Abstract
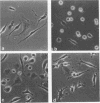
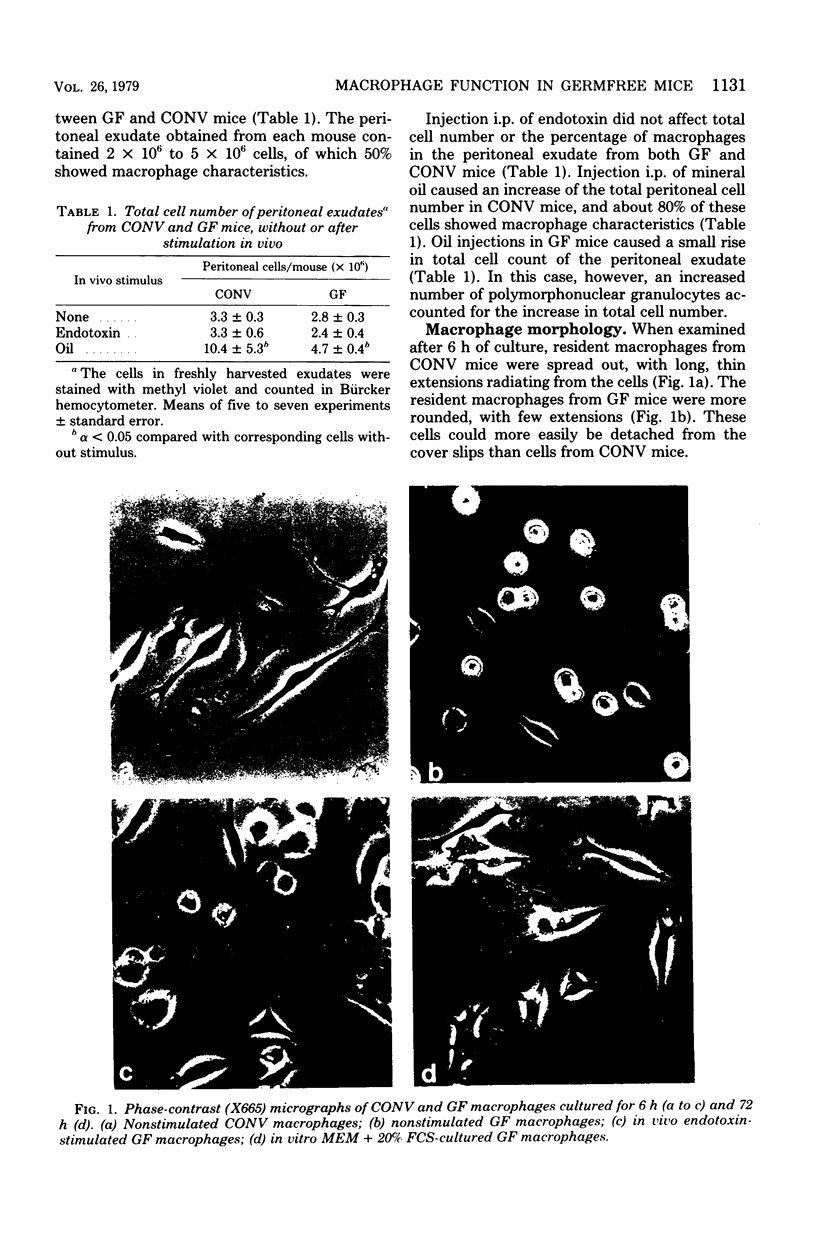
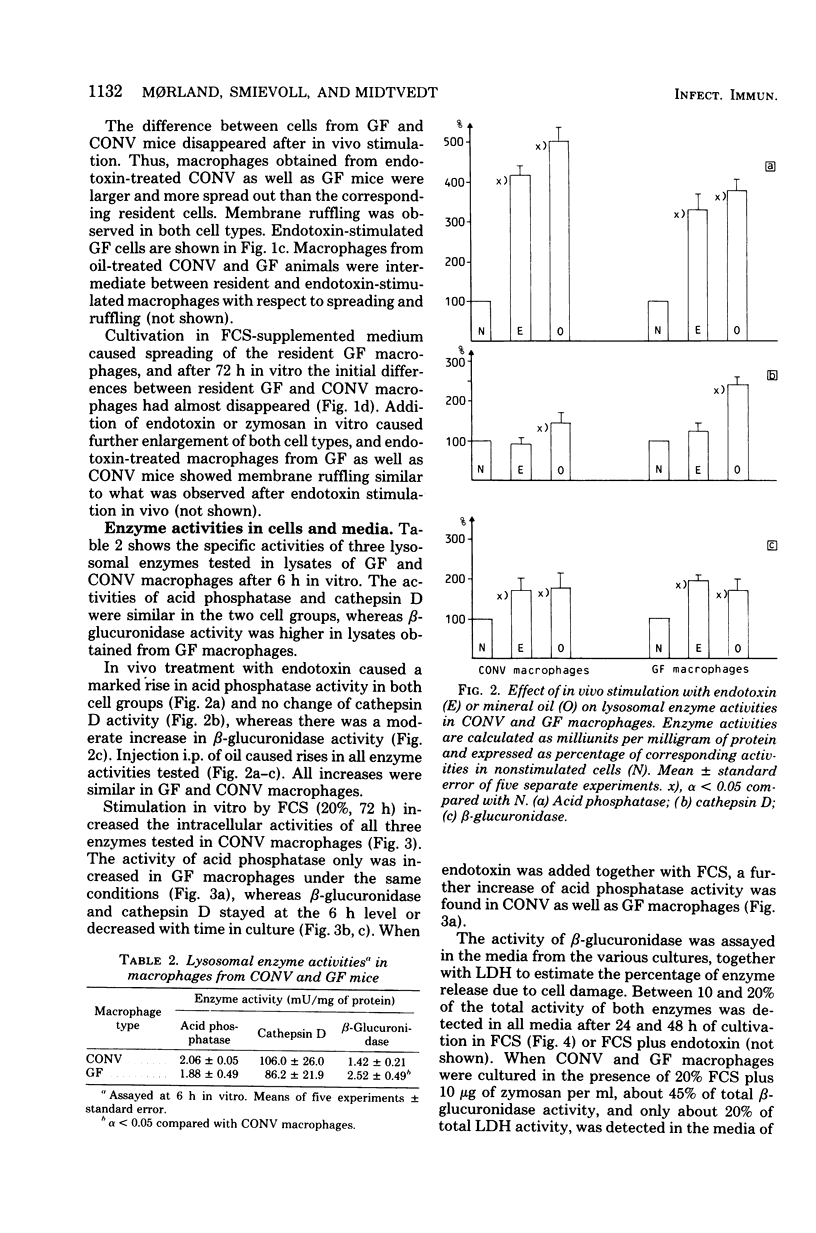
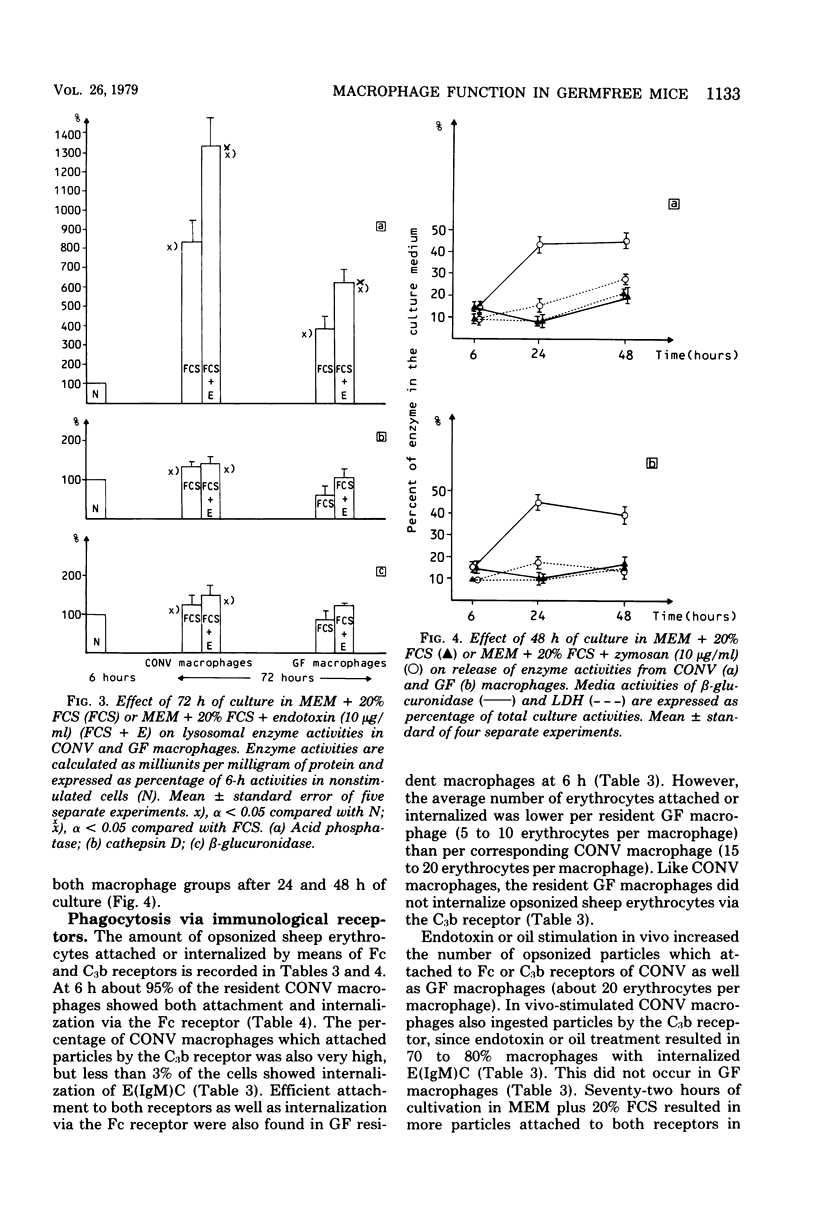
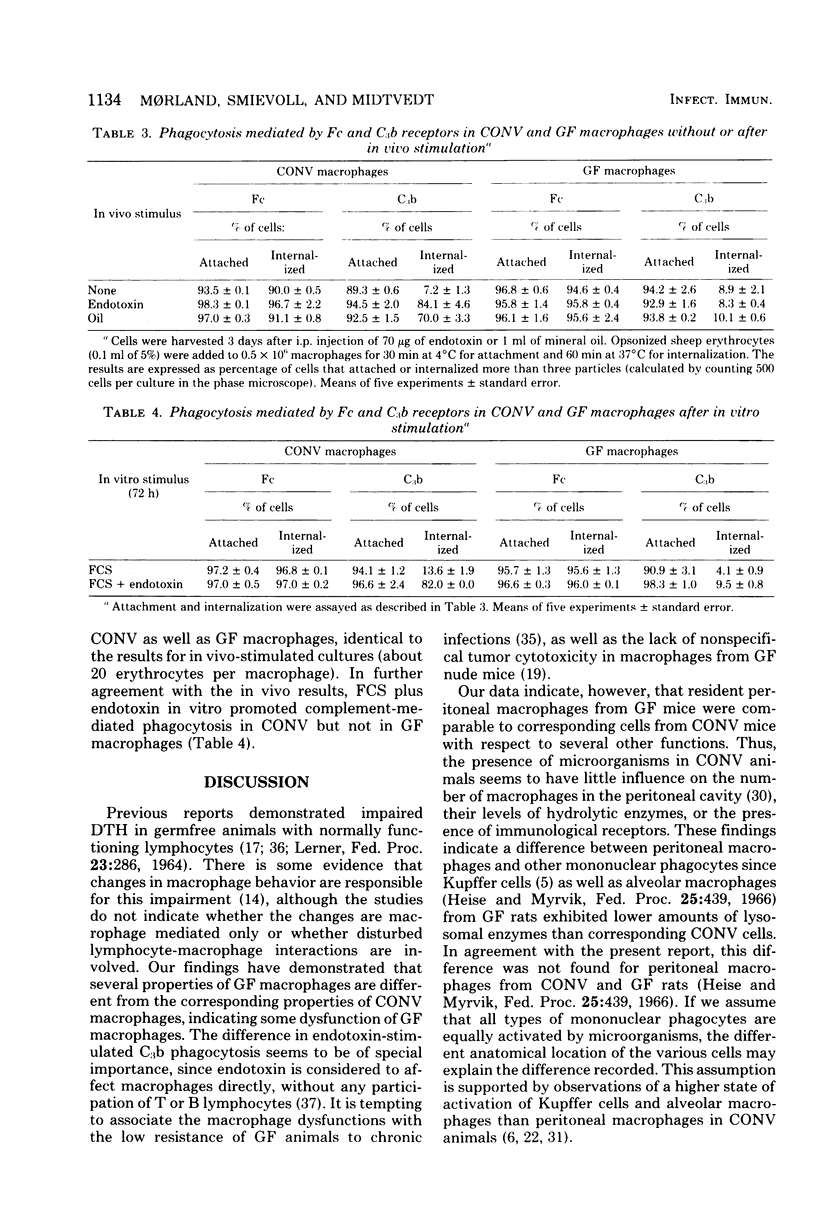
Morphology, lysosomal enzyme activities, and phagocytosis via immunological receptors were tested in peritoneal macrophages from germfree and conventional mice. Nonstimulated macrophages from germfree mice showed less spreading and were more easily detached when seeded on glass than conventional macrophages. The activities of the lysosomal acid phosphatase and cathepsin D were similar in the two cell groups, whereas β-glucuronidase showed higher activity in macrophages from germfree mice. Fc receptor-mediated phagocytosis of opsonized sheep erythrocytes was equally effective in germfree and conventional macrophages, and both cell types attached but did not internalize erythrocytes via the C3b receptor. Intraperitoneal injections of mineral oil caused a significantly higher influx of macrophages in conventional mice than in germfree mice, whereas the influx of polymorphonuclear cells was enhanced in both animals. Stimulation in vivo with oil or Escherichia coli endotoxin increased cell size, spreading ability, membrane ruffling, and lysosomal enzyme activities in macrophages from both conventional and germfree mice. The Fc-mediated phagocytosis was not influenced by stimulation, whereas the capacity to internalize via C3b receptor was triggered in macrophages from conventional mice, but not in corresponding cells from germfree mice. Similar results were obtained after stimulation with endotoxin in vitro. Culture in fetal calf serum for 72 h caused intracellular rises in all three enzyme activities tested in macrophages from conventional mice, whereas only the activity of acid phosphatase was increased in macrophages from germfree mice. Stimulation with zymosan in vitro caused selective release of lysosomal enzyme activity in macrophages from both animal groups. We conclude that peritoneal macrophages from germfree mice share several properties with cells from conventional mice, however, unstimulated β-glucuronidase activity was increased, whereas spreading on glass, chemotactic response, in vitro induction of lysosomal enzymes, and the capacity to internalize via the C3b receptor after stimulation were reduced or absent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abernathy R. S., Landy J. J. Increased resistance to endotoxin in germ-free guinea pigs. Proc Soc Exp Biol Med. 1967 Apr;124(4):1279–1283. doi: 10.3181/00379727-124-31988. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Nichols B. A., Farquhar M. G. Primary lysosomes of blood leukocytes. Front Biol. 1976;45:3–32. [PubMed] [Google Scholar]
- Bauer H., Paronetto F., Burns W. A., Einheber A. The enhancing effect of the microbial flora on macrophage function and the immune response. A study in germfree mice. J Exp Med. 1966 Jun 1;123(6):1013–1024. doi: 10.1084/jem.123.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Midtvedt T. The influence of infection on the content of lysosomal enzymes in rat Kupffer cells and hepatocytes. Acta Pathol Microbiol Scand A. 1976 Sep;84(5):415–420. doi: 10.1111/j.1699-0463.1976.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Berg T., Munthe-Kaas A. C. Lysosomal enzymes in cultured rat Kupffer cells. Exp Cell Res. 1977 Oct 1;109(1):119–125. doi: 10.1016/0014-4827(77)90051-9. [DOI] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T., Barrett A. J. Lysosomes. Essays Biochem. 1976;12:1–40. [PubMed] [Google Scholar]
- Elens A., Wattiaux R. Age-correlated changes in lysosomal enzyme activities: an index of ageing? Exp Gerontol. 1969 Jul;4(2):131–135. doi: 10.1016/0531-5565(69)90036-9. [DOI] [PubMed] [Google Scholar]
- HOROWITZ R. E., BAUER H., PARONETTO F., ABRAMS G. D., WATKINS K. C., POPPER H. THE RESPONSE OF THE LYMPHATIC TISSUE TO BACTERIAL ANTIGEN. STUDIES IN GERMFREE MICE. Am J Pathol. 1964 May;44:747–761. [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W., McGregor D. D. Impaired chemotactic responsiveness of macrophages from gnotobiotic rats. Infect Immun. 1978 Feb;19(2):553–561. doi: 10.1128/iai.19.2.553-561.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Mørland B. Properties of a murine monocytic tumor cell line J-774 in vitro. I. Morphology and endocytosis. Exp Cell Res. 1978 Aug;115(1):53–61. doi: 10.1016/0014-4827(78)90401-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lev M., Battisto J. R. Impaired delayed hypersensitivity in germ-free guinea-pigs. Immunology. 1970 Jul;19(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S. Tumoricidal responses in vitro of peritoneal macrophages from conventionally housed and germ-free nude mice. Cell Immunol. 1976 Mar 1;22(1):176–181. doi: 10.1016/0008-8749(76)90018-6. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Foster R., Pollard M. Colony stimulating activity of serum from germfree normal and leukemic mice. J Cell Physiol. 1967 Aug;70(1):131–132. doi: 10.1002/jcp.1040700118. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Trippestad A. Opsonizing and bactericidal effects of sera from gnotobiotic and conventionalized rats on 32P-labelled E. coli. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(1):1–5. doi: 10.1111/j.1699-0463.1970.tb04264.x. [DOI] [PubMed] [Google Scholar]
- Morland B., Kaplan G. Macrophage activation in vivo and in vitro. Exp Cell Res. 1977 Sep;108(2):279–288. doi: 10.1016/s0014-4827(77)80035-9. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C. Phagocytosis in rat Kupffer cells in vitro. Exp Cell Res. 1976 May;99(2):319–327. doi: 10.1016/0014-4827(76)90589-9. [DOI] [PubMed] [Google Scholar]
- North R. J. The concept of the activated macrophage. J Immunol. 1978 Sep;121(3):806–809. [PMC free article] [PubMed] [Google Scholar]
- Rod T. O., Midtvedt T. Origin of intestinal beta-glucuronidase in germfree, monocontaminated and conventional rats. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):271–276. doi: 10.1111/j.1699-0463.1977.tb01973.x. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: increased lymphokine responsiveness of peritoneal macrophages during acute inflammation. J Immunol. 1978 Mar;120(3):1054–1062. [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorlemmer H. U., Edwards J. H., Davies P., Allison A. C. Macrophage responses to mouldy hay dust, Micropolyspora faeni and zymosan, activators of complement by the alternative pathway. Clin Exp Immunol. 1977 Feb;27(2):198–207. [PMC free article] [PubMed] [Google Scholar]
- Shelton E., Daves S., Hemmer R. Quantitation of strain BALB-c mouse peritoneal cells. Science. 1970 Jun 5;168(3936):1232–1234. doi: 10.1126/science.168.3936.1232. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Stossel T. P. Complement (C3)-activated phagocytosis by lung macrophages. J Immunol. 1978 Apr;120(4):1305–1312. [PubMed] [Google Scholar]
- Smith C. S., Pilgrim H. I., Steinmuller D. The survival of skin allografts and xenografts in germ-free mice. Transplantation. 1972 Jan;13(1):38–41. doi: 10.1097/00007890-197201000-00009. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976 Nov 4;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- Stashak P. W., Baker P. J., Roberson B. S. The serum antibody response to bacteriophage phi chi 174 in germ-free and conventionally reared mice. II. Kinetics of the serum antibody response following primary immunization. Immunology. 1970 Feb;18(2):307–317. [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Impairment and restoration of the delayed type hypersensitivity in germ-free mice. Jpn J Microbiol. 1973 Nov;17(6):533–536. doi: 10.1111/j.1348-0421.1973.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Ueda K., Yamazaki S., Someya S. Studies on tubercle bacillus infection in germ-free mice. J Reticuloendothel Soc. 1972 Nov;12(5):545–563. [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]