Abstract
BACKGROUND
Brain injury is observed on brain magnetic resonance imaging preoperatively in up to 50% of newborns with congenital heart disease. Newer imaging techniques such as diffusion tensor imaging provide sensitive measures of the white matter. The objective of this study was to evaluate the diffusion tensor imaging analysis technique of tract-based spatial statistics in newborns with congenital heart disease.
METHODS
Term newborns with congenital heart disease that would require surgery at less than one month of age were prospectively enrolled (n = 19). Infants underwent preoperative and postoperative brain magnetic resonance imaging with diffusion tensor imaging. Tract-based spatial statistics, an objective whole brain diffusion tensor imaging analysis technique, was used to determine differences in white matter fractional anisotropy between infant groups. Term control infants were also compared to congenital heart disease infants. Postmenstrual age was equivalent between congenital heart disease infant groups and between congenital heart disease and control infants.
RESULTS
Ten infants had preoperative brain injury, either infarct or white matter injury, by conventional brain magnetic resonance imaging. The technique of tract-based spatial statistics showed significantly lower fractional anisotropy (P <0.05, corrected) in multiple major white matter tracts in the infants with preoperative brain injury compared to infants without preoperative brain injury. Fractional anisotropy values increased in the white matter tracts from the preoperative to the postoperative brain magnetic resonance imaging correlating with brain maturation. Control infants showed higher fractional anisotropy in multiple white matter tracts compared to infants with congenital heart disease.
CONCLUSION
Tract-based spatial statistics is a valuable diffusion tensor imaging analysis technique that may have better sensitivity in detecting white matter injury compared to conventional brain magnetic resonance imaging in term newborns with congenital heart disease.
Keywords: white matter injury, congenital heart disease, diffusion tensor imaging, brain magnetic resonance imaging, tract-based spatial statistics, newborn brain injury, fractional anisotropy
INTRODUCTION
Newborns with congenital heart disease (CHD) are at risk for brain injury, preoperatively and postoperatively. The increased preoperative risk can be explained, at least in part, by the structural immaturity of the brain in newborns with CHD.1, 2 Conventional brain magnetic resonance imaging (MRI) may show preoperative injury in the form of ischemic infarcts, white matter injury, and other injury types in up to 50% of newborns requiring surgery as neonates.1, 3, 4 New or increased injury may also be seen postoperatively in 30–40%.1, 5, 6 These injuries may appear fairly small on conventional imaging and may resolve over time.6, 7
Diffusion tensor imaging (DTI) is a quantitative MRI technique that can assess the structural integrity of the cerebral white matter, providing a valuable method to quantitate microstructural brain changes with injury.8 Fractional anisotropy is one parameter that can be measured by DTI. Fractional anisotropy reflects the directionality of water molecule diffusion and thus provides a measure of microstructural integrity, since water molecules tend to diffuse along the direction of axonal tracts while diffusion is restricted by the myelin sheath in the direction perpendicular to axonal tracts. Fractional anisotropy values rise as a newborn’s brain matures correlating with increasing structural complexity.9–11 Fractional anisotropy values, however, are reduced with white matter injury.12–14 In a MRI study of CHD newborns, fractional anisotropy values in many white matter tracts were found to be lower compared to control newborns, but there was no difference between CHD newborns with and without brain injury using a region of interest analysis technique.15 In a separate study, infants with preoperative brain injury were found to have a lower rate of fractional anisotropy increase postoperatively compared to infants with normal preoperative brain MRIs, suggesting delayed development of the white matter tracts in these newborns.16 Fractional anisotropy values may also vary as a function of cardiac anatomy. For example, among infants with a single ventricle type of CHD, a smaller ascending aortic diameter was associated with lower fractional anisotropy values in brain white matter compared to infants with a larger ascending aortic diameter.17
A newer DTI analysis technique is known as tract-based spatial statistics. This technique allows for an objective measure of the brain white matter tracts using a “white matter skeleton” built by the combined subject images, thus removing any subjective selection of brain regions.18 In preterm newborns, this technique has shown areas of white matter injury outside of areas visualized by conventional brain MRI.19 A recent study using this technique was able to demonstrate widespread changes in major white matter tracts in ex-preterm neonates known to have punctate lesions on previous MRI images.12 These changes in white matter microstructure were significantly different when compared to gestational age and sex-matched controls without a history of punctate lesions on MRI. The distribution of reduced fractional anisotropy in the corticospinal tracts with punctate lesions using this technique was more widespread than the visible extent of the lesions on conventional MRI.12 In addition, findings at term equivalent age correlate with neurodevelopment at age 2 years, thus raising the possibility of using tract-based spatial statistics in preterm newborns as a biomarker of neurodevelopmental outcome.20 This technique, however, has not been well explored in newborns with CHD.
The objectives of this study were to: 1) evaluate the DTI technique of tract-based spatial statistics in studying the white matter of newborns with CHD, 2) determine how brain injury in CHD newborns affects fractional anisotropy values in the major white matter tracts, and 3) correlate fractional anisotropy values with brain maturity in CHD newborns. We hypothesized that tract-based spatial statistics would be a valuable technique to analyze white matter injury and brain maturity in newborns with CHD.
METHODS
Subjects
Following informed consent, newborns with CHD were prospectively enrolled into a pilot observational study at Arkansas Children’s Hospital between June 1, 2012 and April 30, 2013. Subjects had to have CHD expected to require surgery at less than one month of age. Infants were excluded if their gestational age at birth was <36 weeks or if they had a major genetic syndrome. Recorded baseline characteristics included demographics, birth history (i.e. birth weight, gestational age, and Apgar scores), and type of CHD. For each infant, details pertaining to the surgery included, age at surgery, type of surgery, Risk Adjustment for CHD Surgery (RACHS)-1 category21, use of cardiopulmonary bypass including total bypass time, and the degree of hypothermia. The RACHS-1 is a consensus based method for risk adjustment for in-hospital mortality in CHD and is based on the surgical procedure performed.21 In addition, data from 11 healthy infants born at term gestational age recruited for other brain MRI research projects were included in this study to serve as controls. This study received Institutional Review Board approval prior to initiation.
Brain MR Imaging
All subjects underwent a brain MRI, preoperatively, on a 1.5 Tesla Achieva scanner (Philips Healthcare, Best, the Netherlands) using a MRI protocol that consisted of 3D T1 weighted images, axial T2 weighted images, T1 inversion recovery, fluid attenuation inversion recovery, diffusion weighted images, susceptibility weighted images, and DTI. The 3D T1 weighted sequence was obtained for anatomic evaluation and volumetric analysis and the T1 weighted inversion recovery was obtained to assess myelination. A single-shot spin echo planar imaging sequence with acquisition voxel size 2 mm × 2 mm × 3 mm and diffusion weighting gradients (b = 700 s/mm2) uniformly distributed in 15 directions was used to acquire the DTI data. Infants were sedated by a pediatric cardiovascular anesthesiologist based on the individual need of each infant, as the MRI was performed immediately prior to transfer to the operating room for CHD surgery, in most cases. Three infants had a preoperative clinical cardiac MRI during which the brain MRI was performed.
Postoperatively, infants had a brain MRI with the same protocol using a non-sedated technique of swaddling the infant to achieve sleep22 using a MedVac Infant Immobilizer (CFI Medical Solutions, Fenton, MI, USA). During the MRI, infants were monitored using a MRI-compatible pulse oximeter and a MRI-compatible camera was attached to the head coil and connected to a screen outside the scanner room to watch for infant movement. For some infants, sequences were repeated due to movement artifact. Most of the MRIs occurred when the infant was stable and close to hospital discharge. One infant had a clinical procedure requiring anesthesia during which the postoperative brain MRI was obtained under sedation. For two infants, the postoperative brain MRI was obtained due to attending physician clinical concern for a neurologic problem and was done under sedation. The control infants had similar MRI examinations including DTI at 0–2 weeks of age (postmenstrual age 39–41 weeks).
The brain MRIs were read by board-certified pediatric neuroradiologists (CMG and RHR) and scored using the CHD MRI Injury Score as reported previously.4 The score incorporates the relative size and number of areas involved of 11 types of imaging abnormalities including white matter injury, gray matter injury, focal cerebral infarction, watershed infarcts, hemorrhages, changes in susceptibility weighted imaging, and brain atrophy, which are seen in newborns with CHD. Brain injury was defined as having an infarct, white matter injury, and/or deep gray matter injury. The isolated finding of a subdural hemorrhage, preoperatively, was considered as “no brain injury” since this can frequently be seen in a newborn due to birth forces following delivery. The level of brain maturity was determined using the validated Total Maturation Score by consensus of the neuroradiologists.1, 2, 23 A MRI physicist (XO) reviewed all DTI data for quality and performed the DTI tract-based spatial statistics analysis.
DTI Tract-Based Spatial Statistics Analysis
For the DTI tract-based spatial statistics analysis, infants were grouped by brain injury status, with either the presence or absence of brain injury by conventional MRI, preoperatively and postoperatively. Infants were also grouped by preoperative Total Maturation Score, with either Total Maturation Score ≤12 vs. >12, to evaluate the effect of brain maturation on fractional anisotropy. A Total Maturation Score of 12 was chosen since it correlates with a term gestational age (38 to 39 weeks) and provided two CHD subject groups of fairly equal size for tract-based spatial statistics analysis of brain maturation.23 CHD infants were also compared to control infants.
The tract-based spatial statistics analyses were carried out by the FMRIB Software Library (FSL) tract-based spatial statistics v1.2.18, 24 First, the fractional anisotropy maps for each subject were computed from the scanner-carried software (Fibertrak, Philips) and were exported to a workstation with FSL installed on a Linux virtual machine (VMware Inc., Palo, Alto, CA, USA). Each fractional anisotropy data set was then aligned to every other fractional anisotropy data set by FSL to identify the one with minimum total warping, and this fractional anisotropy data set was therefore regarded as the most representative one and served as the target fractional anisotropy data set. Nonlinear transforms were then performed to register each fractional anisotropy data set to this target. Afterwards, all fractional anisotropy images were merged, and a fractional anisotropy skeletonization program by FSL was used to create a fractional anisotropy skeleton in which a threshold of fractional anisotropy ≥0.15 was chosen. Subsequent voxel-wise statistics were performed on the normalized and skeletonized fractional anisotropy data.
Statistical Analysis
For demographic and clinical data, the median, lower quartiles, and upper quartiles were computed for continuous variables, frequencies and percentages were computed for ordinal variables. Demographic and clinical similarity between CHD infant groups (preoperative brain injury vs. no brain injury) was estimated using equivalence tests assuming a 20% margin.25, 26 Postmenstrual age at initial MRI was estimated between controls and CHD patients, with and without brain injury as well as in total, using equivalence tests assuming a 10% margin. R statistical software, R version 2.15.1, was used for the creation of Table 1. For the DTI tract-based spatial statistics analysis, voxel-wise comparisons of fractional anisotropy values were performed by t-tests. Threshold-Free Cluster Enhancement option18 was used and the results were fully corrected for multiple comparisons. Clusters with P <0.05 (corrected) were regarded as significant.
Table 1.
Characteristics of Study Infants by Preoperative Brain Injury
| n † | No Brain Injury* n = 9 |
Brain Injury* n = 10 |
Combined* n = 19 |
Equivalence§ | |
|---|---|---|---|---|---|
| Birth gestational age (weeks) | 19 | 38 39 39 | 37 37 38 | 37 38 39 | E |
| Birth weight (grams) | 19 | 3076 3418 3460 | 2663 3192 3494 | 2769 3360 3488 | NE |
| Apgar Score 1-min | 19 | 8 8 9 | 4.5 7 7 | 7 8 8 | NE |
| Apgar Score 5-min | 19 | 8 9 9 | 7.2 8 8 | 8 8 9 | NE |
| Age at MRI 1 (days)‡ | 19 | 6 8 13 | 6 7 8 | 6 8 10.5 | NE |
| Postmenstrual age MRI 1 (weeks) | 19 | 39.71 39.86 40.14 | 37.75 38.50 40.00 | 38.50 39.71 40.21 | E |
| Age at Surgery (days) | 19 | 6.0 8.0 13.0 | 8.0 8.0 13.8 | 6.0 8.0 13.5 | NE |
| Age at MRI 2 (days)‡ | 16 | 18 19 35 | 26 34 36 | 18 32 36 | NE |
| Postmenstrual age MRI 2 (weeks) | 16 | 40.7 41.6 43.3 | 40.3 42.3 43.2 | 40.7 42.1 43.4 | E |
| Days between MRI 1 and MRI 2‡ | 16 | 8.0 12.0 29.0 | 13.0 27.0 30.0 | 9.5 14.5 29.0 | E |
Abbreviations:
MRI = magnetic resonance imaging
E = equivalent
NE= non-equivalent
Specific notes:
a b c represent the lower quartile a, the median b, and the upper quartile c for continuous variables
n is the number of non–missing values
MRI 1 = Preoperative brain MRI; MRI 2 = Postoperative brain MRI
RESULTS
Nineteen of 31 (61%) eligible infants during the study period were enrolled. The clinical characteristics, timing of brain MRIs, and age at surgery for the 19 infants are described in Table 1. The CHD type and surgical RACHS-1 category for each infant is presented in Table 2. None of the infants had seizures preoperatively. Six of 12 infants that had a normal preoperative neurologic exam had preoperative brain injury (Table 2).
Table 2.
Infant CHD Type, Preoperative Brain Injury, and Neurologic Examination
| Infant | CHD Type | RACHS* | Preoperative Brain Injury |
Focal Infarct |
White Matter Injury† |
CHD MRI Injury Score‡ |
Preoperative Neurologic exam§ |
|---|---|---|---|---|---|---|---|
| 1 | Hypoplastic left heart syndrome | 4 | Yes | MCA (left, right) | – | 6 | Limited |
| 2 | d-Transposition of the great arteries | 3 | Yes | – | Minimal | 4 | Limited |
| 3 | Pulmonary atresia with intact ventricular septum | 3 | Yes | MCA (left) | – | 4 | Normal |
| 4 | d-Transposition of the great arteries, double outlet right ventricle, pulmonary artery stenosis | 3 | Yes | ACA (right) MCA (left) | – | 2 | Normal |
| 5 | Tetralogy of Fallot, pulmonary artery stenosis | 3 | No | – | – | 1 | Normal |
| 6 | Coarctation of aorta | 2 | No | – | – | 3 | Normal |
| 7 | Hypoplastic left heart syndrome | 3 | Yes | – | Minimal | 5 | Normal |
| 8 | d-Transposition of the great arteries | 4 | No | – | – | 0 | Normal |
| 9 | Hypoplastic left heart syndrome, coarctation of aorta | 6 | No | – | – | 3 | Abnormal |
| 10 | d-Transposition of the great arteries | 3 | Yes | – | Minimal | 3 | Normal |
| 11 | d-Transposition of the great arteries, hypoplastic left heart syndrome | 3 | No | – | – | 4 | Limited |
| 12 | Hypoplastic left heart syndrome | 3 | Yes | ACA (right) MCA (right) | – | 6 | Abnormal |
| 13 | d-Transposition of the great arteries | 4 | Yes | ACA (left) | Minimal | 6 | Abnormal |
| 14 | d-Transposition of the great arteries | 3 | No | – | – | 0 | Abnormal |
| 15 | d-Transposition of the great arteries | 4 | Yes | – | Moderate | 3 | Normal |
| 16 | Double outlet right ventricle, pulmonary artery stenosis | 3 | Yes | PCA (right) | – | 1 | Normal |
| 17 | Hypoplastic left heart syndrome | 3 | No | – | – | 0 | Normal |
| 18 | Heterotaxy syndrome | NA | No | – | – | 0 | Normal |
| 19 | d-Transposition of the great arteries | 3 | No | – | – | 0 | Normal |
Abbreviations:
RACHS = Risk Adjustment for Congenital Heart Surgery category21
ACA = anterior cerebral artery
MCA = middle cerebral artery
PCA = posterior cerebral artery
Specific Notes:
RACHS-1 category: A consensus-based method for CHD surgical risk. “1” is the lowest score associated with the least amount of risk of in-hospital mortality and “6” is the highest.21 Not all surgeries for CHD have a RACHS-1 category.
White matter injury; Minimal is less than or equal to 3 areas of T1 signal abnormality on brain MRI measuring less than 2 mm in size, Moderate is greater than 3 areas of T1 signal abnormality or the areas measure greater than 2mm but less than 5% of the hemisphere involved.1, 4, 32
CHD MRI Injury Score: A detailed score based on conventional brain MRI for newborns with CHD in which distribution/severity of brain injury is quantified for 11 types of MRI findings. “0” represents a completely normal brain MRI.4
Neurologic examination included an assessment of level of consciousness, cranial nerves, motor strength, tone, muscle stretch reflexes, and newborn reflexes. A normal exam was complete and had normal findings in all areas for age. An abnormal exam was complete, but with at least one abnormal finding for age, and a limited exam was an examination that was performed, but was felt to not be complete due to infant’s level of sedation or medical complexity at the time of the exam.
Brain injury, as defined by having a single focal infarct or multi-focal infarcts and/or white matter injury on conventional MRI, was seen preoperatively in 10 CHD infants (52%, Table 2). Five of the 9 CHD infants without brain injury had completely normal brain MRIs, while the other 4 had a small subdural hemorrhage (n=1), small subdural, choroid plexus, and intraventricular hemorrhages (n=1), which are changes that can be found normally in newborns following birth, and cerebral atrophy (n=2). Six infants with brain injury also had cerebral atrophy. The preoperative CHD MRI Injury Score4 is reported in Table 2. Infants with and without brain injury were equivalent for birth gestational age and postmenstrual age at the preoperative and postoperative brain MRI. Infants with preoperative brain injury had a lower birth weight and lower 1- and 5-minute Apgar scores compared to infants without preoperative brain injury (Table 1).
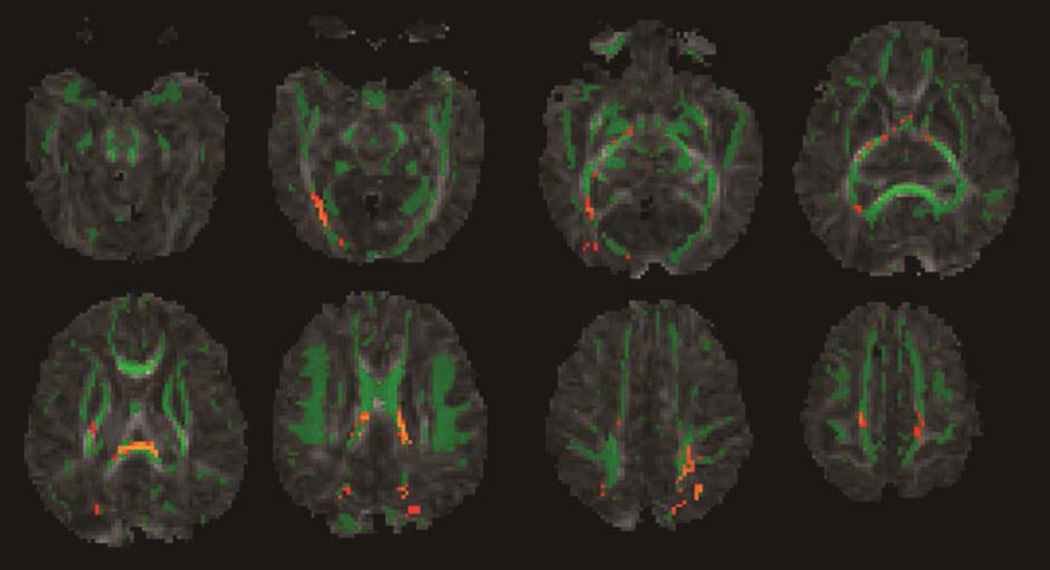
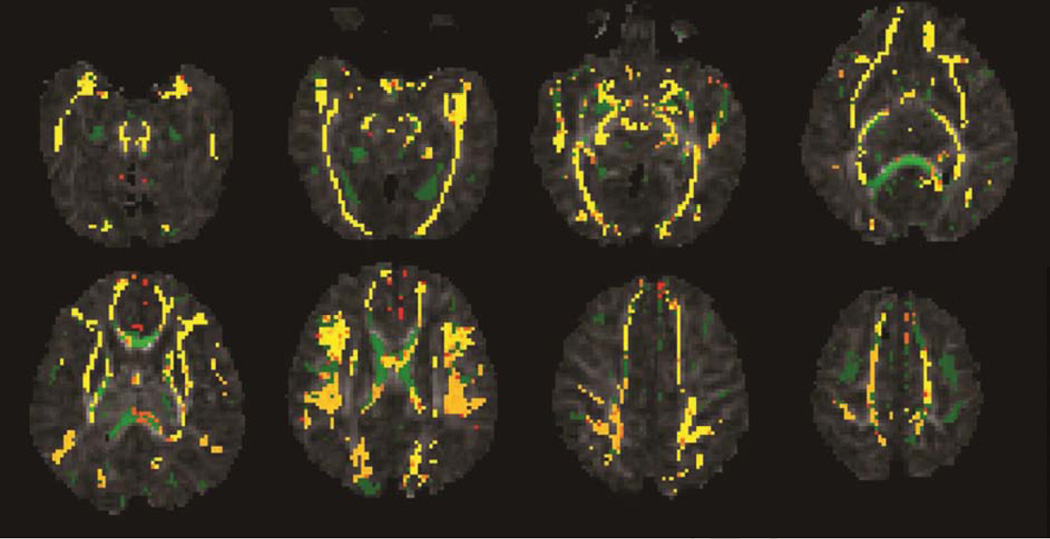
Following Threshold-Free Cluster Enhancement correction for multiple comparisons, tract-based spatial statistics showed significantly lower fractional anisotropy values in many major white matter tracts including the splenium of the corpus callosum, posterior limb of the internal capsule, the corticospinal tracts, and the optic radiations in infants with preoperative brain injury compared to infants without preoperative brain injury (P <0.05, corrected, Figure 1). These changes did not persist postoperatively. Fractional anisotropy values in all major white matter tracts (projection, association, and callosal fibers), showed a significant increase from the preoperative to the postoperative MRI correlating with increasing brain maturity (P <0.05, corrected, Figure 2).
Figure 1.
Mean fractional anisotropy skeleton (green) of 19 CHD infants overlaid on fractional anisotropy maps from preoperative brain MRIs. Yellow/orange color represents brain regions with lower fractional anisotropy values (P <0.05, corrected) in CHD infants with preoperative brain injury compared to CHD infants without preoperative brain injury.
Figure 2.
Mean fractional anisotropy skeleton (green) of 16 CHD infants overlaid on fractional anisotropy maps. Yellow/orange color represents regions with lower fractional anisotropy values (P <0.05, corrected) in the preoperative MRI compared to the postoperative MRI. Almost all major white matter tracts had increased fractional anisotropy values by the time of the postoperative brain MRI.
Total Maturation Scores were equivalent, preoperatively and postoperatively, for infants with and without preoperative brain injury. Two of 3 infants with a preoperative Total Maturation Score of ≤ 10, equivalent to a maturation level or gestational age ≤ 35 weeks, had preoperative brain injury. Infants were grouped by Total Maturation Score ≤12 (correlating with a postmenstrual age of ≤38–39 weeks) vs. >12 (correlating with a postmenstrual age of >39 weeks) for DTI analysis.23 The mean (standard deviation) postmenstrual age at MRI was slightly lower in the Total Maturation Score ≤12 vs. >12 groups, 38.8(0.4) vs. 40.1(0.3) weeks, P = 0.038. There was no difference in white matter fractional anisotropy values for the 12 infants with a Total Maturation Score of ≤12 vs. the 7 infants with a preoperative Total Maturation Score >12. A cutoff value of Total Maturation Score ≤11 vs. >11 was also tried, without finding a significant difference between brain maturation groups. Eight of 10 (80%) infants with preoperative brain injury had a Total Maturation Score ≤12 compared to 4 of 9 (44%) infants without preoperative brain injury (odds ratio 5, 95% confidence interval 0.6553, 38.1532, P = 0.109).
The postmenstrual age for control infants at MRI was statistically equivalent within a 10% margin to CHD with brain injury, CHD infants without brain injury, and CHD infants overall at the time of the preoperative brain MRI. Control infants showed higher fractional anisotropy values in the splenium of the corpus callosum, posterior limb of the internal capsule, the corticospinal tracts, and the optic radiations compared to all CHD infants (P <0.05, corrected). When CHD infants were grouped by preoperative brain injury status, infants with brain injury had widespread areas of lower fractional anisotropy values in the white matter tracts compared to controls, however CHD infants without brain injury had more limited areas of lower fractional anisotropy compared to control infants.
DISCUSSION
This pilot study demonstrates the usefulness of the quantitative MRI technique of DTI with tract-based spatial statistics as a way to thoroughly evaluate the white matter in newborns with CHD and is the first study, to our knowledge, in this population. This technique is better able to detect white matter changes, which may have been missed using DTI with region of interest analysis and changes that are not seen with conventional brain MRI. The study found that fractional anisotropy values in CHD newborns with preoperative brain injury were lower in many major white matter tracts compared to CHD newborns without preoperative brain injury and compared to control infants. Given that much of the injury seen in these infants was small and focal; these findings demonstrate that the distribution of white matter changes are more extensive than is appreciated based on conventional imaging. It can therefore be hypothesized that infants with small focal areas of brain injury by conventional brain MRI that also have multiple white matter tracts involved when imaged with DTI tract-based spatial statistics, would be at higher risk for long-term motor and cognitive/behavioral deficits.
A previous report using DTI with region of interest analysis in CHD infants found that white matter injury was observed in CHD infants compared with control infants.15 We demonstrated the same finding using tract-based spatial statistics analysis comparing CHD infants to control infants and also showed a difference between CHD infants with and without brain injury based on conventional brain MRI. The region of interest method has been a long accepted technique for DTI analysis, but has limitations which may impact results including dependency on operator skill, need for accurate placement of measurement area, reproducibility between operators, and it is fairly time-consuming.8 While region of interest analysis may be a sensitive method to detect white matter abnormalities, the selection of region of interest is inevitably subjective. The definition of white matter regions may vary across different observers, or even vary at different time of measurements by the same observer. On the other hand, tract-based spatial statistics can provide a non-subjective voxel-wise comparison of white matter in the whole brain.18 Therefore, DTI with tract-based spatial statistics analysis may be a more sensitive MRI tool to thoroughly assess the white matter in at risk newborns, since conventional MRI may underestimate the amount of affected white matter, and tract-based spatial statistics is more objective than the region of interest approach.
The cause of early brain injury in CHD newborns is multi-factorial making it difficult to identify a single reason for a particular infant. Potential risk factors include genetics, birth weight, CHD type, prenatal cerebral blood flow, hypoxemia, and demographics.27 Brain injury in newborns with CHD is similar to preterm newborn brain injury, with injury to the white matter a prominent injury type. CHD newborns also may have relative brain immaturity.1, 2 Tract-based spatial statistics has been successfully used in several studies of brain injury in preterm newborns and also in newborns with encephalopathy.12, 19, 20, 28 Newborns with CHD may have chronic hypoxemia and poor tolerance to changes at birth with some degree of neonatal encephalopathy.29 In our study infants, Apgar scores were lower in those with preoperative brain injury compared to those without preoperative brain injury (Table 1). In a DTI study using tract-based spatial statistics in preterm newborns with CHD, widespread microstructural changes were found in the white matter tracts compared to term newborns without CHD.30 The finding that fractional anisotropy values were higher in all major areas postoperatively, mean of 18(13) days from preoperative MRI, showed that the technique used to analyze the data worked well.31 It was expected that there would be an increase in fractional anisotropy values from the preoperative to the postoperative periods as the brain matures. This study was not able to determine if the increase in fractional anisotropy from the preoperative to the postoperative brain MRI was less in infants with preoperative brain injury compared to infants without preoperative brain injury. Based on a similar study evaluating maturation of the pyramidal tracts in newborns with CHD, lower fractional anisotropy postoperatively may be expected in infants with preoperative brain injury.16
Brain maturity using the validated Total Maturation Score in our study infants, mean of 11.95(1.72) correlating with a postmenstrual age of 38–39 weeks, was similar to other studies of CHD newborns.1, 2, 23 Our study found a difference in fractional anisotropy values between infants with Total Maturation Score ≤12 vs. Total Maturation Score >12, but, due to low sample size, lacked sufficient power to survive correction for multiple comparisons. This difference, therefore, may simply be due to a slight age difference, but does confirm that the maturation scoring system is reliable as fractional anisotropy values increased with increasing brain maturity. This study is limited by the small sample size and that not every infant was able to have postoperative brain MRI. Two infants who had preoperative brain injury did not have postoperative MRI. One was discharged soon postoperatively and was unable to have MRI scheduled prior to discharge, and the other infant died postoperatively and was not ever stable enough for the postoperative research MRI. An additional infant had postoperative brain MRI, but the DTI dataset was not able to be analyzed. This may explain the loss of significance in the fractional anisotropy values between the brain injury and no brain injury groups at the time of the postoperative brain MRI. MRIs also occurred at slightly different postnatal ages since the timing was dependent upon the surgery date and postoperatively, was dependent upon the infants’ clinical stability. Importantly though, compared groups were equivalent for postmenstrual age at MRI since fractional anisotropy values increase with age.
The quantitative brain MRI technique of DTI tract-based spatial statistics in newborns with CHD offers a detailed insight into brain white matter changes. Brain injury, even small focal changes in the white matter, may be associated with more diffuse changes to the brains’ white mater tracts. These findings can be used to predict long-term neurodevelopmental outcome. Larger studies evaluating how CHD in newborns with and without brain injury affects the developing white matter, using specialized MRI techniques such as DTI with tract-based spatial statistics analysis, are needed to determine how these tools can best be used clinically and for research.
ACKNOWLEDGEMENTS
This study was supported by the University of Arkansas for Medical Sciences Center for Translational Neuroscience award from The National Institutes of Health (P20 GM103425). REDCap receives institutional support from the University of Arkansas for Medical Sciences Translational Research Institute (NCATS/NIH 1 UL1 RR029884). Dr. Mulkey also receives support from the Arkansas Children’s Hospital Research Institute and the Arkansas Biosciences Institute. The authors appreciate the work of Nupur Chowdhury, MS, CRRP, who entered study data in the database, the research coordinators at the ACH Research Institute, the MRI technicians at ACH, the nurses of the ACH cardiovascular intensive care unit for preparing study infants for MRI, and the pediatric cardiovascular surgery anesthesia department. We also appreciate our study infants and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah B. Mulkey, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Xiawei Ou, Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Raghu H. Ramakrishnaiah, Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Charles M. Glasier, Department of Radiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Christopher J. Swearingen, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Maria S. Melguizo, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Vivien L. Yap, Department of Pediatrics, New York Presbyterian Weill Cornell Medical College, New York, NY, USA.
Michael L. Schmitz, Department of Anesthesiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA.
Adnan T. Bhutta, Department of Pediatrics, University of Maryland, Baltimore, MD, USA.
REFERENCES
- 1.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg. 2010;139:543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licht DJ, Shera DM, Clancy RR, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McQuillen PS, Hamrick SEG, Perez MJ, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 4.Mulkey SB, Swearingen CJ, Melguizo MS, et al. Multi-tiered analysis of brain injury in neonates with congenital heart disease. Pediatr Cardiol. 2013;34:1772–1784. doi: 10.1007/s00246-013-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block AJ, McQuillen PS, Chau V, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Tharac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahle WT, Tavani F, Zimmerman RA, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–I114. [PubMed] [Google Scholar]
- 7.Galli KK, Zimmerman RA, Jarvik GP, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 8.Pannek K, Guzzetta A, Colditz PB, Rose SE. Diffusion MRI of the neonate brain: acquisition, processing and analysis techniques. Pediatr Radiol. 2012;42:1169–1182. doi: 10.1007/s00247-012-2427-x. [DOI] [PubMed] [Google Scholar]
- 9.Dubois J, Dehaene-Lambertz G, Perrin M, et al. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16:621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 12.Bassi L, Chew A, Merchant N, et al. Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res. 2011;69:561–566. doi: 10.1203/PDR.0b013e3182182836. [DOI] [PubMed] [Google Scholar]
- 13.Cheong JL, Thompson DK, Wang HX, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30:623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward P, Counsell S, Allsop J, et al. Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic-ischemic encephalopathy. Pediatrics. 2006;117:e619–e630. doi: 10.1542/peds.2005-0545. [DOI] [PubMed] [Google Scholar]
- 15.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 16.Partridge SC, Vigneron DB, Charlton NN, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59:640–651. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 17.Sethi V, Tabbutt S, Dimitropoulos A, et al. Single-ventricle anatomy predicts delayed microstructural brain development. Pediatr Res. 2013;73:661–667. doi: 10.1038/pr.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Anjari M, Srinivasan L, Allsop JM, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 20.van Kooij BJ, de Vries LS, Ball G, et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol. 2012;33:188–194. doi: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180–184. doi: 10.1053/j.pcsu.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 23.Childs AM, Ramenghi LA, Cornette L, et al. Cerebral maturation in premature infants: quantitative assessment using MR imaging. AJNR Am J Neuroradiol. 2001;22:1577–1582. [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 25.Westlake WJ. Response to T.B.L. Kirkwood: bioequivalence testing - a need to rethink. Biometrics. 1981;37:589–594. [Google Scholar]
- 26.Schuirmann DL. On hypothesis testing to determine if the mean of a distribution is contained in a known interval. Biometrics. 1981;37:617. [Google Scholar]
- 27.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 28.Porter EJ, Counsell SJ, Edwards AD, Allsop J, Azzopardi D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr Res. 2010;68:205–209. doi: 10.1203/PDR.0b013e3181e9f1ba. [DOI] [PubMed] [Google Scholar]
- 29.Mulkey SB, Fontenot E, Imamura M, Yap VL. Therapeutic hypothermia in a neonate with hypoxic-ischemic encephalopathy and transposition of the great arteries. Ther Hypotherm Temp Manage. 2011;1:205–208. doi: 10.1089/ther.2011.0016. [DOI] [PubMed] [Google Scholar]
- 30.Paquette LB, Wisnowski JL, Ceschin R, et al. Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR Am J Neuroradiol. 2013;34:2026–2033. doi: 10.3174/ajnr.A3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hermoye L, Saint-Martin C, Cosnard G, et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 32.McQuillen PS, Barkovich AJ, Hamrick SE, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]