Abstract
Introduction:
Hepatitis B virus (HBV) is a parenterally transmitted viral illness of significant public health importance. The prevalence of HBV related viral hepatitis still remains debatable.
Objectives:
The objective of the following study is to determine the magnitude and pattern of HBV infection in clinically suspected infectious hepatitis at a tertiary care hospital in urban India.
Materials and Methods:
This prospective study was conducted in the Department of Microbiology at Lady Hardinge Medical College, New Delhi, over a period of 1 year from January 2008 to December 2008. All the serum samples taken from subjects (600 study and 200 control) were tested for hepatitis B surface antigen (HBsAg) using commercially available enzyme linked immunosorbent assay kit. Serum samples testing positive for HBsAg were tested for hepatitis B e antigen, immunoglobulin M (IgM) capture anti hepatitis D virus (HDV), IgM anti hepatitis B surface and IgM anti hepatitis B core.
Results:
24 (4%) serum samples tested positive for HBsAg in the study group while 5 (2.5%) tested positive in the control. Maximum seropositivity of HBsAg was in 20-30 years of age group in the study group (7.6%) followed by 11-20 years (4.5%), 0-10 years (2.8%) and >40 years (2.5%). The difference in seropositivity in study and control group was statistically insignificant in all the age groups (P > 0.05). Out of 24 cases positive for HBsAg, 4 cases (16.6%) were co infected with HDV in study group while there were none in control group.
Conclusions:
HBV is a common cause of parenterally transmitted viral hepatitis and hence, it is recommended that measures for public awareness regarding safe infection practices and safe sex practices should be undertaken to limit its spread.
Keywords: Enzyme linked immunosorbent assay, Hepatitis B virus, Hepatitis D virus, Seroprevalence
INTRODUCTION
Hepatitis B virus (HBV) is a deoxyribonucleic acid (DNA) virus from the Hepadnaviridae family transmitted by parenteral, sexual or perinatal mode. HBV is about a hundred times more infectious than human immunodeficiency virus.[1] HBV is a viral illness of significant public health importance. Clinical presentation ranges from asymptomatic or inapparent infection to acute liver failure. Chronic liver disease with cirrhosis and hepato-cellular carcinoma is the other spectrum of the disease.[2]
Globally, HBV related chronic infections are linked to nearly 60% cases of cirrhosis and 80% cases of hepatocellular carcinoma.[3] End stage liver disease accounts for about one in 40 deaths. Hepatitis D virus (HDV) also plays a role in progression to end stage liver disease among individuals infected with HBV.[3] World-wide over 300 million persons are chronically infected with HBV and 75% among these are in Asia alone.[4] The total HBV carrier pool in India is around 43 million and about 1 million new HBV carriers are added to this pool annually.[5] The average estimated carrier rate of HBV in India is 4.7%.[6] The diagnosis of HBV is based on serological demonstration of viral markers namely hepatitis B surface antigen (HBsAg), anti-hepatitis B surface (HBs), immunoglobulin M (IgM) anti hepatitis B core (HBc) antibody and HBV DNA.[7]
The HDV is a defective satellite virus, requiring HBV as helper virus. HDV is transmitted by parenteral, sexual and perinatal routes. Infections can occur as co-infection with HBV or as super infection of an HBsAg carrier.[8] The prevalence of HBV related viral hepatitis still remains debatable in developing and developed countries. There is paucity of data regarding age and sex related prevalence of HBV. Co infection of HBV with HDV could alter the spectrum of disease. However, the epidemiology of this co infection is not precisely known. As HBV is a vaccine preventable disease, the burden of the disease could be reduced to a considerable extent, if its seroprevalence is precisely known in different age groups.
Thus, this study was designed to determine the seroprevalence of HBV infection in clinically suspected acute infectious hepatitis patients. The study also aimed to determine age and sex related HBV prevalence and evidence of co-infection.
MATERIALS AND METHODS
This prospective study was conducted in the Department of Microbiology at Lady Hardinge Medical College, New Delhi which is a tertiary care hospital in urban Northern India, over a period of 1 year from January 2008 to December 2008. Subjects were divided into two groups. The study group consisted of 600 patients with clinically suspected acute infectious hepatitis attending the out-patient department of various specialties in Kalawati Saran and Smt. Sucheta Kriplani Hospital, New Delhi both attached to Lady Hardinge Medical College.
Inclusion criteria were (1) Recent onset of jaundice (<6 months), defined by serum bilirubin level >2.5 mg/dl and/or increase in serum transaminases >5 times the upper limit of normal. (2) Fever in absence of chronic liver disease or past history of jaundice.
Exclusion criteria included history of chronic liver disease or past history of jaundice with duration of illness more than 6 months and acute fatty liver of hepatitis/alcoholic hepatitis/intrahepatic cholestasis.
The control group consisted of 200 age and sex matched patients showing no clinical evidence of acute infectious hepatitis.
Routine blood samples received in the serology section of Department of Microbiology from patients suspected of acute infectious hepatitis were analyzed. The sera were separated and stored frozen (−70°C) until tested for the viral markers. The serum samples taken from subjects (study and control group) were tested for HBsAg using commercially available enzyme linked immunosorbent assay kit (ELISA; Biokit, Barcelona, Spain). Serum samples testing positive for HBsAg were tested for hepatitis B e antigen (HBeAg) (ELISA; Sanofi Diagnostics, Pasteur, France), IgM capture anti HDV (ELISA; Abbott GmBH Diagnostic, Wiesbaden-Delk-enheim, Germany), IgM anti HBs and IgM anti HBc (ELISA; Biokit, Barcelona, Spain). All the serum samples were also tested for the IgM anti hepatitis A virus (HAV) and IgM anti HEV using commercially available enzyme-linked immunosorbent assay kits (ELISA; Biokit, Barcelona, Spain).
Informed consent and institutional review board approval was taken from ethics committee for the study bearing protocol number MIC 07/312. We used SPSS version 10.0 (SPSS Inc., Chicago, Illinois) for the statistical analysis. The means of continuous variables were compared using the Students t-test and categorical variables were compared using the Chi-square test and the Fishers Exact test, as appropriate. P < 0.05 was considered to be significant.
RESULTS
The study group comprised of 362 male and 238 female patients. The overall male to female ratio was 1.5:1 and thus a male preponderance was seen in study group. The control group (n = 200) comprised of 121 males and 79 females with overall male to female ratio of 1.5:1. The study and control group were divided age wise, i.e., 0-10 years, 11-20 years, 21-30 years, 31-40 years and >40 years. The percentage of males was not different between cases and controls (P = 0.125).
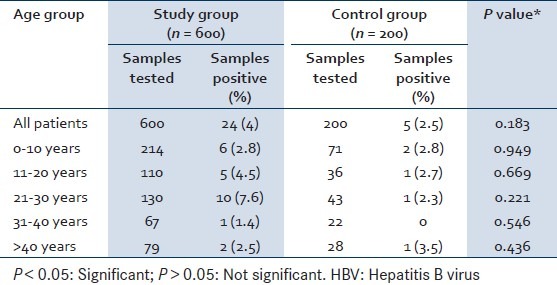
The mean age in the study group was 20.2 ± 15.2 years while in the control group it was 19.65 ± 14.8 years. The mean age of study and control group was not different (P = 0.46). Overall 24 (4%) serum samples tested positive for HBsAg in the study group while 5 (2.5%) tested positive in the control group. On observing age wise seropositivity of HBV it was found that maximum seropositivity of HBsAg was in 20-30 years of age group in the study group (7.6%) followed by 11-20 years (4.5%), 0-10 years (2.8%) and >40 years (2.5%). The seropositivity in study and control group was not different across all the age groups (P > 0.05).
IgM anti HAV was positive in 50 (8.3%) serum samples in the study group against 4 (2%) in the control group showing statistical significance (P = 0.002). IgM anti HEV was positive in 21 (3.5%) serum samples in the study group against 5 (2.5%) in the control group showing no statistical significance (P = 0.735) [Table 1].
Table 1.
Age wise seropositivity of HBV in study and control group
HBV profile in HBsAg positive cases in study and control group
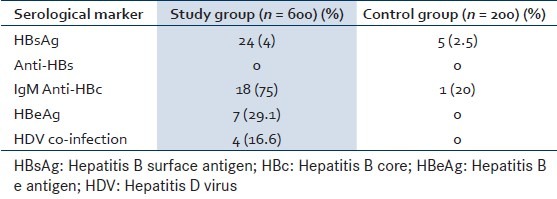
In the study group, out of 24 (4%) HBsAg positive patients, 18 (75%) were also positive for IgM anti HBc indicating acute infection. HBeAg was positive in 7 (29.1%) patients indicating high infectivity and active replication of the virus. Four patients (16.6%) showed coinfection with HDV and none were positive for anti HBs. In control group, 5 (2.5%) patients were positive for HBsAg. Among these only 1 (20%) showed positivity for IgM anti HBc. None of the control individuals was positive for HDV anti HBs or HBeAg [Table 2].
Table 2.
Hepatitis B profile in study and control group
DISCUSSION
HBV related acute viral hepatitis is a global public health concern associated with substantial mortality and morbidity.[9] Our study demonstrated an overall seroprevalence rate of HBV as 4% in the study group while 2.5% in the control group. The HBsAg seroprevalence recorded by different authors in and around Delhi varied from 8 to 42.5% respectively.[10,11,12,13,14] Das et al.[11] (New Delhi) reviewed 75 cases of sporadic viral hepatitis and found HBsAg seroprevalence of 8%. Manoj et al.[12] (New Delhi) conducted a hospital based study in 2493 patients of suspected acute viral hepatitis and found HBsAg seroprevalence of 10.15%. The reduced prevalence rates in our study could be attributed to integration of HBV vaccination into Universal immunization program and free availability of vaccine. Blood bank quality control by stringent screening practices and community awareness campaigns proved pivotal in disease control. Prevention of household or nosocomial spread of HBV infection with awareness of safe injecting practices and safe sex practices reduced the spread of HBV. The seroprevalence rate of HBsAg in general population in our study was 2.5%. Various studies have recorded the seroprevalence rate of HBsAg in general population between 0.97 and 9.5%[12,15,16,17] The lowest prevalence (0.97%) was reported in North India (Chandigarh, 2004) by Gupta et al. while the highest (9.5%) in Delhi by Prakash et al. The reasons for such diversities were attributed to social, economic and health care factors.[16,17]
HBV profile and co-infection
High infectivity and active replication of virus in the study group was indicated by HBeAg positivity. Similarly IgM anti HBc positivity indicated recent infection in the study group. Patients in control group might have presented later in the course of illness such that none showed high infectivity and active replication. HBV DNA was not tested in this study.
HDV being a defective satellite virus, is usually transmitted along with HBV as co-infection as superinfection. A variety of factors such as increase in the sexually transmitted diseases, unsafe injecting practices, intravenous drug abuse, unsafe sex practices and needle stick injuries have a contributory effect on the overall seroprevalence of HDV. HDV seropositivity of 16.6% in this study is comparable with other studies showing seroprevalence between 10.6 and 16% respectively.[18,19,20] It has been suggested that HBV-HDV coinfections are significantly higher in acute hepatitis while super infection is commoner in chronic liver disease.[18] Several studies showed HDV seroprevalence of 0-7% in asymptomatic HBsAg carriers.[21]
The seropositivity of HBV increased gradually with increasing age. This was probably due to continuing increase in risk of exposure with increasing age. Similar findings have been reported by Manoj et al. (New Delhi) who analyzed 2493 serum samples from icteric patients and showed the highest seroprevalence of HBsAg in 20-30 year age group (14.38%).[12] The high prevalence rates of HBsAg in 21-30 years group is due to sexual promiscuity and intravenous drug abuse in this age group. Blood transfusions and tattooing could be other contributory factors. HBV is transmitted through percutaneous and parenteral contact with the infected blood, body fluids etc.[12] Increased exposure to these risk factors could be responsible for increased seropositivity of HBsAg in this age group. In contrast, some authors have demonstrated high prevalence rates of HBsAg in <10 years of age.[22,23] Studies suggested that exposure to virus early in life and vertical transmission plays a major role in viral transmission.[22,23] Thus the high prevalence rate of HBsAg in <10 years of age group could be due to high perinatal transmission rates of HBV. Many of the studies have reported increasing prevalence of HBsAg in pregnant women ranging from 3.74 to 10%.[22,24] The rising prevalence of HBV in pregnant women, is responsible for high perinatal transmission to infants.[24] In our study, the seroprevalence rate of HBV in <10 year age group was 2.8% both in study as well as control group.
CONCLUSION
We conclude that HBV is a common cause of parenterally transmitted viral hepatitis and hence, it is recommended that measures for public awareness regarding safe infection practices and safe sex practices should be undertaken to limit the spread. Furthermore, vaccination programs need to be strengthened with inclusion of HBV vaccine in the National Immunization Program. Vaccination of children and high risk adults is must to interrupt transmission of HBV. Comprehensive HBV prevention strategies should include prevention of nosocomial transmission and preventive strategies for health care workers.
Footnotes
Source of Support: Funding for work is through the Department of Microbiology, Lady Hardinge Medical College, New Delhi.
Conflict of Interest: None declared.
REFERENCES
- 1.Vira l Hepatitis Prevention Board. Antwerp VHPB Report. Editorial. Control of viral hepatitis in Europe. Viral Hepatitis. 1996;4(2) http://hgins.uia.ac.be/esoc/VHPB/vhv4n2.html . [Google Scholar]
- 2.Dienstag L. Harrison's Principles of Internal Medicine. 16th edition. McGraw-Hill Professional; 2004. Acute Viral Hepatitis; pp. 1822–38. [Google Scholar]
- 3.Koff RS. Review article: Vaccination and viral hepatitis-current status and future prospects. Aliment Pharmacol Ther. 2007;26:1285–92. doi: 10.1111/j.1365-2036.2007.03517.x. [DOI] [PubMed] [Google Scholar]
- 4.Margolis HS, Alter MJ, Hadler SC. Hepatitis B: Evolving epidemiology and implications for control. Semin Liver Dis. 1991;11:84–92. doi: 10.1055/s-2008-1040427. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organisation. Children's Vaccines - Safety First. Geneva: WHO; 1999. [Google Scholar]
- 6.Batham A, Narula D, Toteja T, Sreenivas V, Puliyel JM. Sytematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatr. 2007;44:663–74. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Hepatitis B virus. [Last accessed on 2013 Jul 21]. Available from: http://www.cdc.gov/hepatitis/HBV/index.htm .
- 8.Hadziyannis SJ. Review: Hepatitis delta. J Gastroenterol Hepatol. 1997;12:289–98. doi: 10.1111/j.1440-1746.1997.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol Rev. 2006;28:112–25. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 10.Kar P, Budhiraja S, Narang A, Chakravarthy A. Etiology of sporadic acute and fulminant non-A, non-B viral hepatitis in north India. Indian J Gastroenterol. 1997;16:43–5. [PubMed] [Google Scholar]
- 11.Das K, Agarwal A, Andrew R, Frösner GG, Kar P. Role of hepatitis E and other hepatotropic virus in aetiology of sporadic acute viral hepatitis: A hospital based study from urban Delhi. Eur J Epidemiol. 2000;16:937–40. doi: 10.1023/a:1011072015127. [DOI] [PubMed] [Google Scholar]
- 12.Manoj J, Pradhan SK, Randhawa V, Jyotsna K. Age-wise seroprevalence of hepatitis B infection in clinical cases of jaundice attending a tertiary health care institute of Delhi. J Commun Dis. 2005;37:255–8. [PubMed] [Google Scholar]
- 13.Hussain Z, Das BC, Husain SA, Murthy NS, Kar P. Increasing trend of acute hepatitis A in north India: Need for identification of high-risk population for vaccination. J Gastroenterol Hepatol. 2006;21:689–93. doi: 10.1111/j.1440-1746.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaur R, Gur R, Berry N, Kar P. Etiology of endemic viral hepatitis in urban North India. Southeast Asian J Trop Med Public Health. 2002;33:845–8. [PubMed] [Google Scholar]
- 15.Chowdhury A, Santra A, Chakravorty R, Banerji A, Pal S, Dhali GK, et al. Community-based epidemiology of hepatitis B virus infection in West Bengal, India: Prevalence of hepatitis B e antigen-negative infection and associated viral variants. J Gastroenterol Hepatol. 2005;20:1712–20. doi: 10.1111/j.1440-1746.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- 16.Gupta N, Kumar V, Kaur A. Seroprevalence of HIV, HBV, HCV and syphilis in voluntary blood donors. Indian J Med Sci. 2004;58:255–7. [PubMed] [Google Scholar]
- 17.Prakash C, Sharma RS, Bhatia R, Verghese T, Datta KK. Prevalence of North India of hepatitis B carrier state amongst pregnant women. Southeast Asian J Trop Med Public Health. 1998;29:80–4. [PubMed] [Google Scholar]
- 18.Chakraborty P, Kailash U, Jain A, Goyal R, Gupta RK, Das BC, et al. Seroprevalence of hepatitis D virus in patients with hepatitis B virus-related liver diseases. Indian J Med Res. 2005;122:254–7. [PubMed] [Google Scholar]
- 19.Singh V, Goenka MK, Bhasin DK, Kochhar R, Singh K. A study of hepatitis delta virus infection in patients with acute and chronic liver disease from northern India. J Viral Hepat. 1995;2:151–4. doi: 10.1111/j.1365-2893.1995.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 20.Amarapurkar DN, Vishwanath N, Kumar A, Shankaran S, Murti P, Kalro RH, et al. Prevalence of delta virus infection in high risk population and hepatitis B virus related liver diseases. Indian J Gastroenterol. 1992;11:11–2. [PubMed] [Google Scholar]
- 21.Smedile A, Lavarini C, Farci P, Aricò S, Marinucci G, Dentico P, et al. Epidemiologic patterns of infection with the hepatitis B virus-associated delta agent in Italy. Am J Epidemiol. 1983;117:223–9. doi: 10.1093/oxfordjournals.aje.a113533. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee A, Chakravarty R, Mondal PN, Chakraborty MS. Hepatitis B virus genotype D infection among antenatal patients attending a maternity hospital in Calcutta, India: Assessment of infectivity status. Southeast Asian J Trop Med Public Health. 2005;36:203–6. [PubMed] [Google Scholar]
- 23.Beniwal M, Kumar A, Kar P, Jilani N, Sharma JB. Prevalence and severity of acute viral hepatitis and fulminant hepatitis during pregnancy: A prospective study from north India. Indian J Med Microbiol. 2003;21:184–5. [PubMed] [Google Scholar]
- 24.Robinson WS. Hepatitis B virus and hepatitis D virus. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone; 1995. pp. 1406–39. [Google Scholar]


