Abstract
Background
Intraperitoneal adenosine reduces abdominal adhesions. However, because of the ultra-short half-life and low solubility of adenosine, optimal efficacy requires multiple dosing.
Aim
Here, we compared the ability of potential adenosine prodrugs to inhibit post-surgical abdominal adhesions after a single intraperitoneal dose.
Methods
Abdominal adhesions were induced in mice using an electric toothbrush to damage the cecum. Also, 20 μL of 95 % ethanol was applied to the cecum to cause chemically induced injury. After injury, mice received intraperitoneally either saline (n = 18) or near-solubility limit of adenosine (23 mmol/L; n = 12); 5′-adenosine monophosphate (75 mmol/L; n = 11); 3′-adenosine monophosphate (75 mmol/L; n = 12); 2′-adenosine monophosphate (75 mmol/L; n = 12); 3′,5′-cyclic adenosine monophosphate (75 mmol/L; n = 19); or 2′,3′-cyclic adenosine monophosphate (75 mmol/L; n = 20). After 2 weeks, adhesion formation was scored by an observer blinded to the treatments. In a second study, intraperitoneal adenosine levels were measured using tandem mass spectrometry for 3 h after instillation of 2′,3′-cyclic adenosine monophosphate (75 mmol/L) into the abdomen.
Results
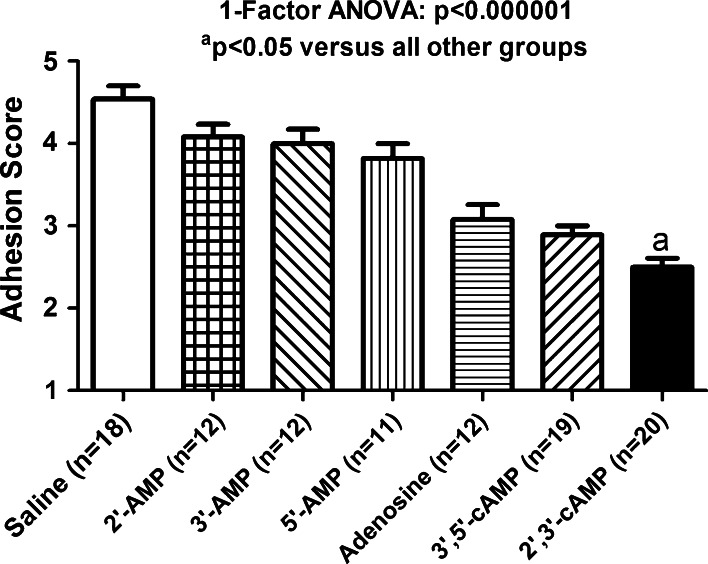
The order of efficacy for attenuating adhesion formation was: 2′,3′-cyclic adenosine monophosphate > 3′,5′-cyclic adenosine monophosphate ≈ adenosine > 5′-adenosine monophosphate ≈ 3′-adenosine monophosphate ≈ 2′-adenosine monophosphate. The groups were compared using a one-factor analysis of variance, and the overall p value for differences between groups was p < 0.000001. Intraperitoneal administration of 2′,3′-cAMP yielded pharmacologically relevant levels of adenosine in the abdominal cavity for >3 h.
Conclusion
Administration of 2′,3′-cyclic adenosine monophosphate into the surgical field is a unique, convenient and effective method of preventing post-surgical adhesions by acting as an adenosine prodrug.
Keywords: Post-surgical adhesions; Adenosine; Adenosine prodrug; 2′,3′-Cyclic adenosine monophosphate
Introduction
Abdominal adhesions are internal fibrous bands that form between abdominal tissues and organs and represent an exaggerated healing response by the body secondary to surgically induced trauma following open and laparoscopic procedures. Post-surgical adhesions remain a common clinical problem occurring in 93 % of patients undergoing abdominal surgery, resulting in infertility, obstruction of the small bowel and chronic pelvic pain [1, 2]. Adhesions from previous surgeries also increase the risk, difficulty and complications of subsequent surgery. Therefore, adhesions account for a large financial burden resulting in an approximately $1.18 billion for inpatient care based on a survey of hospitalizations (National Hospital Discharge Survey) between 1998–2002 [1, 2].
Current therapies to prevent post-surgical abdominal adhesions are inadequate, and finding solutions remains elusive and is an important focus of health care research [1, 2]. The difficulty partially resides in understanding the exact pathogenesis of adhesion formation so that reversal of the mechanisms responsible does not impede normal healing processes or result in excessive bleeding. Although the etiology is complex, a unifying concept proposes that surgically induced trauma and microvascular ischemia evoke a robust inflammatory response leading to activation of the coagulation system with subsequent development of fibrin deposits [3–5]. Although there are numerous therapies, including solid and fluid/gel barriers, no FDA-approved device or pharmacologic agent appears to decrease the clinical consequences of adhesions [2, 6, 7]. Clearly, novel treatments for the prevention of post-surgical adhesions and tissue protection are needed, particularly those that can be easily and conveniently administered as a solution during minimally invasive surgery and are able to protect tissues both at the surgical site and throughout the abdominal cavity.
Adenosine is an endogenous nucleoside that is an agonist for four G-protein coupled receptors (A1, A2A, A2B, A3) that reduce inflammation [8–11], particular in the intestines [12, 13], and inhibit fibroblast migration, proliferation and collagen formulation [14–18]. Previous studies from our laboratory demonstrate that adenosine significantly inhibits post-surgical adhesion formation in rodents [19] and pigs (preliminary data) following severe peritoneal abrasion. However, with adenosine per se, there are a number of practical limitations that reduce its potential efficacy as an optimal anti-adhesion agent. These include its limited solubility (approximately 23 mmol/L) and short half-life (in rats adenosine resides in the peritoneal cavity for only a few minutes after instillation), which together necessitate frequent administration of adenosine to elicit a maximal therapeutic response [19]. Although high peritoneal adenosine concentrations of adenosine do not affect other organ systems [20], it is conceivable that accelerated absorption of adenosine may occur in the setting of an inflamed peritoneum.
We hypothesize that administration of a non-cell-membrane permeable adenosine prodrug would provide more sustained levels in the abdominal cavity and would avoid potential toxicity on other organs. In this regard, our recent studies show that 2′,3′-cyclic adenosine monophosphate (2′,3′-cAMP), a positional isomer of 3′,5′-cyclic adenosine monophosphate (3′,5′-cAMP) and formed from the metabolism of mRNA, is metabolized extracellularly to adenosine by organ systems [21–30]. Because 2′,3′-cAMP is even more hydrophilic than 3′,5′-cAMP [31], the absorption of 2′,3′-cAMP would be limited because of lack of diffusion across cell membranes. The conversion of 2′,3′-cAMP to adenosine involves first the metabolism of 2′,3′-cAMP to 2′-adenosine monophosphate (2′-AMP) and 3′-adenosine monophosphate (3′-AMP) by 2′,3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) and 2′,3′-cyclic nucleotide-2′-phosphodiesterase, respectively, followed by conversion of these AMPs to adenosine. Therefore, if metabolism of 2′,3′-cAMP is slow due to low levels of these enzymes in the peritoneal cavity, the anti-adhesive effects of adenosine would be enhanced because of the prolonged duration of 2′,3′-cAMP in the peritoneal cavity. Also, since 2′,3′-cAMP is highly hydrophilic, it is therefore highly water soluble, being stable in aqueous solutions of 75 mmol/L. Thus, high concentrations of 2′,3′-cAMP can be readily instilled into the peritoneal cavity which would also prolong the duration of action. Similarly 3′,5′-cAMP could also function as an adenosine prodrug since it is also water soluble and can be converted to adenosine [32–39].
The purpose of the present study was to improve upon our adenosine-based strategy for attenuating the formation of post-surgical adhesions. We therefore compared the efficacy of both cAMP compounds and their metabolites with a single dose of adenosine in a mouse model of abdominal adhesion formation (i.e., physical abrasion and chemical damage to the intestines). In this regard, we used the limit of solubility for these compounds because our main interest was whether maximal possible concentrations of the cAMPs and AMPs (75 mmol/L) were more efficacious that the maximum possible concentration of adenosine (25 mmol/L), i.e., our focus was not on potency, but rather efficacy (a clinically more important parameter). Our findings demonstrate that maximal concentrations of a one-time application of 2′,3′-cAMP are more efficacious than adenosine, 3′,5′-cAMP, 2′-AMP, 3′-AMP or 5′-AMP in reducing adhesion formation.
Materials and Methods
Animals
The experiments used 6-week-old C57BL/6 mice (Taconic Farms; Germantown, NY) fed Prolab RMH 3000 rodent diet (PMI Nutrition Inc.; St. Louis, MO) and allowed access to tap water. Light cycle, relative humidity and room temperature were 7:00 am to 7:00 pm, 55 % and 22 °C, respectively. The University of Pittsburgh Institutional Animal Care and Use Committee approved the study.
Drugs
All test drugs used in this study were obtained from Sigma-Aldrich (St. Louis, MO).
Surgery
Mice were fasted overnight and then anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg). The abdomen was shaved, and the incision site was cleaned with betadine solution. Surgery was performed under a laminar flow hood using instruments sterilized in a glass bead sterilizer. The abdomen was opened via a midline incision of approximately 1 cm. The cecum was exposed and placed on sterile gauze. A sterile pediatric electric toothbrush was used to damage both sides of the cecum (brushing for 1.5 min on each side). In addition, 20 μL of 95 % ethanol was applied to the cecum to cause further chemically induced tissue injury. After 30 s, ethanol was washed from the cecum with 1 mL of test solution. Next, the cecum was returned to the peritoneal cavity. Then 1 mL of test solution was placed in the peritoneal cavity, and the abdominal incision was sutured. Test solutions were applied by Mr. Gillespie who was blinded as to which test solution a given mouse received. Twenty mice were randomly assigned to the control (saline only) group and to each of the two cAMP groups; 12 mice were randomly assigned to each of the AMP groups. However, two mice in the control group, one mouse in the 5′-AMP group and one mouse in the 3′,5′-cAMP group died within a few days of surgery and were therefore excluded because no adhesion scoring was possible in these mice. Mice were treated with antibiotics [penicillin benzathine (20 mg/mL, 0.05 mL injected into the thigh) and enrofloxacin (1.15 mg/mL, 0.15 mL injected subcutaneously)], placed under a warming lamp and returned to their housing once they regained consciousness.
Scoring of Adhesions
Fourteen days later, each mouse was re-anesthetized with pentobarbital, and the abdomen was opened to provide a clear view of the intestines. The severity of adhesion formation was assessed on a scale from 1 to 5, taking into account the extent of adhesion formation. Adhesions were identified visually and by noting the difficulty of separating adjacent surfaces. Adhesion scores were assigned by Mr. Gillespie, who was blinded as to the test solutions. The scoring was based on the extent of adhesion of the cecum to itself and to adjacent tissues or organs. A score of 1 was assigned to animals when there was only minimal (approximately >10 to <20 %) adhesion of the cecum to itself (that is within the cecum core) with no adhesion of the cecum to other adjacent structures (abdominal wall, liver or intestines). A score of 2 was assigned when there was mild (approximately >20 to <30 %) adhesion of the cecum to itself or when there was minimal (approximately >10 to <20 %) adhesion of the cecum to itself but in addition minimal adhesion of the cecum to adjacent structures. A score of 3 was assigned to animals when the adhesion of the cecum to itself was moderate (approximately >30 to <50 %), in the absence or presence of minimal adhesion of the cecum to adjacent structures. A score of 4 was assigned to animals when the adhesion of the cecum to itself was severe (approximately >60 to <80 %) or when there was moderate adhesion to adjacent structures or both. A score of 5 was assigned to animals when adhesion of the cecum to itself was so extensive that it existed as one entity (complete adhesion within itself) or when there was severe adhesion of the cecum to itself but in addition severe adhesion of the cecum to adjacent structures.
Pharmacokinetic Study
Mice (n = 4) were anesthetized with an intraperitoneal injection of thiobutabarbital (100 mg/kg), and body temperature was monitored and maintained using a thermostatically regulated heating plate. The trachea was cannulated with PE-90, a PE-10 catheter was inserted into the jugular vein and an infusion of 2.45 % albumin in 0.9 % saline was initiated at 10 μL/min. With the mouse in the supine position, a suture was secured into the muscle of the mid-abdominal wall and utilized to suspend the abdominal wall in the shape of a tent. A hole was carefully made at the apex of the tent, and a PE-50 catheter was positioned deep in the peritoneal cavity. One milliliter a solution of 2′,3′-cAMP (75 mmol/L) was instilled into the peritoneal cavity. Next, utilizing a new PE-50 catheter for each sample, 0.1 mL of fluid was withdrawn at 1, 15, 30, 60, 120 and 180 min following instillation and immediately placed on ice. 2′,3′-cAMP, 2′-AMP, 3′-AMP, adenosine, inosine and hypoxanthine concentrations were measured with high-performance liquid chromatography–tandem mass spectrometry using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra; Thermo Fisher Scientific, Waltham, MA) as previously described [27].
Statistical Analysis
Adhesion scores passed all tests for normality and equality of variances. Accordingly, the data were analyzed with a one-way analysis of variance, followed by a protected Fisher’s least significant difference multiple-comparisons test if the overall analysis of variance was significant. Statistical analyses were conducted with the NCSS software (version 6.0; Kaysville, UT).
Results
Six groups were treated with test solutions containing chemical agents (purines) of interest. Each compound was administered into the peritoneal cavity at a concentration that was approximately the limit of solubility for that compound (23 mmol/L for adenosine and 75 mmol/L for all other purines.) In addition, there was a seventh group (control group) in which the test solution was only the vehicle (0.9 % saline) in which the chemical agents of interest were dissolved. The order of efficacy for attenuating adhesion formation was as follows (Fig. 1): 2′,3′-AMP (adhesion score of 2.5 ± 0.11) which was better than 3′,5′-cAMP (adhesion score of 2.89 ± 0.11) which was approximately the same as adenosine (adhesion score of 3.08 ± 0.18) which was better than 5′-AMP (adhesion score of 3.82 ± 0.18) which was approximately the same as 3′-AMP (adhesion score of 4.00 ± 0.17) which was approximately the same as 2′-AMP (adhesion score of 4.08 ± 0.15). The seven groups were compared using a one-factor analysis of variance with independent groups. The overall p value for differences between the groups was p < 0.000001. Post-hoc analysis with a protected Fisher’s least significant difference multiple-comparisons test was used to identify which groups were different. All treatment groups had adhesions scores that were significantly (p < 0.05) less than (better than) the saline control group (adhesion score of 4.54 ± 0.16). However, the adhesion scores for 5′-AMP, 3′-AMP and 2′-AMP were nominally similar, not significantly different from each other and only slightly less than (better than) that for the saline control group. This indicates that although these three adenosine monophosphates decreased adhesion formation, the effect was small. The adhesion scores for adenosine and 3′,5′-cAMP were nominally similar, not significantly different from each other, less than (better than) 5′-AMP (p < 0.05), 3′-AMP (p < 0.05) and 2′-AMP (p < 0.05), but more than (not as good as) 2′,3′-cAMP (p < 0.05). Finally, the analysis showed that 2′,3′-cAMP significantly (p < 0.05) improved adhesion scores better than all other treatments. Although not formally scored, it was also observed that the structures within the abdominal cavity in the group treated with 2′,3′-cAMP and 3′,5′-cAMP appeared less inflamed and generally healthier than the other groups.
Fig. 1.
Bar graph shows the adhesion scores of mice treated with a single intraperitoneal dose of the indicated treatment. Each compound was administered into the peritoneal cavity at a concentration that was approximately the limit of solubility for that compound (23 mmol/L for adenosine and 75 mmol/L for all other purines). Values are means and SEMs
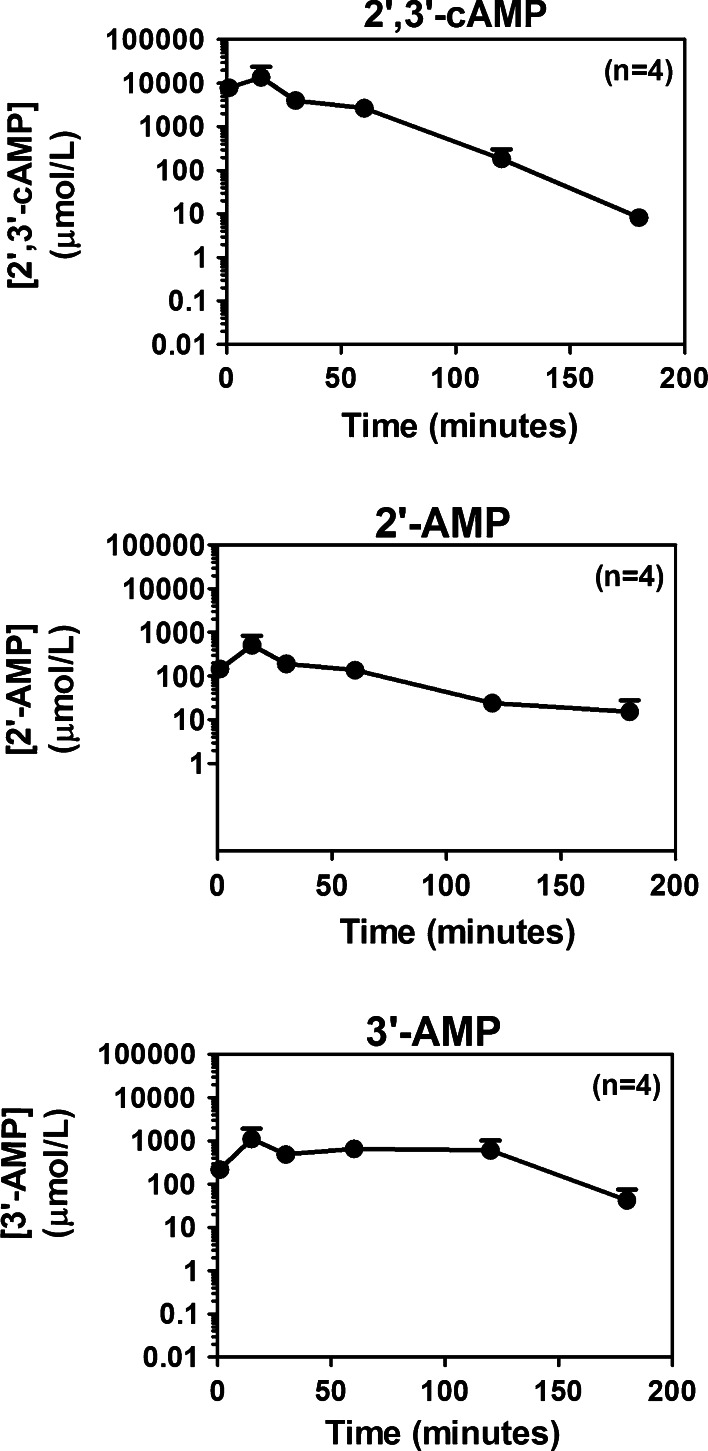
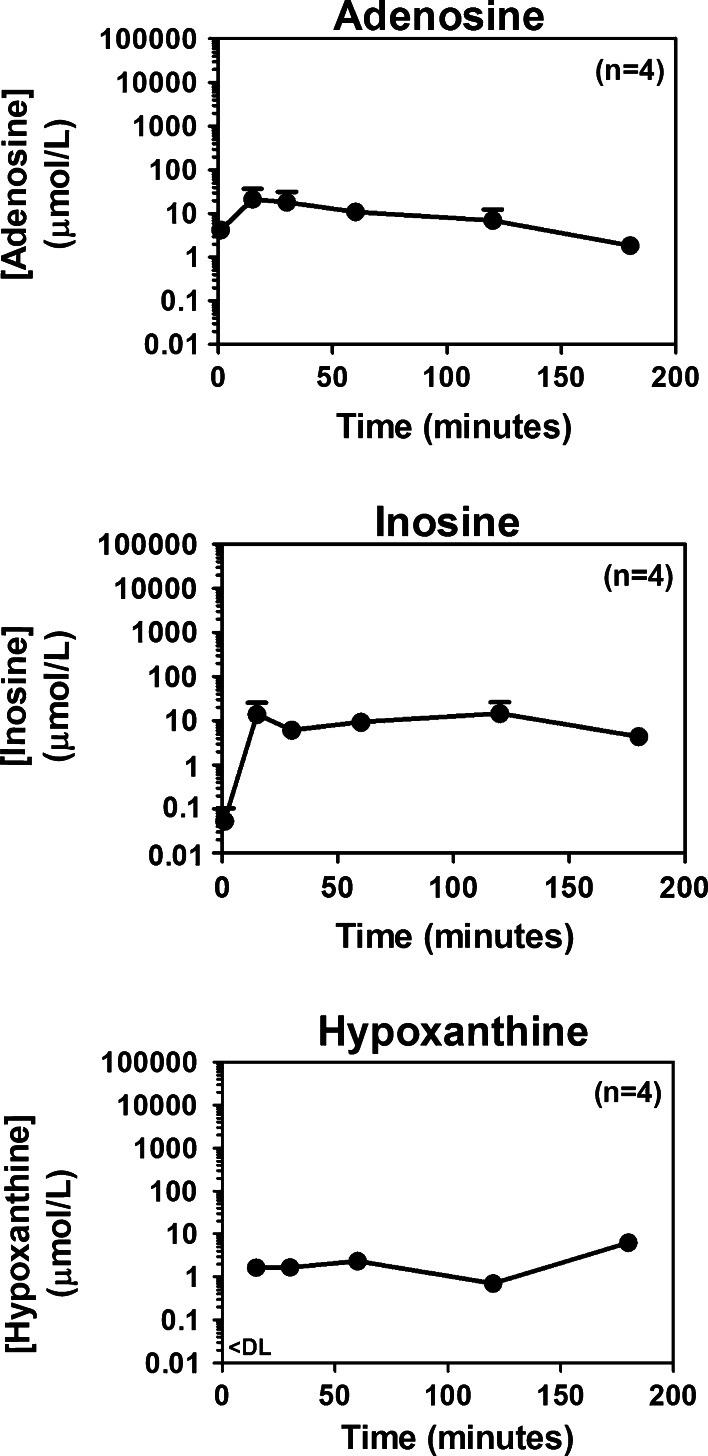
The metabolism of 2′,3′-cAMP in the peritoneal cavity is shown in Figs. 2 and 3. Although concentrations of 2′,3′-cAMP dropped rapidly after administration, because of the high starting concentrations, 3 h after the administration of 2′,3′-cAMP concentrations of 2′,3′-cAMP in the peritoneal cavity were still substantial (i.e., approximately 10 μmol/L; Fig. 2, top panel). Within a minute of application of 2′,3′-cAMP, levels of 2′-AMP (Fig. 2, middle panel) and 3′-AMP (Fig. 2, bottom panel) achieved concentrations of >100 μmol/L, which were relatively maintained for 2 h, and even at 3 h post administration of 2′,3′-cAMP, levels of 2′-AMP and 3′-AMP remained above 10 μmol/L. More importantly, within 1 min after administering 2′,3′-cAMP, adenosine levels in the peritoneal cavity exceeded 1 μmol/L (Fig. 3, top panel) and quickly rose to above 10 μmol/L, and even at 3 h, adenosine levels in the peritoneal cavity were pharmacological significant (>1 μmol/L, which is a concentration that activates adenosine receptors [40] ). One minute after administration of 2′,3′-cAMP, levels of inosine (Fig. 3, middle panel) were extremely low (<0.1 μmol/L), and levels of hypoxanthine (Fig. 3, bottom panel) were below detection limit. However, within 15 min, inosine levels increased to >10 μmol/L, and hypoxanthine concentrations increased to >1 μmol/L. Both inosine and hypoxanthine remained increased for >3 h.
Fig. 2.
Line graphs illustrate the relationship between time (1–180 min) after administration into the peritoneal cavity of 2′,3′-cAMP (1 mL of a 75 mmol/L solution) and concentrations of 2′,3′-cAMP, 2′-AMP and 3′-AMP. Values are means and SEMs
Fig. 3.
Line graphs illustrate the relationship between time (1–180 min) after administration into the peritoneal cavity of 2′,3′-cAMP (1 mL of a 75 mmol/L solution) and concentrations of adenosine, inosine and hypoxanthine. The level of hypoxanthine at 1-min post-administration was below the assay’s detection limit (<DL). Values are means and SEMs
Discussion
The most striking finding of this study is that a single intraperitoneal dose of 2′,3′-cAMP is highly effective in attenuating adhesion formation in a small animal model subjected to extreme tissue injury (3 min of brushing the cecum with an electric toothbrush plus chemical injury with ethanol). This study also confirms prior results from our group that a single adenosine dose is anti-adhesive [19]; however, the present results show that 2′,3′-cAMP is more efficacious than adenosine per se. Although a lower concentration of adenosine was used compared to 2′,3′-cAMP, this is a valid comparison for clinical efficacy since higher concentrations of adenosine cannot be dissolved in saline; therefore, an important advantage of 2′,3′-cAMP is its higher aqueous solubility, thus yielding a higher achievable (in practice) efficacy. We conclude that 2′,3′-cAMP may be a preferred therapy for reducing the formation of post-surgical adhesions.
Adenosine activates A1, A2A, A2B and A3 receptors, and inosine is a partial agonist at A1 and A3 receptors [40]. The present study shows that a one-time intraperitoneal dose of 2′,3′-cAMP utilized for adhesion studies results in concentrations of adenosine and inosine in the abdominal cavity of >1 μmol/L for >3 h. These are pharmacologically active concentrations of adenosine and inosine [40]. Indeed, because adenosine can bind to A1 and A2A receptors at low nmol/L concentrations [40], it is likely that pharmacologically active levels of adenosine are achieved for much longer than 3 h. Therefore, it is likely that 2′,3′-cAMP functions as an adenosine prodrug to provide prolonged levels of adenosine (and inosine) in the peritoneal cavity, thus avoiding the necessity of multiple applications of adenosine to optimize adenosine’s anti-adhesive effects as previously demonstrated in the rat model [19].
Our recently published in vivo studies demonstrate the important role of CNPase in the conversion of 2′,3′-cAMP to adenosine [29]. Since CNPase is a rate-limiting enzyme for the metabolism of 2′,3′-cAMP to adenosine, we speculate that low CNPase levels may be present in the abdomen, which serves to slow the metabolism of 2′,3′-cAMP and prolong its duration of action. Indeed, our preliminary studies in rats confirm that protein levels of CNPase are low in the peritoneum and small intestines compared with the brain. Therefore, 2′,3′-cAMP may be an ideal slow-drug release technology because it is slowly metabolized extracellularly in the abdomen to adenosine, a potent anti-adhesive agent.
The marked beneficial effect of 2′,3′-cAMP in preventing abdominal adhesions is most likely secondary to increased levels of adenosine in the peritoneal cavity for hours following injury. While the pathogenesis of adhesions is complex and multifactorial, adenosine via activation of numerous extracellular receptors modifies a number of the proposed cellular and biochemical pathways involved [19]. Adenosine would reverse the initial event of microvascular ischemia induced by surgical trauma via vasodilatation through activation of adenosine receptors. Indeed, our recently published studies show that in vivo 2′,3′-cAMP causes vasodilation via activation of adenosine receptors [30]. In addition to vasodilation, 2′,3′-cAMP, via adenosine, stimulates the proliferation of vascular endothelial cells, which would accelerate vascular repair [41]. Moreover, 2′,3′-cAMP, via adenosine, enhances the proliferation of epithelial cells [41], which would hasten re-epithelialization of the mesothelial surface. Although the effects of 2′,3′-cAMP on the immune system are unknown, adenosine is well known to reduce the secondary inflammatory response to tissue injury via activation of adenosine receptors on various inflammatory cells including neutrophils, lymphocytes and macrophages [9, 11, 12]. It is also well accepted that adenosine inhibits release of cytotoxic oxygen free radicals and proteolytic enzymes from neutrophils, reduces lymphocyte proliferation and increases suppressor subtypes and inhibits macrophage production of various inflammatory cytokines—such as TNFα and interleukins 6 and 8 [9, 11, 12]. Finally, studies show that adenosine would also modify the final stage of adhesion formation due to inhibition of fibroblast proliferation via activation of A2B receptors [14–18].
Why was 2′,3′-cAMP more efficacious than 2′-AMP and 3′-AMP even though 2′,3′-cAMP is metabolized to adenosine via the intermediate AMPs (i.e., 2′-AMP and 3′-AMP)? It is possible that very high concentrations of these AMPs are pro-inflammatory and cause vasoconstriction. In this regard, our preliminary (unpublished) data in human CD4+ T cells suggest that very high concentrations of 2′-AMP stimulate TNFα production. Also, our preliminary (unpublished) data in the rat kidney indicate that high concentrations of 2′-AMP and 3′-AMP can cause vasoconstriction. Therefore, when using mM concentrations of 2′-AMP and 3′-AMP, the direct pro-inflammatory and vasoconstrictor effects of 2′-AMP and 3′-AMP likely attenuate any protection by generated adenosine. In contrast, when 2′,3′-cAMP is administered, 2′,3′-cAMP acts as a “reservoir” of adenosine yet generates much lower concentrations of the intermediate AMPs (too low to cause adverse effects).
As recently reviewed by Eltzschig, Sitkovsky and Robson [42], adenosine signaling affords, via A2A and A2B receptors, both anti-inflammatory and barrier-protective actions during intestinal inflammation. Importantly, inflammation and hypoxia, via hypoxia-dependent transcription factors, upregulate A2A and A2B receptors, increase the expression of CD39 (metabolizes ATP to ADP and 5′-AMP) and CD73 (metabolizes 5′-AMP to adenosine) and decrease the expression of equilibrative nucleoside transporters [43]. These concepts suggest that endogenous adenosine may importantly protect against post-surgical adhesions because surgery no doubt induces inflammation, which would activate the aforementioned mechanisms that accelerate adenosine formation and slow adenosine removal from the extracellular compartment. By analogy, it is conceivable that the enzymes involved in 2′,3′-cAMP metabolism to adenosine are also up-regulated by intestinal injury, hypoxia and inflammation, which would make 2′,3′-cAMP an even more effective in combating the formation of post-surgical adhesions.
Currently approved therapies for prevention of adhesions in humans are based on application of liquid or solid barriers to physically separate damaged tissue. These devices have a number of limitations and do not appear to prevent small bowel obstruction [1, 6]. Therefore, there is a dire need to develop new, safe and easily administered drug technologies to prevent adhesions following abdominal surgical procedures [4]. There are numerous reports of drug therapies, tested in animals, targeting specific pathways in adhesion formation [1, 2, 4, 44, 45]. Current consensus demonstrates that these agents have limited success and have significant adverse effects. Following the discovery of 2′,3′-cAMP, we postulated that 2′,3′-cAMP would be a promising anti-adhesive agents for the following reasons: First, 2′,3′-cAMP would function as an adenosine prodrug since it is metabolized to adenosine. Second, because the metabolism of 2′,3′-cAMP could be slow in tissues expressing only low levels of CNPase, 2′,3′-cAMP could result in significant and prolonged levels of adenosine in the peritoneal cavity and hence would require only a one-time administration. Third, adenosine per se is likely superior to other pharmacological agents since adenosine modulates numerous pathways postulated to produce adhesions. Fourth, 2′,3′-cAMP is cheap and highly water soluble allowing for instillation of high concentrations into the abdominal cavity. Fifth, the hydrophilicity of 2′,3′-cAMP and its slow metabolism to adenosine would avoid rapid absorption of 2′,3′-cAMP or adenosine, thus limiting adverse effects on other organ systems.
In summary, single administration of 2′,3′-cAMP markedly inhibits adhesion formation in a mouse model of extreme tissue injury. Although not formally scored, structures in the abdominal cavity appeared less inflamed and healthier in animals treated with 2′,3′-cAMP. Biochemical studies showed that 2′,3′-cAMP results in a significant elevation of intraperitoneal adenosine for >3 h. Further studies are warranted to examine the potential of this inexpensive, water soluble, and easily formulated compound to inhibit surgical adhesions in large animal models.
Conflict of interest
The University of Pittsburgh is the applicant of a pending patent application entitled “Cyclic Adenosine Monophosphates for Reducing the Formation of Adhesions”, and E. K. Jackson is listed as the inventor on this patent application.
Contributor Information
Mervyn B. Forman, Email: formanm@bellsouth.net
Delbert G. Gillespie, Email: dgg3@pitt.edu
Dongmei Cheng, Email: doc14@pitt.edu.
Edwin K. Jackson, Phone: +1-412-6481505, FAX: +1-412-6245070, Email: edj@pitt.edu
References
- 1.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Brochhausen C, Schmitt VH, Rajab TK, et al. Intraperitoneal adhesions—an ongoing challenge between biomedical engineering and the life sciences. J Biomed Mater Res A. 2011;98:143–156. doi: 10.1002/jbm.a.33083. [DOI] [PubMed] [Google Scholar]
- 3.Hellebrekers BWJ, Kooistra T. Pathogenesis of postoperative adhesion formation. Br J Surg. 2011;98:1503–1516. doi: 10.1002/bjs.7657. [DOI] [PubMed] [Google Scholar]
- 4.Arung W, Meurisse M, Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J Gastroenterol. 2011;17:4545–4553. doi: 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimomura M, Hinoi T, Ikeda S, et al. Preservation of peritoneal fibrinolysis owing to decreased transcription of plasminogen activator inhibitor-1 in peritoneal mesothelial cells suppresses postoperative adhesion formation in laparoscopic surgery. Surgery. 2013;153:344–356. doi: 10.1016/j.surg.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 6.Geiger TM, Roberts PL, Read TE, Marcello PW, Schoetz DJ, Ricciardi R. Has the use of anti-adhesion barriers affected the national rate of bowel obstruction? Am Surg. 2011;77:773–777. [PubMed] [Google Scholar]
- 7.van der Wal JBC, Iordens GIT, Vrijland WW, van Veen RN, Lange J, Jeekel J. Adhesion prevention during laparotomy: long-term follow-up of a randomized clinical trial. Ann Surg. 2011;253:1118–1121. doi: 10.1097/SLA.0b013e318217e99c. [DOI] [PubMed] [Google Scholar]
- 8.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–2234. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008;83:447–455. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 11.Ohta A, Sitkovsky M. The adenosinergic immunomodulatory drugs. Curr Opin Pharmacol. 2009;9:501–506. doi: 10.1016/j.coph.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–175. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets. 2009;13:1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.HYP.31.4.943. [DOI] [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation. 1997;96:2656–2666. doi: 10.1161/01.CIR.96.8.2656. [DOI] [PubMed] [Google Scholar]
- 16.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension. 2000;36:337–342. doi: 10.1161/01.HYP.36.3.337. [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001;37:1095–1100. doi: 10.1161/01.HYP.37.4.1095. [DOI] [PubMed] [Google Scholar]
- 18.Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension. 2001;37:716–721. doi: 10.1161/01.HYP.37.2.716. [DOI] [PubMed] [Google Scholar]
- 19.Jackson EK. Intraperitoneal administration of adenosine inhibits formation of abdominal adhesions. Dis Colon Rectum. 2004;47:1390–1396. doi: 10.1007/s10350-004-0578-z. [DOI] [PubMed] [Google Scholar]
- 20.Jackson EK, Swamy RS, Herzer WA, Mi Z. Local and systemic effects of peritoneal lavage with high concentrations of adenosine in rats. Aliment Pharmacol Ther. 2000;14:1371–1380. doi: 10.1046/j.1365-2036.2000.00830.x. [DOI] [PubMed] [Google Scholar]
- 21.Jackson EK. The 2′,3′-cAMP-adenosine pathway. Am J Physiol Renal. 2011;301:F1160–F1167. doi: 10.1152/ajprenal.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP-adenosine pathway in proximal tubular, thick ascending limb, and collecting duct epithelial cells. Am J Physiol Renal. 2013;304:F49–F55. doi: 10.1152/ajprenal.00571.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson EK, Gillespie DG, Dubey RK. 2′-AMP and 3′-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. J Pharmacol Exp Ther. 2011;337:444–450. doi: 10.1124/jpet.110.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson EK, Ren J, Cheng D, Mi Z. Extracellular cAMP-adenosine pathways in the mouse kidney. Am J Physiol Renal Physiol. 2011;301:F565–F573. doi: 10.1152/ajprenal.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson EK, Ren J, Gillespie DG. 2′,3′-cAMP, 3′-AMP, and 2′-AMP inhibit human aortic and coronary vascular smooth muscle cell proliferation via A2B receptors. Am J Physiol Heart Circ Physiol. 2011;301:H391–H401. doi: 10.1152/ajpheart.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension. 2010;56:151–158. doi: 10.1161/HYPERTENSIONAHA.110.152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem. 2009;284:33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verrier JD, Exo JL, Jackson TC, et al. Expression of the 2′,3′-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem. 2011;118:979–987. doi: 10.1111/j.1471-4159.2011.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verrier JD, Jackson TC, Bansal R, et al. The brain in vivo expresses the 2′,3′-cAMP-adenosine pathway. J Neurochem. 2012;122:115–125. doi: 10.1111/j.1471-4159.2012.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson EK, Mi Z. In vivo cardiovascular pharmacology of 2′,3′-cAMP, 2′-AMP, and 3′-AMP in the rat. J Pharmacol Exp Ther. 2013;346:190–200. doi: 10.1124/jpet.113.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther. 2009;328:855–865. doi: 10.1124/jpet.108.146712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackson EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension. 2000;35:262–266. doi: 10.1161/01.HYP.35.1.262. [DOI] [PubMed] [Google Scholar]
- 33.Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension. 1996;28:765–771. doi: 10.1161/01.HYP.28.5.765. [DOI] [PubMed] [Google Scholar]
- 34.Hong KW, Shin HK, Kim HH, Choi JM, Rhim BY, Lee WS. Metabolism of cAMP to adenosine: role in vasodilation of rat pial artery in response to hypotension. Am J Physiol. 1999;276:H376–H382. doi: 10.1152/ajpheart.1999.276.2.H376. [DOI] [PubMed] [Google Scholar]
- 35.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal. 2001;281:F597–F612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- 36.Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther. 2000;295:23–28. [PubMed] [Google Scholar]
- 37.Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther. 1997;283:177–182. [PubMed] [Google Scholar]
- 38.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol. 2004;66:571–599. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- 39.Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. J Pharmacol Exp Ther. 1998;287:926–930. [PubMed] [Google Scholar]
- 40.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 41.Jackson EK, Gillespie DG. Extracellular 2′,3′-cAMP and 3′,5′-cAMP stimulate proliferation of preglomerular vascular endothelial cells and renal epithelial cells. Am J Physiol Renal Physiol. 2012;303:F954–F962. doi: 10.1152/ajprenal.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poth JM, Brodsky K, Ehrentraut H, Grenz A, Eltzschig HK. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J Mol Med. 2013;91:183–193. doi: 10.1007/s00109-012-0988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meisel JA, Fallon EM, Le HD, et al. Sunitinib inhibits postoperative adhesions in a rabbit model. Surgery. 2011;150:32–38. doi: 10.1016/j.surg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Chu DI, Lim R, Heydrick S, et al. N-acetyl-L-cysteine decreases intra-abdominal adhesion formation through the upregulation of peritoneal fibrinolytic activity and antioxidant defenses. Surgery. 2011;149:801–812. doi: 10.1016/j.surg.2011.02.015. [DOI] [PubMed] [Google Scholar]