Abstract
Objective
To measure trends in the pulmonary tuberculosis burden between 2002 and 2011 and to assess the impact of the DOTS (directly observed treatment, short-course) strategy in Cambodia.
Methods
Cambodia’s first population-based nationwide tuberculosis survey, based on multistage cluster sampling, was conducted in 2002. The second tuberculosis survey, encompassing 62 clusters, followed in 2011. Participants aged 15 years or older were screened for active pulmonary tuberculosis with chest radiography and/or for tuberculosis symptoms. For diagnostic confirmation, sputum smear and culture were conducted on those whose screening results were positive.
Findings
Of the 40 423 eligible subjects, 37 417 (92.6%) participated in the survey; 103 smear-positive cases and 211 smear-negative, culture-positive cases were identified. The weighted prevalences of smear-positive tuberculosis and bacteriologically-positive tuberculosis were 271 (95% confidence interval, CI: 212–348) and 831 (95% CI: 707–977) per 100 000 population, respectively. Tuberculosis prevalence was higher in men than women and increased with age. A 38% decline in smear-positive tuberculosis (P = 0.0085) was observed with respect to the 2002 survey, after participants were matched by demographic and geographical characteristics. The prevalence of symptomatic, smear-positive tuberculosis decreased by 56% (P = 0.001), whereas the prevalence of asymptomatic, smear-positive tuberculosis decreased by only 7% (P = 0.7249).
Conclusion
The tuberculosis burden in Cambodia has declined significantly, most probably because of the decentralization of DOTS to health centres. To further reduce the tuberculosis burden in Cambodia, tuberculosis control should be strengthened and should focus on identifying cases without symptoms and in the middle-aged and elderly population.
Résumé
Objectif
Mesurer les tendances de la prévalence de la tuberculose pulmonaire entre 2002 et 2011 et évaluer l'impact de la stratégie DOTS (traitement de brève durée sous surveillance directe) au Cambodge.
Méthodes
La première étude sur la tuberculose à l'échelle nationale, axée sur la population cambodgienne et basée sur un échantillonnage par grappes et à plusieurs degrés a été menée en 2002. La deuxième étude sur la tuberculose, incluant 62 grappes, a ensuite été menée en 2011. Les participants, âgés de 15 ans ou plus, ont été testés pour la tuberculose pulmonaire active avec une radiographie pulmonaire et/ou des symptômes tuberculeux. Pour confirmer le diagnostic, des frottis et des cultures d'expectoration ont été effectués pour les participants dont les résultats de dépistage se sont avérés positifs.
Résultats
Sur les 40 373 sujets éligibles, 37 417 (92,6%) ont participé à l'étude; 103 cas à frottis positif et 211 cas à culture positive et à frottis négatif ont été identifiés. Les prévalences pondérées de la tuberculose à frottis positif et de la tuberculose bactériologiquement positive étaient de 271 (intervalle de confiance de 95%, IC: 212-348) et de 831 (IC de 95%: 707–977) pour 100 000 habitants, respectivement. La prévalence de la tuberculose était plus élevée chez les hommes que chez les femmes et augmentait avec l'âge. Une baisse de 38% de la tuberculose à frottis positif (P = 0,0085) a été observée par rapport à l'étude de 2002, après que les participants ont été associés à des caractéristiques démographiques et géographiques. La prévalence de la tuberculose symptomatique à frottis positif a diminué de 56% (P = 0,001), alors que la prévalence de la tuberculose asymptomatique à frottis positif a diminué de seulement 7% (P = 0,7249).
Conclusion
Au Cambodge, la prévalence de la tuberculose a fortement diminué, probablement à cause de la décentralisation du DOTS vers les centres de santé. Pour continuer à réduire la prévalence de la tuberculose au Cambodge, la lutte antituberculoseuse doit être renforcée et se concentrer sur l'identification des cas sans symptômes et dans la population d'âge moyen et âgée.
Resumen
Objetivo
Medir las tendencias de la carga de tuberculosis pulmonar entre 2002 y 2011, y evaluar el impacto de la estrategia DOTS (tratamiento directamente observado, curso corto) en Camboya.
Métodos
En 2002 se realizó la primera encuesta de la tuberculosis demográfica a nivel nacional de Camboya, basada en pruebas de grupos multietapas. La segunda encuesta de la tuberculosis, que abarcó 62 grupos, se llevó a cabo en 2011. Se realizaron pruebas de detección de la tuberculosis pulmonar activa mediante radiografía de tórax o de síntomas de la tuberculosis a participantes mayores de 15 años. Para confirmar el diagnóstico, se realizó una baciloscopia y un cultivo del esputo en aquellos participantes cuyos resultados de la evaluación fueron positivos.
Resultados
De los 40 423 participantes idóneos, 37 417 (92,6%) participaron en la encuesta. Se identificaron 103 casos bacilíferos y 211 casos no bacilíferos con cultivo positivo. Las prevalencias ponderadas de tuberculosis bacilífera y tuberculosis bacteriológicamente positiva fueron 271 (intervalo de confianza del 95%, IC: 212–348) y 831 (95% CI: 707–977) por 100 000 población, respectivamente. La prevalencia de la tuberculosis fue mayor en hombres que en mujeres y aumentó con la edad. Se observó una disminución del 38% en la tuberculosis bacilífera (P = 0,0085) con respecto a la encuesta de 2002 tras agrupar a los participantes por características demográficas y geográficas. La prevalencia la tuberculosis bacilífera y sintomática disminuyó en un 56% (P = 0,001), mientras que la prevalencia de la tuberculosis bacilífera asintomática solo se redujo en un 7% (P = 0,7249).
Conclusión
La carga de tuberculosis en Camboya ha disminuido considerablemente, lo cual probablemente se debe a la descentralización de la estrategia DOTS en los centros de salud. Para reducir aún más la carga de la tuberculosis en Camboya, debe reforzarse el control de la tuberculosis y centrarse en la identificación de casos sin síntomas y en la población anciana y de mediana edad.
ملخص
الغرض
قياس الاتجاهات في عبء السل الرئوي في الفترة بين عامي 2002 و2011، وتقييم أثر استراتيجية DOTS (استراتيجية المعالجة قصيرة الأمد تحت الإشراف المباشر) في كمبوديا.
الطريقة
تم إجراء المسح السكاني الأول للسل على الصعيد الوطني في كمبوديا، استناداً إلى عينات عنقودية متعددة المراحل، في عام 2002. وتلاه المسح الثاني للسل، الذي اشتمل على 62 مجموعة، في عام 2011. وتم فحص المشاركين الذين تبلغ أعمارهم 15 عاماً فأكبر لرصد السل الرئوي النشط باستخدام التصوير الإشعاعي و/أو لرصد أعراض السل. ومن أجل تأكيد التشخيص، تم إجراء اختبار لطاخة البلغم ومزرعة للأشخاص الذين كانت نتائج فحصهم إيجابية.
النتائج
شارك 37417 (92.6 %) من إجمالي 40373 مريضاً مؤهلاً في المسح؛ وتم تحديد 103 حالة إيجابية للطاخة و211 حالة سلبية للطاخة وإيجابية للمزرعة. وبلغت معدلات الانتشار المرجح للسل الإيجابي للطاخة والسل الإيجابي جرثومياً 271 حالة (فاصل الثقة 95 %، فاصل الثقة: 212 - 348) و831 حالة (فاصل الثقة: 95 %، فاصل الثقة: 707 - 977) لكل 100 ألف نسمة، على التوالي. وكان معدل انتشار السل مرتفعاً لدى الرجال عنه لدى النساء وازداد مع زيادة العمر. ولوحظ حدوث انخفاض بنسبة 38 % في السل الإيجابي للطاخة (الاحتمال = 0.0085) فيما يتعلق بالمسح الذي أجري في عام 2002، بعد مضاهاة المشاركين حسب الخصائص الديمغرافية والجغرافية. وانخفض معدل انتشار السل الإيجابي للطاخة المقترن بأعراض بنسبة 56 % (الاحتمال = 0.001)، بينما انخفض معدل انتشار السل الإيجابي للطاخة غير المقترن بأعراض بنسبة 7 % (الاحتمال = 0.7249).
الاستنتاج
انخفض عبء السل في كمبوديا بشكل كبير، ويعزى ذلك في الغالب إلى اللامركزية في استراتيجية المعالجة قصيرة الأمد تحت الإشراف المباشر في المراكز الصحية. ولتحقيق قدر أكبر من الانخفاض في عبء السل في كمبوديا، ينبغي تعزيز مكافحة السل وأن تركز على تحديد الحالات التي لا تقترن بأعراض والحالات التي تندرج تحت فئة أواسط العمر وكبار السن.
摘要
目的
衡量柬埔寨2002至2011年肺结核负担的趋势并评估DOTS(短期直接观察治疗)策略的影响。
方法
2002年,柬埔寨在多级集群抽样的基础上开展了第一次基于人群的全国肺结核病调查。2011年,该国进行了第二次肺结核调查(包括62个集群)。对年满15岁的参与者进行带有胸片和/或肺结核症状的活跃肺结核病筛查。为确认诊断,对筛查结果阳性的参与者进行痰涂片镜检和培养。
结果
在40423名符合条件的受试者中,37417(92.6%)名参与了调查;确认了103例涂片阳性病例和211例涂片阴性、培养阳性病例。涂片阳性肺结核病和病菌阳性肺结核病的加权患病率分别是每10万人口271(95%置信区间,CI:212-348)和831(95% CI:707-977)例。男性的肺结核患病率比女性高,且随年龄增长。将参与者按照人口统计和地理特征匹配之后,发现较之 2002 年的调查,涂片阳性肺结核患病率下降38%(P = 0.0085)。有症状的涂片阳性肺结核患病率降低56%(P = 0.001),无症状的涂片阳性肺结核患病率仅降低7%(P = 0.7249)。
结论
柬埔寨的肺结核负担显著降低,大部分是因为将DOTS分散到卫生中心。 要进一步降低柬埔寨的肺结核负担,应加强肺结核控制,并且以识别中年以及老年人群中无症状的病例为重点。
Резюме
Цель
Определить тенденции в бремени легочного туберкулеза в период 2002-2011 годов и оценить воздействие стратегии DOTS (лечение под непосредственным наблюдением коротким курсом) в Камбодже.
Методы
Первое общенациональное обследование населения для выявления туберкулеза методом многоступенчатой кластерной выборки было проведено в Камбодже в 2002 году. Второе обследование по туберкулезу, охватывающее 62 кластера, было выполнено в 2011 году. Участники в возрасте от 15 лет и старше были обследованы на наличие активного туберкулеза легких с помощью рентгенографии грудной клетки и/или выявления симптомов туберкулеза. Для подтверждения диагноза у людей с положительным результатом скрининга было проведен анализ мокроты и ее посев на выделение культуры.
Результаты
Из 40 373 человек, имеющих право на участие в обследовании, в нем приняли участие 37 417 человек (92,6%); было выявлено 103 случая заболевания с положительным результатом микроскопии мокроты и 211 случаев — с отрицательным результатом для микроскопии мокроты и положительным для посева на выделение культуры. Средневзвешенная распространенность туберкулеза с положительным результатом мазка мокроты и с открытой формой (БК+) составила 271 случай (95% доверительный интервал, ДИ: 212-348) и 831 случай (95%-ный ДИ: 707-977) на 100 000 населения соответственно. Распространенность туберкулеза была выше у мужчин, чем у женщин, и увеличивалась с возрастом. По сравнению с обследованием 2002 года наблюдалось снижение на 38% количества случаев туберкулеза с положительным результатом мазка (р = 0,0085), после того как участники были сопоставлены по демографическим и географическим характеристикам. Распространенность симптоматического туберкулеза с положительным мазком снизилась на 56% (р = 0,001), в то время как распространенность бессимптомного туберкулезом с положительным мазком снизилась всего на 7% (P = 0,7249).
Вывод
Бремя туберкулеза в Камбодже значительно снизилось, что скорее всего связано с децентрализацией стратегии DOTS до уровня медицинских центров. В целях дальнейшего сокращения бремени туберкулеза в Камбодже борьба с туберкулезом должна быть усилена и сосредоточена на выявлении бессимптомных случаев среди населения среднего и старшего возраста.
Introduction
Tuberculosis remains a major global health problem, with 8.6 million estimated incident cases and 1.3 million estimated deaths in 2012.1 Cambodia ranks second among the 22 countries with a high tuberculosis burden. Since the World Health Organization (WHO) adopted the DOTS (directly observed treatment, short-course) strategy in 1994,2 which is based on the passive detection of cases of smear-positive tuberculosis, this policy has been the foundation of global tuberculosis control. Cambodia’s national tuberculosis control programme introduced DOTS in hospitals in 1994 and decentralized it to primary care health centres between 1999 and 2004 with the technical support of WHO and the Japan International Cooperation Agency.
To evaluate the impact of the national tuberculosis control programme in Cambodia, Cambodia conducted its first national tuberculosis prevalence survey in 2002 (hereafter referred to as the 2002 survey), during the early stage of DOTS decentralization. The results revealed a prevalence of smear-positive pulmonary tuberculosis of 362 cases per 100 000 people aged 10 years or older for (269 per 100 000 overall). For bacteriologically-positive (i.e. smear-positive tuberculosis case and/or smear-negative, culture-positive tuberculosis case) tuberculosis among individuals aged 10 years or older, the prevalence was shown to be 1208 per 100 000.3
In 2005, the year after DOTS became available at all health centres throughout the country (i.e. not just in the 141 tuberculosis units that existed already, primarily in hospitals), the rate of notification of new smear-positive tuberculosis cases peaked and subsequently stagnated for three years.4 In the following years treatment success rates were consistently above 90% thanks to the efforts of the national tuberculosis control programme.1 The question then is whether the prevalence of tuberculosis declined, and if so, by how much with respect to the first tuberculosis survey. To respond to these questions, the national tuberculosis control programme conducted a second national tuberculosis prevalence survey in 2010–2011(hereafter referred to as the 2011 survey). The objectives of the study presented here were to measure the current prevalence of smear-positive pulmonary tuberculosis and bacteriologically-positive pulmonary tuberculosis and to evaluate the change in tuberculosis prevalence in Cambodia between the 2002 and 2011 surveys.
Methods
Survey design
A population-based, cross-sectional survey was conducted in 2010–2011. Like the 2002 survey, it was based on multistage cluster sampling and stratified by urban, rural and remote areas.5,6
To be able to meet the study objectives, we needed to detect a statistically significant reduction of 42% or more, with a relative precision of 25%, in the prevalence of smear-positive tuberculosis among adults (i.e. people 15 years of age or older) between surveys, we calculated that a sample of approximately 38 400 adults was needed. This was based on a 5% margin of error for an observed prevalence of 256 per 100 000, a design effect of 1.43 and an assumed participation rate of 90%. The target cluster size was set to 640. Thus, the number of clusters was calculated to be 60 for urban and rural clusters. In addition, two remote clusters in the four provinces that were excluded from the 2002 survey, because they were difficult to reach and because of security concerns, were included.
Multistage sampling with stratification was conducted as follows. First the country was stratified into urban, rural and remote areas. Within each stratum, first districts, then communes and finally villages were selected, with sampling probability proportional to size, based on the 2008 population census.7 Accordingly, 13 clusters from urban areas, 47 clusters from rural areas and two clusters from remote areas were selected.
The population eligible for the survey included all residents aged 15 years or older in the selected clusters who were present during the survey. A resident was defined as a person who had lived in the selected household for at least two weeks.
Survey procedures
Two teams performed the fieldwork from December 2010 to September 2011. In each cluster, data were collected during a seven-day period. First, the survey team conducted a house-to-house census based on the household list provided by the village authorities. The purpose was to collect information on family members, check for eligibility and inform survey participants of the purpose of the survey and the procedures involved. For families that were not available during the visit of the team, authorities or neighbours informed the team if these families were eligible for the survey. Eligible participants who were capable of walking were invited to the field operation site. Invited participants who did not show up at the site were classified as absent. Eligible disabled people were visited by the interviewers in their homes, where they were questioned and asked to submit two samples for sputum examination irrespective of their respiratory symptoms. No chest X-rays were performed at home.
At the field operation site, participants were interviewed by a trained interviewer, mainly nurses and pharmacists, using a structured questionnaire that included a section on tuberculosis symptoms, health seeking behaviour and treatment history. Chest X-rays were performed by radiological technicians at the site with a portable machine. The participants were then screened for eligibility for sputum collection, which depended on the results of chest X-rays and on the presence or absence of symptoms of tuberculosis (cough for two weeks or longer and/or blood in the sputum, as recommended by WHO).5 Anyone with abnormal findings in the lungs or mediastinum – except for a single small calcified nodule or unilateral pleural adhesions – was considered eligible for sputum collection. Over interpretation of chest X-rays was encouraged. Eligible participants were asked to submit two sputum specimens: one was obtained after the interview and the other was collected by the survey participant early the following morning in a cup provided by the laboratory staff. Survey participants who did not turn up to the field operation site the next morning with the second sputum sample were visited at home by the team. Participants who gave two samples received a small gift. The sputum quality was later determined in the laboratory.
Diagnostic procedure
Sputum specimens collected in the field were stored on ice, transferred to one of two designated culture centres and examined by smear and culture within five days of collection. All sputum smears were screened using fluorescence microscopy. Two solid culture media (3% Ogawa and Kudoh)8 were used for each specimen. The Capilia tuberculosis assay was used to identify Mycobacterium tuberculosis.9 To compare the prevalence of smear-positive tuberculosis in the two surveys, we re-examined all the microscopy slides from the subjects with smear-positive and/or culture-positive results as well as with a chest X-ray suggestive of active tuberculosis by conventional microscopic examination with Ziehl-Neelsen staining.
Two respiratory physicians and/or radiologists interpreted the chest X-rays. A third expert resolved discrepancies in their interpretation. A central medical panel including international experts on respiratory diseases determined the final diagnosis, with reference to the survey tuberculosis case definitions in Box 1, on the basis of both the bacteriological examinations and of the findings on chest X-ray.
Box 1. Case definitions for the 2011 national tuberculosis prevalence survey, Cambodia.
Smear-positive tuberculosis case:
two positive sputum smears and a sputum culture-negative for mycobacteria other than Mycobacterium tuberculosis, or
one positive sputum smear plus a sputum culture-positive for M. tuberculosis, or
one positive sputum smear and a chest X-ray consistent with tuberculosis.
Sputum smear-negative, culture-positive tuberculosis case:
negative sputum smears and at least one sputum culture-positive for five or more colonies of M. Tuberculosis, or
negative sputum smear and one sputum culture-positive for four or fewer colonies of M. tuberculosis, and a chest X-ray consistent with tuberculosis.
Bacteriologically-positive tuberculosis case:
smear-positive tuberculosis case or smear-negative, culture-positive tuberculosis case.
Data analysis
All data were entered into EpiInfo 3.5 (Centers for Disease Control and Prevention, Atlanta, United States of America). Some key data were double-entered for quality control; discrepancies were resolved by checking against the raw data. Prevalences, odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated by using logistic regression models incorporating sampling designs (stratification, clusters and weights) in STATA version 12 (StataCorp LP, College Station, USA). ORs are commonly used for cross-sectional studies data such as prevalence surveys.10 For comparison of prevalences between the surveys, we used OR as approximation of prevalence ratio because odds [prevalence/(1−prevalence)] is an approximation of prevalence, since tuberculosis is a rare disease. Weights defined as the inverse of the number of participants at each cluster were applied to the 2011 survey data. For comparison in prevalence between 2002 and 2011 surveys, four different strata (urban and rural for both 2002 and 2011) were incorporated in the models. Adjusted weights incorporating the proportion of the eligible subjects aged 15 years or older at each cluster were applied to the 2002 survey data in the models, because the sampling of the 2002 survey was based on all age population.3
Ethics approval
The survey protocol was approved by the National Ethics Committee for Health Research, Ministry of Health, Cambodia. Written informed consent was obtained from each survey participant or his or her guardian.
Comparison of survey results
To compare the results between the 2002 and 2011 surveys, we used data only from people aged 15 years or older who were residents of 20 of Cambodia’s 24 provinces (the four remote provinces were excluded). Because tuberculosis cases in the youngest age group were so few, the youngest group spanned from 15 to 29 years and the remaining age groups had a 10-year span: 30–39, 40–49, 50–59, 60–69 and over 70 years.
Results
Prevalence
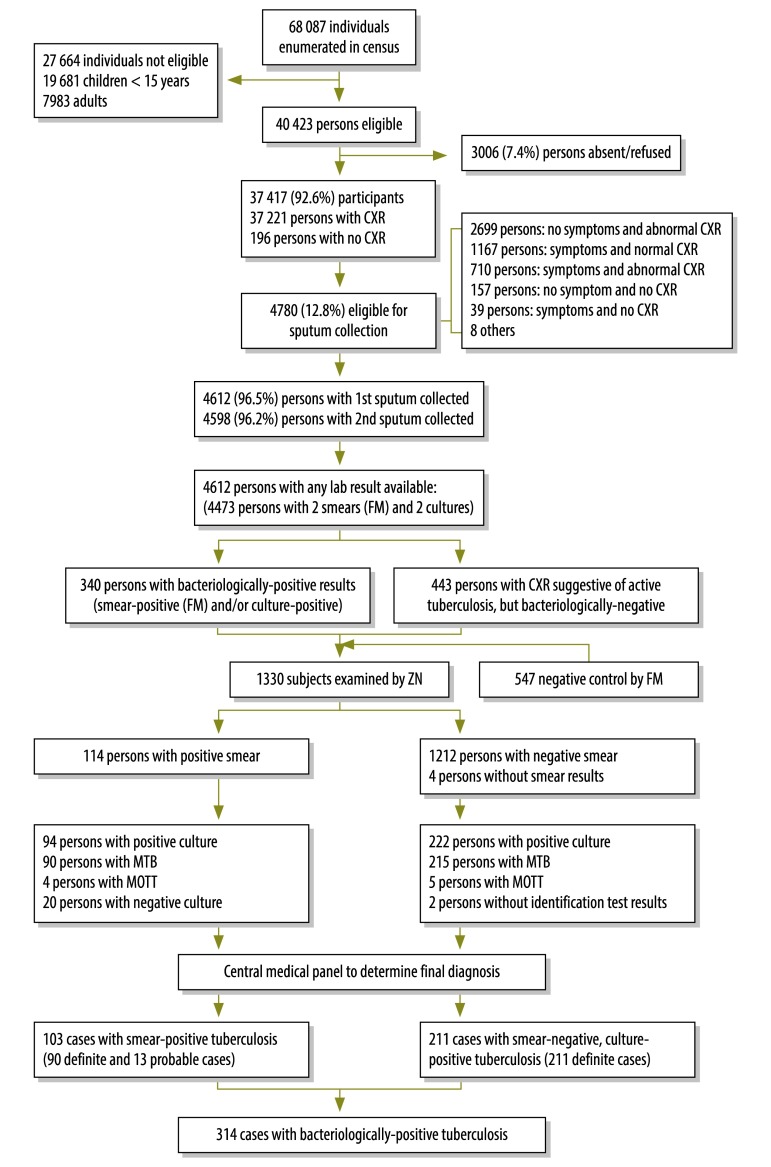
Of the 40 423 eligible subjects, a total of 37 417 (92.6%) participants (17 007 males and 20 410 females) were screened for active pulmonary tuberculosis. Of these, 12.8% (4780/37 417) were considered eligible for sputum examination. At least one sputum specimen was provided by 96.5% (4612/4780) of those eligible. A total of 114 subjects were smear-positive; 222 were smear-negative but culture-positive. Although in nine cases the culture was positive for mycobacteria other than Mycobacterium tuberculosis, these subjects were assessed to see if they met the survey case definitions. In the end, the central medical panel determined that 103 smear-positive tuberculosis cases and 211 smear-negative, culture-positive tuberculosis cases met the survey case definitions (Fig. 1).
Fig. 1.
Outline of the second national tuberculosis cross-sectional survey in Cambodia, 2011
CXR: chest X-ray; FM: fluorescent microscopy; MOTT, mycobacteria other than Mycobacterium tuberculosis; MTB: Mycobacterium tuberculosis; ZN: Ziehl-Neelsen.
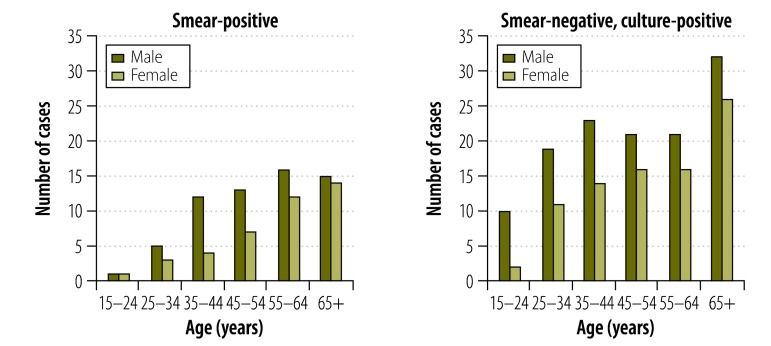
The number of smear-negative, culture-positive tuberculosis cases identified was double the number of smear-positive tuberculosis cases. The ratio of males to females among smear-positive tuberculosis cases was 15.1:10, and participants 55 years or older (16.8% of all participants) accounted for 55.3% (57/103) of such cases. Among cases with smear-negative, culture-positive tuberculosis, the ratio of males to females was 14.8:10, and cases 55 years or older accounted for 45% (95/211) of the total (Fig. 2). Only 43.7% (45/103) of the smear-positive tuberculosis cases and 22.7% (48/211) of the smear-negative, culture-positive tuberculosis cases presented symptoms of pulmonary tuberculosis. Of all the bacteriologically-positive tuberculosis cases, 8.3% (26/314) had a history of tuberculosis, 1.9% (6/314) were receiving treatment and 22.6% (71/314) did not report any cough.
Fig. 2.
Age and sex distribution of identified tuberculosis cases in Cambodia, 2011
The prevalence of smear-positive tuberculosis among individuals 15 years or older was 271 per 100 000. Prevalence was higher in men than in women: 361 versus 197 cases per 100 000, respectively. The prevalence rate for bacteriologically-positive tuberculosis was 831 cases per 100 000 people overall, 1097 cases in men and 609 in women. Taken into account that barely any children aged 10–14 years had smear-positive tuberculosis in the 2002 survey, we assumed that no children younger than 15 years had smear-positive tuberculosis in the 2011 survey. Therefore we estimated the overall prevalence of smear-positive tuberculosis to be 183 per 100 000 people (Table 1).
Table 1. Tuberculosis prevalence in the second national tuberculosis cross-sectional survey, Cambodia, 2011.
| Diagnostic confirmation by age | Prevalence per 100 000 peoplea (95% CI) | No. of estimated casesb |
|---|---|---|
| ≥ 15 years of age | ||
| Smear-positive | ||
| All | 271 (212–348) | 26 204 |
| Male | 361 (265–493) | |
| Female | 197 (127–303) | |
| Smear-negative, culture-positive | ||
| All | 560 (458–685) | 54 031 |
| Male | 736 (587–922) | |
| Female | 413 (319–533) | |
| Bacteriologically-positive | ||
| All | 831 (707–977) | 80 234 |
| Male | 1097 (895–1344) | |
| Female | 609 (486–763) | |
| All agesc | ||
| Smear-positive | 183 (142–234) |
CI: confidence interval.
a Prevalence was calculated for the participants and then extrapolated to per 100 000 people.
b The population aged 15 years or older was 9 654 382.11
c Estimated on the assumption that there was no smear-positive tuberculosis in children aged less than 15 years, and using 67.26% as the proportion of the adults aged 15 years or older based on the survey census data
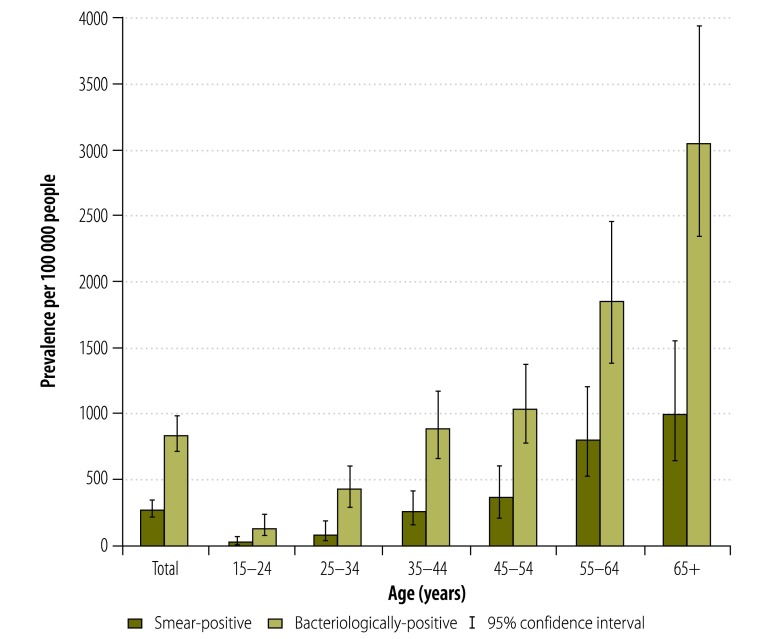
The prevalence of both smear-positive and bacteriologically-positive tuberculosis increased sharply with age, from 17.5 (95% CI: 4.3–71.2) and 130 cases per 100 000 (95% CI: 74–227), respectively, in people aged 15 to 24 years, to 1007 (95% CI: 653–1550) and 3046 (95% CI: 2352–3936) cases per 100 000, respectively, in those aged 65 years or older. Among people aged 35 to 44 years, the prevalence of bacteriologically-positive tuberculosis reached almost 1% (Fig. 3).
Fig. 3.
Tuberculosis prevalence by age, Cambodia, 2011
2002 survey versus 2011 survey
When comparing the same age groups and provinces, the prevalence of smear-positive tuberculosis was reduced by 38%: from 437 cases per 100 000 in 2002 to 272 in 2011 (P = 0.0085). Similarly, the prevalence of bacteriologically-positive tuberculosis was reduced by 45%, from 1497 to 820 cases per 100 000 (P < 0.0001) (Table 2).
Table 2. Tuberculosis prevalence in people aged 15 years or older in national cross-sectional surveys in Cambodia, 2002 and 2011.
| Diagnostic confirmation | Prevalence per 100 000 people (95% CI) |
Reduction (%) | |
|---|---|---|---|
| 2002a | 2011a | ||
| Smear-positive | 437 (342–558) | 272 (211–351) | 37.7 |
| Bacteriologically-positive | 1497 (1238–1808) | 820 (694–968) | 45.2 |
CI: confidence interval.
a Matched group aged 15 years or older in 20 provinces.
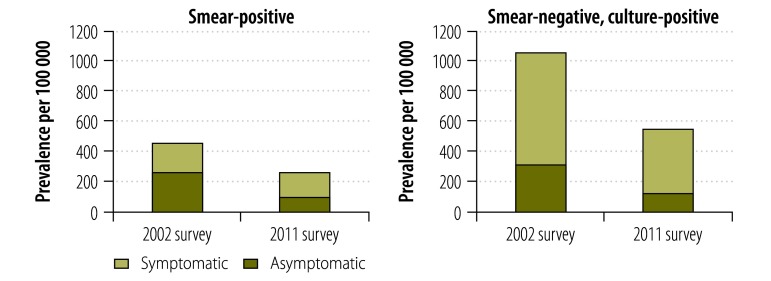
The prevalence of smear-positive tuberculosis cases with symptoms of presumptive tuberculosis decreased by 56%, from 272 to 120 cases per 100 000 (P = 0.001), whereas the prevalence of smear-positive tuberculosis without symptoms decreased by only 7%, from 165 to 153 cases per 100 000 (P = 0.7249) (Fig. 4). Among smear-negative, culture-positive tuberculosis cases, significant reductions were observed in cases with and without symptoms of pulmonary tuberculosis. Prevalence dropped by 60% (from 314 to 126 cases per 100 000, P = 0.0002) and by 44% (from 746 to 421 cases per 100 000, P = 0.0011), respectively (Fig. 4).
Fig. 4.
Tuberculosis prevalence in national tuberculosis surveys, Cambodia, 2002 and 2011
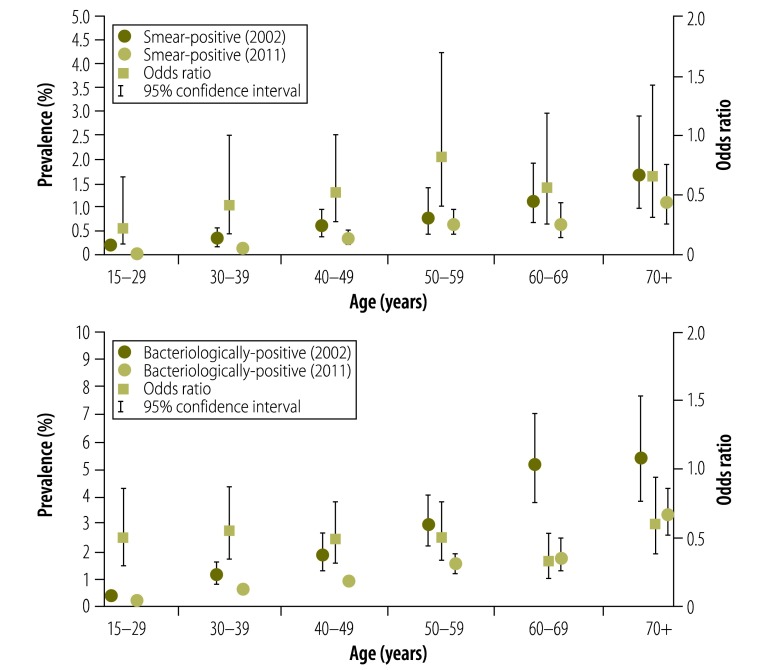
The prevalence of tuberculosis cases in the 2011 survey and ORs of the age-specific prevalence in 2011 to that in 2002 are shown in Fig. 5. Among people aged 15–29 years, a significant reduction in the prevalence of smear-positive tuberculosis was noted (OR: 0.23; 95% CI: 0.08–0.66), while no significant reductions were observed among people aged 30 years or older, although the ORs were less than 1.0. The prevalence of bacteriologically-positive tuberculosis declined significantly in all age groups.
Fig. 5.
Tuberculosis prevalence by age in two national tuberculosis surveys, Cambodia, 2002 and 2011
Discussion
The present study shows that the prevalence of tuberculosis has declined significantly over the past nine years in Cambodia. This decline took place between the initial and final stages of decentralization of the DOTS programme. The 2002 and 2011 tuberculosis surveys in Cambodia are repeat national surveys, which offer the opportunity to assess the epidemiological impact of decentralization of the DOTS strategy by a consistent, proper screening method. Only two studies based on repeat surveys after the DOTS strategy was deployed have been published. One of them, conducted in the Philippines,12 focused on DOTS expansion with the involvement of the private sector in tuberculosis control; the other one was a study conducted in a rural area in southern India.13 Several studies have evaluated the impact of DOTS by using mathematical models14,15or surveillance data.16,17 The expected decline of 5–10% per year in incidence has not been achieved in 22 countries with a high tuberculosis burden that have implemented DOTS, but tuberculosis prevalence in 15 of these countries has decreased.18
Although our study design does not allow conclusions to be drawn about the cause of the reduction in tuberculosis prevalence, this may be attributable, at least partly, to the decentralization of DOTS to health centres between 1999 and 2004 and to a consistently high (> 90%) treatment success rate. The following facts suggest that this may be the case. First, during DOTS decentralization the national tuberculosis control programme aimed to detect new smear-positive tuberculosis cases. In the beginning notification of such cases increased dramatically, from 14 570 in 1998 to 21 001 at the peak, in 2005. However, the increase in the notification of new smear-positive cases was followed by stagnation and a decline to 15 812 in 2011. This occurred despite continuing efforts to increase case detection through community-based DOTS, public–private mixed DOTS, cross-referrals for tuberculosis cases co-infected with human immunodeficiency virus (HIV) and strengthened case-finding to detect smear-negative tuberculosis cases by means of chest X-rays. The decline in notifications might reflect the reduction in tuberculosis prevalence that the 2011 survey showed. Second, the public sector has played a major role in tuberculosis detection and treatment in Cambodia. The 2011 survey showed that 92.5% (74/80) of tuberculosis cases undergoing treatment at the time of the survey and 92.6% (1369/1478) of previous tuberculosis cases had been treated at public hospitals or health centres.6 In Cambodia, the DOTS programme was initiated in the public sector after the long civil war in the 1970s and the 1980s and before the large-scale development of the private sector. Finally, the prevalence of symptomatic tuberculosis decreased much more than that of asymptomatic tuberculosis. It is plausible that the DOTS strategy, which relies mainly on passive case detection, is more effective at reducing symptomatic cases than cases without symptoms of tuberculosis.
Factors other than DOTS decentralization may have contributed to the reduction in tuberculosis prevalence. HIV sero-prevalence among patients with either smear-positive, smear-negative, or extra-pulmonary tuberculosis declined from 11.8% in 2003 to 6.3% in 2009.19–21 Also, socioeconomic factors, such as per capita gross domestic product, increased from 286 United States dollars (US$) in 1999 to US$ 830 in 2010.22 Also, the development of multidrug-resistant tuberculosis was minimized thanks to the consistently high treatment success rate.23,24
This study confirms that Cambodia still has one of the highest tuberculosis prevalences in the world. The majority of the cases detected in the 2011 survey did not meet the criteria for symptomatic pulmonary tuberculosis. The prevalence of tuberculosis cases with symptoms of pulmonary tuberculosis declined by more than half between surveys, whereas that of cases without symptoms declined by only a small fraction. Furthermore, only one third of the bacteriologically confirmed tuberculosis cases identified had positive results on sputum smear microscopy, a fact that underscores the limitations of the DOTS strategy, which focuses on symptomatic patients who independently seek care and relies primarily on smear microscopy for diagnosis. The reduction in tuberculosis prevalence suggests that the current set of tuberculosis control strategies is, in general, valid and should continue until new feasible diagnostic tools become readily available. However, the findings also suggest the need for different approaches to accelerate the decline in tuberculosis prevalence and incidence in certain groups. Active case detection among individuals not likely to seek care early after symptom onset or not likely to be correctly diagnosed by smear microscopy, or those without typical chronic tuberculosis symptoms should be considered. Chest X-ray screening, combined with sensitive diagnostic tools such as GeneXpert MTB/RIF,25 might prove cost-effective for such individuals. Strategies such as these should produce an accelerated decline in prevalence.18,26,27 However, further studies are needed to assess the impact of active case detection on health outcomes and tuberculosis transmission because not enough direct evidence exists so far.28
The prevalence of smear-positive tuberculosis decreased more in people aged 15 to 29 years than in people of other ages. This suggests that under the current programme for tuberculosis control, tuberculosis cases among younger adults are more easily detected than among older people. Epidemiological indicators in the younger population might reflect the effectiveness of tuberculosis control in adults, because most active tuberculosis in younger people is caused by a recent infection rather than reactivation of a remote, latent infection.29
The prevalence of tuberculosis increased with age and the decline in smear-positive tuberculosis was smaller in older age groups than in younger ones. More than half of the prevalence of smear-positive tuberculosis corresponded to the group of individuals aged 55 years or older, perhaps because older patients experience higher tuberculosis recurrence from endogenous reactivation or more vague or complex symptoms than younger people. Perhaps they also have a higher tolerance for symptoms.30 A study in Cambodia showed that a substantial proportion of cases occurred in individuals with abnormal findings on chest X-ray. The study revealed a high annual incidence rate (8.5%) of bacteriologically-positive tuberculosis among individuals with chest X-rays suggestive of active tuberculosis and of 2.9% among individuals with chest X-rays indicative of inactive tuberculosis.31 Other strategies than DOTS may be needed for countries where the populations infected with tuberculosis are ageing.
This study has limitations. First, the study spans over several years and other factors than DOTS decentralization were not controlled for, which could have been partly responsible for the changes in the tuberculosis prevalence we observed. Second, as recommended by WHO,5 the study included no individuals younger than 15 years or people with extra-pulmonary tuberculosis. Therefore, we were unable to measure the prevalence of all forms of tuberculosis. Third, we did not investigate the presence of HIV co-infection among survey participants. However, since HIV seroprevalence surveys in Cambodia have shown a downward trend,19,20we believe that the effect of HIV co-infection on tuberculosis prevalence rates is small. In addition, successful HIV control in Cambodia has contributed to tuberculosis control by reducing HIV infection rates and rates of co-infection and by expanding use of antiretroviral therapy.21
Acknowledgements
The authors thank K Lönnroth and I Law for their helpful comments in the preparation of this manuscript. The authors also thank C Sismanidis for advice on statistical analysis of the data.
Funding:
The 2011 survey, which was funded jointly by the Global Fund to Fight AIDS, Tuberculosis and Malaria, the United States Agency for International Development and WHO, was carried out as part of a technical cooperation project by the Japan International Cooperation Agency.
Competing interests:
None declared.
References
- 1.Global tuberculosis report 2013. Geneva: World Health Organization; 2013.
- 2.Framework for effective tuberculosis control. Geneva: World Health Organization; 1994. [Google Scholar]
- 3.National Center for Tuberculosis and Leprosy Control. National TB Prevalence Survey, 2002 Cambodia. Phnom Penh: Ministry of Health, Cambodia; 2005. [Google Scholar]
- 4.National Center for Tuberculosis and Leprosy Control. Annual Statistics of Tuberculosis in Cambodia 2005. Phnom Penh: Ministry of Health, Cambodia; 2005. [Google Scholar]
- 5.Tuberculosis prevalence surveys: a handbook. Geneva: World Health Organization; 2011.
- 6.National Center for Tuberculosis and Leprosy Control. Second National Tuberculosis Prevalence Survey, Cambodia 2011. Phnom Penh: Ministry of Health, Cambodia; 2012. [Google Scholar]
- 7.National Institute of Statistics. The 2008 general population census. Phnom Penh: Ministry of Planning Cambodia; 2009 [Google Scholar]
- 8.Fujiki A. TB bacteriology examination to Stop TB. Tokyo: Research Institute of Tuberculosis; 2005 [Google Scholar]
- 9.Hillemann D, Rüsch-Gerdes S, Richter E. Application of the Capilia TB assay for culture confirmation of Mycobacterium tuberculosis complex isolates. Int J Tuberc Lung Dis. 2005;9(12):1409–11 [PubMed] [Google Scholar]
- 10.dos Santos SI. Cancer epidemiology: principles and methods. Lyon: International Agency for Research on Cancer; 1999 [Google Scholar]
- 11.National Institute of Statistics. Cambodia socio-economic survey 2011 [Internet]. Phnom Penh: Ministry of Planning Cambodia; 2014. Available from: http://nada.nis.gov.kh/index.php/catalog/24 [cited 2014 May 25].
- 12.Tupasi TE, Radhakrishna S, Chua JA, Mangubat NV, Guilatco R, Galipot M, et al. Significant decline in the tuberculosis burden in the Philippines ten years after initiating DOTS. Int J Tuberc Lung Dis. 2009;13(10):1224–30 [PubMed] [Google Scholar]
- 13.Subramani R, Radhakrishna S, Frieden TR, Kolappan C, Gopi PG, Santha T, et al. Rapid decline in prevalence of pulmonary tuberculosis after DOTS implementation in a rural area of South India. Int J Tuberc Lung Dis. 2008;12(8):916–20 [PubMed] [Google Scholar]
- 14.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet. 1998;352(9144):1886–91 10.1016/S0140-6736(98)03199-7 [DOI] [PubMed] [Google Scholar]
- 15.Dowdy DW, Chaisson RE. The persistence of tuberculosis in the age of DOTS: reassessing the effect of case detection. Bull World Health Organ. 2009;87(4):296–304 10.2471/BLT.08.054510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293(22):2767–75 10.1001/jama.293.22.2767 [DOI] [PubMed] [Google Scholar]
- 17.Huong NT, Duong BD, Co NV, Quy HT, Tung LB, Broekmans JF, et al. Tuberculosis epidemiology in six provinces of Vietnam after the introduction of the DOTS strategy. Int J Tuberc Lung Dis. 2006;10(9):963–9 [PubMed] [Google Scholar]
- 18.Lönnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375(9728):1814–29 10.1016/S0140-6736(10)60483-7 [DOI] [PubMed] [Google Scholar]
- 19.Tamura M, Eam KK, Kimura K, Yoshihara N, Miura T, Yanai H, et al. National HIV prevalence surveillance among TB patients through periodic surveys: experience in Cambodia. Int J Tuberc Lung Dis. 2008;12(3) Suppl 1:20–5 [PubMed] [Google Scholar]
- 20.Khun KE, Tonjing J, Okada K, Tsurugi Y, Yoshihara N, Yadav R, et al. The 4th national HIV sero-prevalence survey among TB patients in Cambodia. Int J Tuberc Lung Dis. 2010;14(11) Suppl 2:S184 Available from: http://www.theunion.org/what-we-do/journals/ijtld/body/ABSTRACT_BOOK_2010_Web.pdf [cited 2014 May 25]. [Google Scholar]
- 21.Eang MT, Vun MC, Eam KK, Sovannarith S, Sopheap S, Bora N, et al. The multi-step process of building TB/HIV collaboration in Cambodia. Health Res Policy Syst. 2012;10(1):34. 10.1186/1478-4505-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Statistics [Internet]. Ministry of Planning, Royal Government of Cambodia. Available from: http://www.nis.gov.kh/nis/NA/NA2012.html [cited 2014 Feb 14].
- 23.Yamada N, Saorith K, Yamakami K, Onozaki I, Boran S, Fujiki A, et al. The national tuberculosis drug resistance survey in Cambodia, 2000–2001. Int J Tuberc Lung Dis. 2007December;11(12):1321–7 [PubMed] [Google Scholar]
- 24.National Center for Tuberculosis and Leprosy Control. National Tuberculosis Drug Resistance Survey, 2006–2007. Phnom Penh: Ministry of Health; 2010. [Google Scholar]
- 25.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363(11):1005–15 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376(9748):1244–53 10.1016/S0140-6736(10)61425-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9(11):1183–203 [PMC free article] [PubMed] [Google Scholar]
- 28.Systematic screening for active tuberculosis: principles and recommendations. Geneva: World Health Organization; 2013 [PubMed] [Google Scholar]
- 29.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(s3) Suppl 3:S184–94 10.1086/651490 [DOI] [PubMed] [Google Scholar]
- 30.Bhushan B, Kajal NC, Maske A, Singh SP. Manifestations of tuberculosis in elderly versus young hospitalised patients in Amritsar, India. Int J Tuberc Lung Dis. 2012;16(9):1210–3 10.5588/ijtld.11.0778 [DOI] [PubMed] [Google Scholar]
- 31.Okada K, Onozaki I, Yamada N, Yoshiyama T, Miura T, Saint S, et al. Epidemiological impact of mass tuberculosis screening: a 2-year follow-up after a national prevalence survey. Int J Tuberc Lung Dis. 2012;16(12):1619–24 10.5588/ijtld.12.0201 [DOI] [PubMed] [Google Scholar]