, with a number of studies demonstrating enhanced immunopathology with defective or depleted Treg cells. It also seems that the induction of Treg cells during infection is an immune subversion strategy engaged by certain pathogens in order to last their survival in the host [26].
In agreement with other studies [11,27], we found that the percentage expression of IL-2 receptor α on peripheral CD4+lymphocytes in the healthy group was <10% (data not shown). Also, in the current study, > 90% of FoxP3+ cells in all three groups were CD4+FoxP3+ (data not shown), which implies that the majority of FoxP3+Treg cells in healthy individuals, AB, and CB patients manifest surface CD4 molecules.
In our study, the percentage of peripheral CD4+CD25+(low or high) T-lymphocytes was increased in AB patients, as compared to controls, suggesting that helper T-cells are activated in AB. This finding adds further support to data presented by other researchers [11,28,29] and it approves that CD25 is upregulated in AB patients. CB patients showed significantly increased percentage of CD25 expression on peripheral CD4+CD25high lymphocytes in comparison to AB patients. This seems to be in contrast with a previous study by Skendros et al [11], which showed diminished percentage in CD25 expression on peripheral CD4+T cells in compared with AB. But, they use PHA in the culture of obtained PBMC for activating and proliferating of lymphocytes and for this the acute brucellosis group has the higher level of CD4+CD25low cells as CD4+T-helper cells, were activated and proliferated more than other groups. By contrast, the increase of CD4+CD25high cells in PB of CB patients and induced suppression of T cells by this Treg cells, appears to be a substantial reason for the significant reduction in the percentage of PHA-proliferating TCD4+CD25+ cells and anergy induction in CB [30].
In addition, in our research, there were significant increases in the proportions of CD4+FoxP3+cells and their FoxP3high subpopulation, as well as CD4+CD25+FoxP3+ cells and their CD25high or FoxP3high subsets in CB in comparison to AB patients and healthy controls. Also, CD4+CD25high Treg cells in the AB group showed a significant increase in frequencies compared with the control group.
According to Curiel et al [31], FoxP3 expression in low levels can cause differentiation of Treg cells to Th2 or Th17 cells. So, the intensity of FoxP3 expression is crucial in the state of Treg suppressive activity. In fact, the presence of FoxP3 molecule maybe not only function as an on-and-off switch to offer suppressive activity of Treg cells but also control the regulatory capacity of these cells owing to the decreased function of Treg cells due to attenuated FoxP3 expression [32]. Consequently, our report about an increase of FoxP3+CD4+Treg subsets in AB and CB patients versus control volunteers indicates a significant rising trend in suppressive activities of the immune response in parallel with an ongoing infection such as brucellosis.
By contrast, we found the relationship between CD4+CD25high and CD4+FoxP3+ cells displayed a stronger power of correlation coefficient than CD4+CD25low and CD4+FoxP3+ cells in all three groups. This positive correlation verifies the direct relationship between the high-level intensity of CD25 (CD25high) and FoxP3 expression. Also, the AB group (R = 0.817) showed a better correlation of CD4+CD25high and CD4+FoxP3+ cells compared with the two other groups [R(healthy group) = 0.792; R(CB) = 0.661], which may be due to the larger sample size of the AB group (Figure 4).
In conclusion, our results demonstrate that there is a marked increase in circulating CD4+ CD25+, CD4+FoxP3+, and CD4+CD25+FoxP3+ Tregs and their CD25high or FoxP3high subpopulations in AB and CB patients. Tregs play a negative role not only in modulating the effectors of immune responses by inhibiting interferon-γ secretion and cellular proliferation upon Brucella antigen stimulation, but also in influencing the disease persistence in CB. These findings suggest that modulation of CD4+ CD25+ Tregs might be one potential therapeutic strategy for the treatment of the chronic form of this infection. However, this study was limited by analysis of the PB compartment only, and further detailed investigation of the level and function of CD4+ CD25+ and CD4+FoxP3+Tregs in brucellosis needs to be carried out.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
This study was financed by the vice chancellor for research, Tehran University of Medical Sciences 90-62-30-13976. We would like to thank all participants who took part in our study and helped us.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Pappas G., Akritidis N., Bosilkovski M. Brucellosis. N Engl J Med. 2005 Jun 2;352(22):2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Pappas G., Papadimitriou P., Akritidis N. The new global map of human brucellosis. Lancet Infect Dis. 2006 Feb;6(2):91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 3.Seleem M.N., Boyle S.M., Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010 Jan 27;140(3):392–398. doi: 10.1016/j.vetmic.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Skendros P., Boura P., Kamaria F. CD80/CD28 co-stimulation in human brucellosis. Clin Exp Immunol. 2006;146(3):400–408. doi: 10.1111/j.1365-2249.2006.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memish Z.A., Balkhy H.H. Brucellosis and international travel. J Travel Med. 2004 Jan–Feb;11(1):49–55. doi: 10.2310/7060.2004.13551. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez de Bagues M.P., Dudal S., Dornand J. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clin Immunol. 2005 Mar;114(3):227–238. doi: 10.1016/j.clim.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Golding B., Scott D.E., Scharf O. Immunity and protection against Brucella abortus. Microbes Infect. 2001 Jan;3(1):43–48. doi: 10.1016/s1286-4579(00)01350-2. [DOI] [PubMed] [Google Scholar]
- 8.Yingst S., Hoover D. T cell immunity to brucellosis. Crit Rev Microbiol. 2003;29(4):313–331. doi: 10.1080/713608012. [DOI] [PubMed] [Google Scholar]
- 9.Giambartolomei G.H., Delpino M.V., Cahanovich M.E. Diminished production of T helper 1 cytokines correlates with T cell unresponsiveness to Brucella cytoplasmic proteins in chronic human brucellosis. J Infect Dis. 2002 Jul 15;186(2):252–259. doi: 10.1086/341449. [DOI] [PubMed] [Google Scholar]
- 10.Rafiei A., Ardestani S.K., Kariminia A. Dominant Th1 cytokine production in early onset of human brucellosis followed by switching towards Th2 along prolongation of disease. J Infect. 2006 Nov;53(5):315–324. doi: 10.1016/j.jinf.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Skendros P., Boura P., Chrisagis D. Diminished percentage of CD4+ T-lymphocytes expressing interleukine-2 receptor alpha in chronic brucellosis. J Infect. 2007 Feb;54(2):192–197. doi: 10.1016/j.jinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Mills K.H., McGuirk P., editors. Antigen-specific regulatory T cells—their induction and role in infection. Seminars in immunology. Elsevier; Philadelphia: 2004. [DOI] [PubMed] [Google Scholar]
- 13.Vigouroux S., Yvon E., Biagi E. Antigen-induced regulatory T cells. Blood. 2004 Jul 1;104(1):26–33. doi: 10.1182/blood-2004-01-0182. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005 Apr;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 15.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003 Feb 14;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 16.Bacchetta R., Gambineri E., Roncarolo M.G. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007 Aug;120(2):227–235. doi: 10.1016/j.jaci.2007.06.023. quiz 236–7. [DOI] [PubMed] [Google Scholar]
- 17.Lim H.W., Hillsamer P., Banham A.H. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005 Oct 1;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 18.Azuma T., Takahashi T., Kunisato A. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003 Aug 1;63(15):4516–4520. [PubMed] [Google Scholar]
- 19.Romagnani C., Della Chiesa M., Kohler S. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur J Immunol. 2005 Aug;35(8):2452–2458. doi: 10.1002/eji.200526069. [DOI] [PubMed] [Google Scholar]
- 20.Trzonkowski P., Szmit E., Myśliwska J. CD4+ CD25+T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004 Sep;112(3):258–267. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Miyara M., Yoshioka Y., Kitoh A. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009 Jun 19;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Sales P.A., Jr., Golgher D., Oliveira R.V. The regulatory CD4+ CD25+ T cells have a limited role on pathogenesis of infection with Trypanosoma cruzi. Microbes Infect. 2008;10(6):680–688. doi: 10.1016/j.micinf.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Irmak H., Buzgan T., Karahocagil M.K. The effect of levamisole combined with the classical treatment in chronic brucellosis. Tohoku J Exp Med. 2003 Dec;201(4):221–228. doi: 10.1620/tjem.201.221. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S., Sakaguchi N., Asano M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995 Aug;155(3):1151–1164. [PubMed] [Google Scholar]
- 25.McGuirk P., Mills K.H. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002 Sep;23(9):450–455. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- 26.Mills K.H. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4(11):841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 27.Bueno L.L., Morais C.G., Araujo F.F. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010 Mar 10;5(3):e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Refik M., Mehmet N., Durmaz R. Cytokine profile and nitric oxide levels in sera from patients with brucellosis. Braz J Med Biol Res. 2004 Nov;37(11):1659–1663. doi: 10.1590/s0100-879x2004001100010. [DOI] [PubMed] [Google Scholar]
- 29.Makis A.C., Galanakis E., Hatzimichael E.C. Serum levels of soluble interleukin-2 receptor alpha (sIL-2Rα) as a predictor of outcome in brucellosis. J Infect. 2005 Oct;51(3):206–210. doi: 10.1016/j.jinf.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Boura P., Tsapas G., Kountouras J. Interferon-A 2a administration in chronic anergic brucellosis. Hepatogastroenterology. 1994 Nov–Dec;42(6):919–922. [PubMed] [Google Scholar]
- 31.Curiel T.J. Regulatory T-cell development: is Foxp3 the decider? Nat Med. 2007 Mar;13(3):250–253. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 32.Wan Y.Y., Flavell R.A. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]


