Abstract
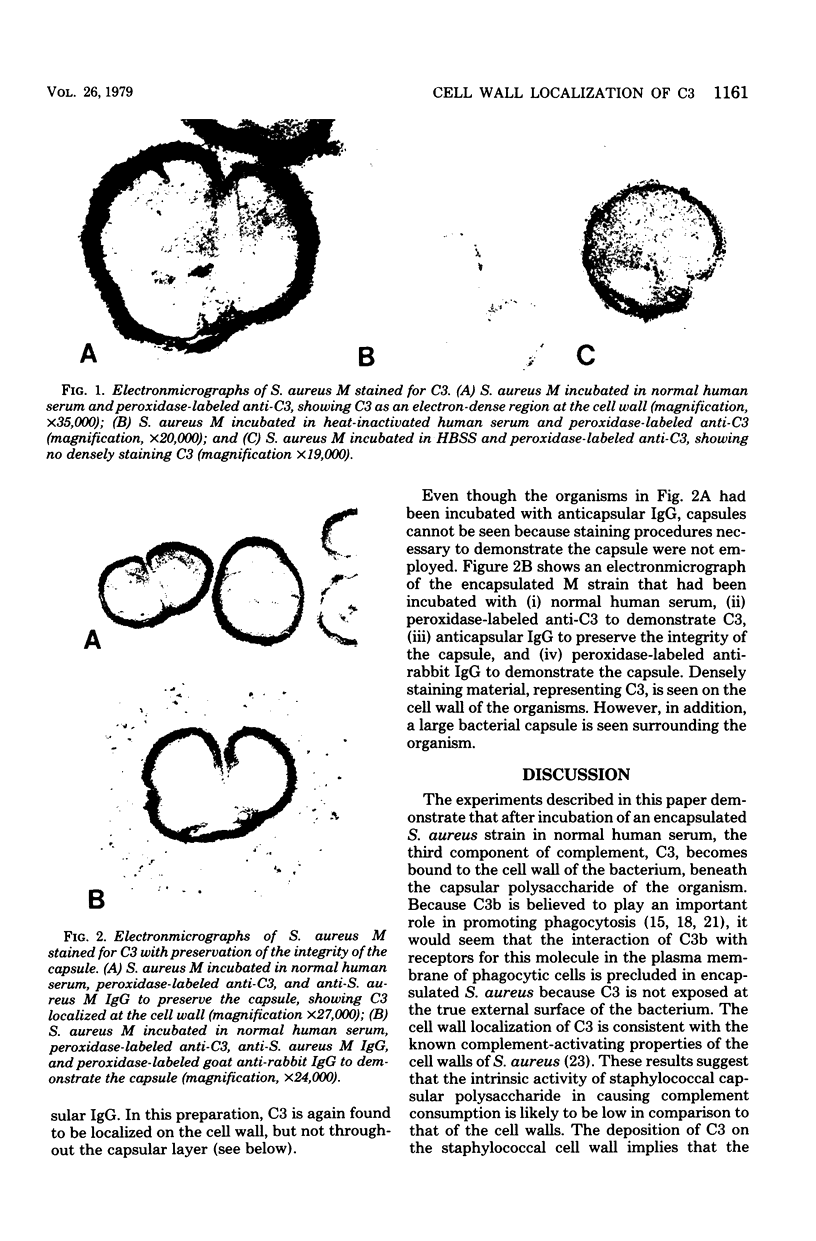
Encapsulated Staphylococcus aureus strains are more virulent than unencapsulated staphylococci, and this phenomenon has been associated with decreased opsonization of encapsulated bacteria by normal human serum. Peptidoglycan, a major cell wall component of S. aureus, has been shown to promote opsonization of this bacterial species by certain components of the serum complement system. However, when the processes of complement activation and opsonization of encapsulated staphylococci have been studied, it has been found that encapsulated bacteria activate complement efficiently and C3 is bacteria associated, yet these organisms are not efficiently phagocytized by human polymorphonuclear leukocytes. In this study, the hypothesis was tested that opsonically active molecules are not on the true external surface of encapsulated organisms but rather are cell wall associated and thus "hidden" from human polymorphonuclear leukocytes. By using immunoelectronmicroscopy, C3 was found to be localized on the cell wall of an encapsulated S. aureus strain after incubation of the organism in normal human serum. When shrinkage of the capsule was prevented by treatment with anticapsular antibody, the C3 was again shown to be cell wall associated and located beneath the bacterial capsule. These results suggest that encapsulated S. aureus may resist phagocytosis because opsonically active C3 molecules are not exposed at the true external surface of the bacterium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Fish A. J., Meuwissen H. J., Frommel D., Good R. A. Localization of immunological complexes fixing beta1C (C3) in germinal centers of lymph nodes. J Exp Med. 1969 Dec 1;130(6):1367–1393. doi: 10.1084/jem.130.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Karakawa W. W., Young D. A. Immunological specificity of heat-stable opsonins in immune and nonimmune sera and their interaction with non-encapsulated and encapsulated strains of Staphylococcus aureus. Infect Immun. 1979 Jul;25(1):175–186. doi: 10.1128/iai.25.1.175-186.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J., NILSSON U., ARONSSON T. Isolation and characterization of two beta1-glycoproteins of human serum. J Exp Med. 1960 Feb 1;111:201–215. doi: 10.1084/jem.111.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaw T. G., Kozel T. R. Opsonization of Cryptococcus neoformans by human immunoglobulin G: masking of immunoglobulin G by cryptococcal polysaccharide. Infect Immun. 1979 Jul;25(1):262–267. doi: 10.1128/iai.25.1.262-267.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R. H., Michael A. F. Properdin anc C3 proactivator: alternate pathway components in human glomerulonephritis. J Clin Invest. 1973 Mar;52(3):634–644. doi: 10.1172/JCI107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melly M. A., Duke L. J., Liau D. F., Hash J. H. Biological properties of the encapsulated Staphylococcus aureus M. Infect Immun. 1974 Aug;10(2):389–397. doi: 10.1128/iai.10.2.389-397.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. C. A capsulate Staphylococcus aureus. J Med Microbiol. 1969 Aug;2(3):253–260. doi: 10.1099/00222615-2-3-253. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Van Oss C. J. Immunoglobulins as aspecific opsonins. II. The influence of specific and aspecific immunoglobulins on the in vitro phagocytosis of noncapsulated, capsulated, and decapsulated bacteria by human neutrophils. J Reticuloendothel Soc. 1971 May;9(5):503–512. [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Human polymorphonuclear leucocyte receptors for staphylococcal opsonins. Immunology. 1977 Aug;33(2):231–239. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Holmes K. M. Staphylococcus aureus cell surface: capsule as a barrier to bacteriophage adsorption. Infect Immun. 1979 Feb;23(2):549–552. doi: 10.1128/iai.23.2.549-552.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]