Abstract
Introduction:
Dengue is one of the most important arboviral infections caused by one of the four dengue serotypes, 1-4.
Objective:
To study the applicability of different diagnostic methods in diagnosis of dengue viral infection.
Materials and Methods:
A total of 2101 blood samples were collected for confirmation of dengue viral infection. All the samples were tested by dengue-specific IgM ELISA, of which 111 were also tested for NS1 antigen detection and 27 acute samples (≤5 days) were further subjected for viral RNA detection by RT-PCR and isolation in C6/36 cell line. To detect the sensitivity of NS1 antigen for different dengue virus serotypes, four dengue serotype 1 and 12 dengue 3 were subjected for the NS1 antigen assay.
Results:
Most common age group affected was 16-45 years, with male to female ratio of 2.8:1. During first 3 days of illness virus isolation and RT-PCR were the most sensitive (83%) followed by NS1 antigen detection (75%) and IgM detection (37.5%). The positivity of IgM detection was found to be significantly higher as compared to NS1 detection during 4 to 5 days and also after 5 days of illness (P < 0.05). Dengue serotypes 1 and 3 were found to be co-circulated, dengue 1 being the predominant serotype.
Conclusion:
Virus isolation and RT-PCR were the most sensitive tests during the early period of illness whereas beyond third day, IgM antibody detection was found to be the most sensitive method of dengue diagnosis.
Keywords: Dengue, Diagnosis, IgM antibody, NS1 Ag, RT-PCR
INTRODUCTION
Dengue viral infection is caused by one of the dengue virus types (DENV 1-4) belonging to the family Flaviviridae. A majority of patients suffer from self-limiting febrile illness (dengue fever; DF), whereas some may progress to severe life-threatening dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS).[1,2]
Several diagnostic tests are presently available for diagnosis of dengue viral infection. Isolation of virus is confirmative but less sensitive and time consuming. The hemagglutination inhibition (HI) and complement fixation test (CFT) were previously the recommended tests by World Health Organisation (WHO). As these tests are time consuming, requires paired samples and tedious to perform presently replaced with dengue IgM antibody detection by MAC ELISA and dengue NS1 antigen by ELISA tests. Molecular techniques like RT-PCR, multiplex RT-PCR and real-time PCR system have also been reported for early detection of dengue RNA to warrant as acute dengue infection.[2] Thus, different diagnostic tests available for dengue viral infection testing are applicable at different phases of illness. Considering the above-mentioned kinetics, the present study compared the positivity of different tests during different phases of dengue viral illness in order to guide the microbiologists and clinicians to follow the appropriate diagnostic strategy.
MATERIALS AND METHODS
The study was carried out in Dept. of Virology, Postgraduate Institute of Medical Education and Research, Chandigarh, which is one of the apex centers for advanced diagnosis of dengue, Japanese encephalitis, chikungunya and other arboviral infections under National Vector Borne Disease Control Programme, Ministry of Health and Family Welfare.
A total of 2101 blood sample were received from clinically suspected dengue cases from Chandigarh and neighboring states during 2010. The inclusion criteria for dengue cases were considered as per the WHO criteria.[2] All these samples were tested for dengue IgM antibody by MAC ELISA as a routine diagnostic service of the department. One hundred and eleven samples were subjected to NS1 antigen ELISA whose information on duration of illness was available. Samples collected within 5 days of illness were further subjected for viral RNA detection and virus isolation in C6/36 cell lines. Dengue IgM was performed by MAC ELISA (National Institute of Virology, Pune, India) and dengue NS1 antigen detected by NS1 by ELISA (Panbio, Australia) as per manufacturer's instructions. The samples tested positive by any of the four mentioned tests were considered as positive for dengue viral infection and were included for analysis.
To detect the sensitivity of NS1 antigen for different dengue virus serotypes four dengue serotype 1 and 12 dengue serotype 3 (2 from 2010 and 10 from 2008) were subjected for NS1 antigen detection.
Virus isolation
Isolation of dengue virus was done as described before.[3]
Detection of dengue viral RNA
Briefly, viral RNA was extracted from 140 μL of serum sample and P2 supernatant fluid using QIAamp Viral RNA Mini Kit (Qiagen, USA). RNA was eluted in 50 μL of elution buffer and used as a template for cDNA synthesis. The extracted RNA was reverse transcribed on the same day with RevertAid™ First Strand cDNA Synthesis Kit (MBI Fermentas, USA) as per the manufacturer instructions.
Single-tube dengue multiplex RT-PCR
The synthesized cDNA was then subjected to single tube dengue multiplex PCR as described previously.[4] Briefly, viral cDNA was amplified using dengue-specific D1 upstream primer and four serotype-specific downstream primers TS1, TS2, TS3 and TS4. The thermal profile of PCR amplification cycle followed was: 35 cycles of denaturation at 95°C for 30 sec; annealing at 55°C for 45 sec and extension at 72° C for 2 min. The PCR products were visualized by 2% agarose gel electrophoresis under the digital gel documentation system (Alpha Innotech, San Leandro, CA).
Gel elution
The PCR positive samples were subjected to gel purification using the QIAquick Gel Extraction Kit (Qiagen, Germany) and the purified product was eluted in 30 μl of elution buffer which was used as a template for sequencing reaction.
Nucleotide sequencing
The purified PCR products were cycle sequenced in an ABI PRISM 310 genetic analyzer (PE Applied Biosystems Inc., Foster City, CA, USA) using an ABI PRISM Big Dye Terminator cycle sequencing kit (PE Applied Biosystems Inc., Foster City, CA). For confirmation of the sequencing product, BLAST programme was implemented.
Nucleotide sequence analysis
The obtained sequences were edited followed by BLAST search to confirm the identity of the sequences. The phylogenetic and molecular evolutionary analyses were conducted using MEGA version 4. The phylogenetic tree was drawn by using the Neighbor-Joining method with bootstrap analysis of 1000 replicates. The sequences of dengue serotype 1 from different geographical regions were retrieved from GenBank and their accession numbers for genotyping and sub-typing.
Statistical analysis
All the data was analyzed using SPSS 17.0 software. The statistical analysis was performed at a significance level of P value <0.05 using the chi-square test.
RESULTS
Of the 2101 dengue suspected serum samples tested for IgM antibody, 745 (35.5%) were found to be positive. A majority of them were in the age group of 16-45 years (61%), with a male to female ratio of 2.8:1. The cases of dengue occurred from August through December, with a peak in October. Of the 111 tested samples 79 were positive by one of the four diagnostic tests applied and thus were included for analysis.
Result of samples collected within 1 to ≤3 days of illness
A total of eight samples were collected from patients with ≤3 days of illness of which six samples were tested by all the four tests where as two samples could not be subjected for virus isolation and RT-PCR due to less sample volume. Virus isolation and RT-PCR could detect maximum number of samples during this period with a positivity of 83.3% (5/6) followed by NS1 antigen detection (75%: 6/8), and IgM antibody detection (37.5%:3/8 (P = 0.180) [Table 1].
Table 1.
Day wise positivity of different diagnostic tests for dengue viral infection
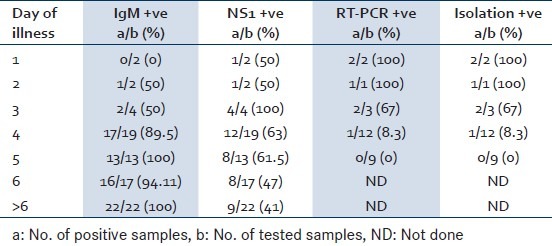
The RT-PCR product revealed dengue type1 in majority (4/6) and type 3 in two samples. All the type 1 samples were confirmed by nucleotide sequencing.
Result of samples collected during 4-5 days of illness
Thirty-two samples collected from patients with 4-5 days of illness, of which 21 samples were tested by all four tests and the remaining 11 samples were tested only for IgM antibody and NS1 antigen detection.
IgM antibody could be detected in 30 of 32 samples tested during this period of illness (98%) whereas NS1 antigen could be detected in 20 of these samples with a sensitivity of 62.5%. The overall positivity of IgM antibody and NS1 antigen detection was found to be 90%, 54% respectively. The positivity of IgM antibody was found to be significantly higher than NS1 antigen detection (P = 0.005). The detail of dengue IgM and NS1 antigen positivity is depicted in Table 2.
Table 2.
Dengue IgM antibody and NS1antigen positivity during different period of dengue viral infection

Result of samples collected after 5 days of illness
A total of 39 samples collected after 5 days of illness were tested for IgM antibody and NS1 antigen detection. The positivity of dengue IgM antibody detection was found to be significantly higher as compared to that of NS1 antigen detection (97% Vs 44%; P < 0.05).
Result of NS1 antigen in different dengue serotypes
NS1 antigen could be detected in all the 4 serum samples of dengue type 1 and 4 of 12 dengue type 3 giving a sensitivity of 100% and 33.3% for type1 and type 3, respectively.
Genotyping and sub typing of dengue viruses
The dendogram showed the sequences of the present study isolates were clustered along with the genotype III and subtype 2 when compared among the reference sequences of dengue serotype 1 [Figure 1].
Figure 1.
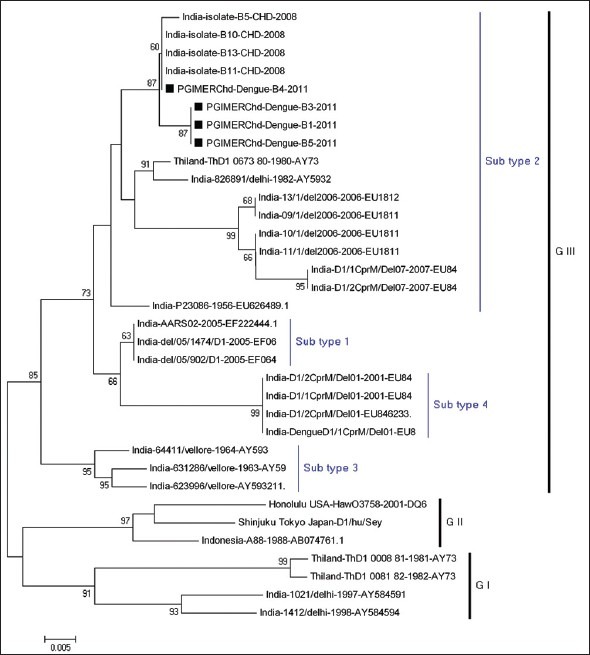
Phylogenetic analysis of dengue virus
DISCUSSION
Dengue is a disease with wide spectrum of clinical manifestations mimicking several other illnesses. Early diagnosis of disease is of importance, as with timely intervention case fatality can be reduced to <1% in severe cases.[2]
In our study 35.5% of cases were serologically positive for dengue infection. The higher positivity among young adult males (61%) is consistent with previous dengue reports by the authors, as well as other Indian studies.[5,6,7,8] In several other studies pediatric population was most commonly affected.[9,10]
The knowledge of seasonal trends is important for timely implementation of effective control and preventive measures. Dengue cases are usually reported during post monsoon months as the climatic conditions during this period are considered favorable for breeding of Aedes aegypti mosquitoes.[5]
Confirmation of suspected dengue cases can be done by isolation of virus, viral RNA detection, viral antigen or IgM antibody detection in patients’ blood sample.[2] Among these, serological test for IgM antibody detection is most widely employed because of its wide availability, high sensitivity and specificity and also as the immunoglobulin are stable at tropical room temperature, specimen transport does not pose any problem.[2]
Because of transient viremia and time required for antibody development, it is widely considered that dengue infection of <5 days illness may be diagnosed by virus isolation, viral nucleic acid detection and recently by viral NS1 antigen detection either by ELISA or rapid tests.[2] The present study found virus isolation and RT-PCR as the most sensitive during first 3 days of illness, followed by NS1 antigen and IgM antibody detection. However, the difference was not found to be significant (P = 0.180) which could be due to less number of sample size. Whereas after day 3 of illness, positivity of IgM antibody detection was found to be significantly higher compared to NS1 antigen detection (P < 0.05). The early appearance of IgM antibody has been reported in secondary dengue infections as compared to primary dengue infection.[11] As Chandigarh is known for its endemo-epidemic status for dengue virus infection with sero-prevalence more than 70%, majority of dengue cases are considered to be secondary infections.[5] Secondly, the lower positivity of NS1 antigen after 3 days of illness in the present study could also be due to the secondary nature of dengue infection in this region due to endemicity. High titers of pre-existing IgG antibodies have been attributed to the lower positivity of NS1 antigen detection in acute secondary dengue infection as compared to that of acute primary infection.[12,13,14,15,16] Considering the observation of the present study and available literature, authors have tried to provide a practical approach to dengue diagnosis for endemic countries [Figure 2].
Figure 2.
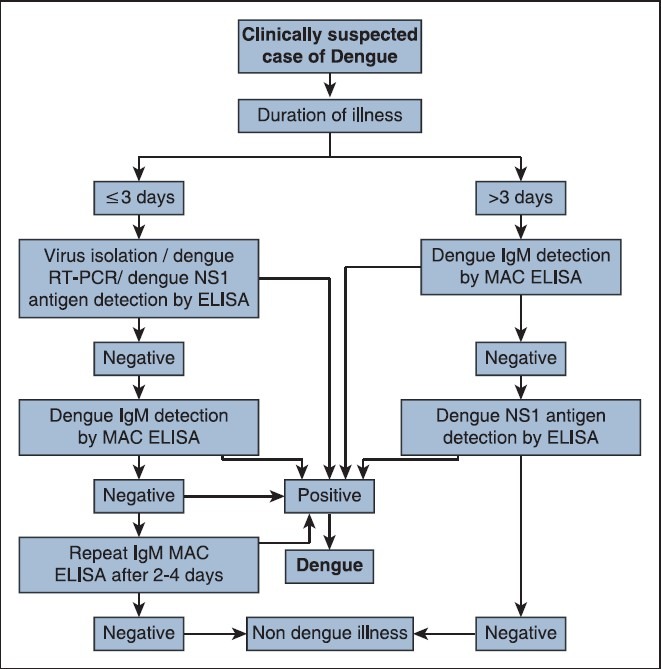
Algorithm for laboratory diagnosis of clinically suspected dengue cases in endemic setting
Diagnosis of dengue based on IgM antibody detection by MAC ELISA from single serum specimen in absence of positive RT-PCR in acute phase sample has been classified as recent probable dengue infection.[17] This could be either due to the persistence of dengue IgM antibody for 2-3 months after acute illness or due to the cross reactivity with other Flaviviruses. However, a late acute phase sample (around day 5 of illness) from a suspected dengue patient if found negative for IgM antibody as well as by RT-PCR is labeled as a laboratory-indeterminate case and in such cases a either a convalescent sample should be asked to confirm the diagnosis[17] or sample may be subjected for NS1 antigen detection.
The sensitivity of NS1 antigen testing in different dengue virus serotypes infection has been reported variedly in different studies.[18,19] Studies from Venezuela and Vietnam have shown lower NS1 sensitivity for dengue virus-2 infections.[16,20] The present study found lower sensitivity of NS1 for dengue virus-3 serotypes which needs to be confirmed with large number of samples from both primary and secondary dengue infection.
The role of viral RNA detection by RT-PCR though limited only to the early part of dengue diagnosis, the method has the potency of dengue virus typing which is important to assess the severity of outbreak and to take timely appropriate control measures.
Circulation of various dengue types have been reported in the past decade. Dengue types 1, 2 and 3 have been found to be circulated in Chandigarh over the last few years.[3,4,5,21] The present outbreak revealed the co-circulation of dengue types 1 and 3 with the predominance of dengue type 1, upon phylogenetic analysis revealed they were belong to subtype 2 under genotype III [Figure 1].
The present study thus concludes that wherever facilities for isolation and molecular techniques are available, diagnosis of acute dengue infection should be done by RT-PCR or virus isolation. Though NS1 antigen is a good early marker, the samples negative for NS1 antigen during acute phase should be tested for IgM antibody detection where secondary dengue infections are common, before reporting as dengue negative.
ACKNOWLEDGEMENTS
Dte. NVBDCP, MOH,GOI for providing dengue kits. Mrs Kanwalpreet & Mrs Ragini for technical assistance.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Guzmán MG, Kouri G. Dengue diagnosis, advances and challenges. Int J Infect Dis. 2004;8:69–80. doi: 10.1016/j.ijid.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New Edition. Geneva: World Health Organization; 2009. WHO Guidelines Approved by the Guidelines Review Committee. [PubMed] [Google Scholar]
- 3.Mishra B, Sharma M, Pujhari SK, Ratho RK, Gopal DS, Kumar CN, et al. Utility of multiplex reverse transcriptase-polymerase chain reaction for diagnosis and serotypic characterization of dengue and chikungunya viruses in clinical samples. Diagn Microbiol Infect Dis. 2011;71:118–25. doi: 10.1016/j.diagmicrobio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Mishra B, Sharma M, Pujhari SK, Appannanavar SB, Ratho RK. Clinical applicability of single-tube multiplex reverse-transcriptase PCR in dengue virus diagnosis and serotyping. J Clin Lab Anal. 2011;25:76–8. doi: 10.1002/jcla.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratho RK, Mishra B, Kaur J, Kakkar N, Sharma K. An outbreak of dengue fever in periurban slums of Chandigarh, India, with special reference to entomological and climatic factors. Indian J Med Sci. 2005;59:518–26. [PubMed] [Google Scholar]
- 6.Gupta E, Dar L, Narang P, Srivastava VK, Broor S. Serodiagnosis of dengue during an outbreak at a tertiary care hospital in Delhi. Indian J Med Res. 2005;121:36–8. [PubMed] [Google Scholar]
- 7.Ukey P, Bondade S, Paunipagar P, Powar R, Akulwar S. Study of seroprevalence of dengue fever in central India. Indian J Community Med. 2010;35:517–9. doi: 10.4103/0970-0218.74366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Rao CR, Pandit V, Shetty S, Bammigatti C, Samarasinghe CM. Clinical manifestations and trend of dengue cases admitted in a tertiary care hospital, Udupi district, Karnataka. Indian J Community Med. 2010;35:386–90. doi: 10.4103/0970-0218.69253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah GS, Islam S, Das BK. Clinical and laboratory profile of dengue infection in children. Kathmandu Univ Med J (KUMJ) 2006;4:40–3. [PubMed] [Google Scholar]
- 10.Anderson KB, Chunsuttiwat S, Nisalak A, Mammen MP, Libraty DH, Rothman AL, et al. Burden of symptomatic dengue infection in children at primary school in Thailand: A prospective study. Lancet. 2007;369:1452–9. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez S, Ruiz D, Barrero R, Ramirez R, Calzada N, del Rosario Peña B, et al. Kinetics of dengue virus NS1 protein in dengue 4-confirmed adult patients. DiagnMicrobiol Infect Dis. 2010;68:46–9. doi: 10.1016/j.diagmicrobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Tricou V, Vu HT, Quynh NV, Nguyen CV, Tran HT, Farrar J, et al. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC Infect Dis. 2010;10:142. doi: 10.1186/1471-2334-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dussart P, Petit L, Labeau B, Bremand L, Leduc A, Moua D, et al. Evaluation of two new commercial tests for the diagnosis of acute dengue virus infection using NS1 antigen detection in human serum. PLoS Negl Trop Dis. 2008;2:e280. doi: 10.1371/journal.pntd.0000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaterji S, Allen JC, Jr, Chow A, Leo YS, Ooi EE. Evaluation of the NS1 rapid test and the WHO dengue classification schemes for use as bedside diagnosis of acute dengue fever in adults. Am J Trop Med Hyg. 2011;84:224–8. doi: 10.4269/ajtmh.2011.10-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumarasamy V, Chua SK, Hassan Z, Wahab AH, Chem YK, Mohamad M, et al. Evaluating the sensitivity of a commercial dengue NS1 antigen-capture ELISA for early diagnosis of acute dengue virus infection. Singapore Med J. 2007;48:669–73. [PubMed] [Google Scholar]
- 16.Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3:e360. doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laboratory Guidance and Diagnostic Testing. [Last accessed on 2013 Aug 14]. http://www.cdc.gov/dengue/clinicallab/laboratory.html .
- 18.Lima Mda R, Nogueira RM, Schatzmayr HG, dos Santos FB. Comparison of three commercially available dengue NS1 antigen capture assays for acute diagnosis of dengue in Brazil. PLoS Negl Trop Dis. 2010;4:e738. doi: 10.1371/journal.pntd.0000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duyen HT, Ngoc TV, Ha do T, Hang VT, Kieu NT, Young PR, et al. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: Differential effects according to serotype and immune status. J Infect Dis. 2011;203:1292–300. doi: 10.1093/infdis/jir014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez AH, Moros Z, Comach G, Zambrano J, Bravo L, Pinto B, et al. Evaluation of dengue NS1 antigen detection tests with acute sera from patients infected with dengue virus in Venezuela. Diagn Microbiol Infect Dis. 2009;65:247–53. doi: 10.1016/j.diagmicrobio.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Singh MP, Majumdar M, Singh G, Goyal K, Preet K, Sarwal A, et al. NS1 antigen as an early diagnostic marker in dengue: Report from India. Diagn Microbiol Infect Dis. 2010;68:50–4. doi: 10.1016/j.diagmicrobio.2010.04.004. [DOI] [PubMed] [Google Scholar]


