Abstract
Mucormycosis is a rare life-threatening fungal infection mostly affecting immunocompromised hosts. The main categories of human disease with the Mucorales are sinusitis/rhinocerebral, pulmonary, cutaneous/subcutaneous, gastrointestinal and disseminated disease. Other disease states occur with a much lower frequency and include cystitis, vaginitis; external otitis and allergic disease. We report a diabetic patient with comorbidities, who developed gastric perforation clinically indistinguishable from perforated peptic ulcer due to invasive gastric mucormycosis complicated by spleen infarction.
Keywords: Gastric ulcer, Infarction of spleen, Mucormycosis, Perforation, Zygomycosis
INTRODUCTION
Mucormycosis is a fungal infection caused by fungi in the order Mucorales. Among the Mucoraceae, Rhizopus oryzae (Rhizopus arrhizus) is by far the most common, inhabiting soil, animal feces and decaying vegetative matter.[1,2,3] Other less frequently isolated species of the Mucoraceae family that cause a similar spectrum of infections include Rhizopus microsporus var. rhizopodiformis, Absidia corymbifera, Apophysomyces elegans, Mucor species and Rhizomucor pusillus.[2]
CASE REPORT
The present case report is about a 54-year-old male patient, with well-controlled diabetes mellitus, hypertension, ischemic heart disease with poor ejection fraction (35%), decompensated congestive heart failure, anasarca and chronic kidney disease. He presented with 2 days history of passage of black tarry stool, progressive abdominal distention and New York Heart Association class III-IV dyspnea. He denied history of alcohol intake and did not have history of diabetic ketoacidosis over the past 3 years. The patient did not use proton pump inhibitors, H2 blockers, non-steroidal anti-inflammatory drugs, or broad spectrum antibiotics in the past 6 months. On clinical examination, there was gross ascites with diffuse abdominal tenderness. Serum level of glucose was 6.3 mmol/l; HbA1c 6.2; sodium 124 mmol/l; potassium 6.1 mmol/l; bicarbonate 10.8; creatinine 388 μmol/l; urea 42.3 mmol/l; pro-brain natriuretic peptide 1498 pmol/l; white blood cells 9.3 × 109/l, Hb 6.3 g/dl; C-reactive protein 3.5 mg/l; hepatitis B, C and HIV serology were negative. Two sets of blood cultures were also negative.
Esophagogastroduodenoscopy revealed an ulcer over the ampullary fold around which adrenalin was injected. The patient continued to have massive hematemesis and melena with drop in hemoglobin, requiring more than 4 units of packed red blood cells. He was resuscitated after cardiac arrest, but continued to have upper gastrointestinal bleeding with abdominal distention and signs of peritonitis. Abdominal computed tomography (CT) with contrast was requested to rule out bowel ischemia. CT showed free air in the abdomen posterior to the fundus of the stomach representing perforation of posterior wall and acute infarction of spleen. An emergency laparotomy revealed a large posterior gastric wall perforation at cardio-fundal area with abscess formation, trimming of the necrotic edge with two layers, closure of the gastric wall and splenectomy were performed. Small and large bowels were found normal. Post-operatively, the patient developed disseminated intravascular coagulopathy and died in the early post-operative period.
Histopathology of the edge of the posterior gastric wall ulcer and spleen showed branching non-septated fungal hyphae with an extensive necrosis [Figure 1]. Deep sections showed non-septated fungal hyphae and spores invading blood vessel and spleen parenchyma with extensive tissue necrosis.
Figure 1.
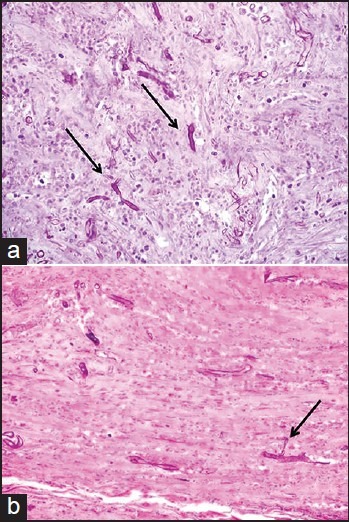
(a) Periodic acid Schiff (PAS) after diastase digestion (×40): Non-septated fungal hyphae with branching (arrow). Background shows extensively necrotic gastric wall. (b) PAS after diastase digestion (×40) revealing non-septated fungal hyphae with branching and extensive necrosis (arrow) — spleen
A diagnosis of spontaneous gastric perforation due to invasive gastric mucormycosis with acute spleen infarction was made. As the diagnosis was not suspected during the surgery, no specimen was submitted for fungal culture.
DISCUSSION
Mucor species are opportunists. Similar to the other members of the family Mucoraceae, infections are seen with a variety of disease states that cause immunosuppression.
Cases have been identified associated with leukemia, aplastic anemia, organ or bone marrow transplantation, diabetes mellitus, renal disease, iron overload, asthma, gastric cancer, burns and prednisone therapy.[2] Diabetes mellitus was seen as the major risk factor for developing rhinocerebral zygomycosis.[4] In the original publication by Gregory et al.,[4] two out of the three patients reported had presented with diabetic ketoacidosis and the third patient was thought to have an undiagnosed case of diabetes. In another report, a 17-year-old woman with diabetic ketoacidosis and severe epigastric pain due to an extensive stomach ulcer; found to have invasive mucormycosis. Treatment with amphotericin B was initiated, but severe persistent gastrointestinal bleeding resulted in the patient's death.[5]
Mucormucosis of the gastrointestinal tract is an unusual form of the disease, accounting for only 7% of all reported cases.[6,7] Only 25% of cases of gastrointestinal mucormycosis are diagnosed ante-mortem; mortality is high, primarily due to bowel perforation. The ingestion of fermented milk with dried bread products or fermented porridges and alcoholic drinks derived from corn may play a role in promoting gastric zygomycosis.[2] Spore-contaminated herbal or homeopathic remedies have likewise been linked to gastrointestinal disease. The stomach is the most common site of gastrointestinal mucormycosis, followed by the colon and ileum. The symptoms of gastrointestinal mucormycosis are varied and depend on the affected site. Non-specific abdominal pain and distention associated with nausea and vomiting are the most common symptoms. Fever and hematochezia may also occur. The patient is often thought to have an intra-abdominal abscess.[8] Emphysematous gastritis associated with invasive gastric mucormycosis is an extremely rare condition associated with heavy alcohol abuse and diabetes mellitus.[9]
Fungal elements are frequently noted overlying the base of chronic peptic ulcers of the stomach and it has been suggested that the fungi enhance the degree of necrosis and that these cases have protracted disease and deeper ulcers with more perforations.
A study by Al-Rikabi et al., described a very rare case of invasive mucormycosis occurring in the base of a chronic gastric ulcer in a diabetic male which was clinically and radiologically been mistaken for a gastric carcinoma. The ulcer was complicated by perforation and fungal septicemia with subsequent fatal outcome.[10]
In a series of 20 patients, Thomson et al.,[11] classified gastrointestinal mucormycosis into 3 histological categories: Colonization, infiltration and vascular invasion. In 10 patients, mucormycosis complicated peptic ulcer disease (PUD), 3 with colonization, with 5 infiltration and 2 with vascular invasion. Mucormycosis had a less aggressive course when complicating PUD than when it occurred in association with other gut diseases. None of the patients with PUD died compared to the other 10 patients who had infection associated with other gastrointestinal diseases: Post-traumatic peritonitis (4 patients), transmural amoebiasis (2 patients), tuberculosis (1 patient), gastroenteritis (1 patient), gastric carcinoma (1 patient) and diabetes (1 patient). Eight patients had a significant infection and only one survived.
Clinical diagnosis is difficult as symptoms are nonspecific, however gastrointestinal mucormycosis can be diagnosed with endoscopic biopsy of the lesions, with histopathologic demonstration of the characteristic broad non-septated hyphae in the affected tissues. Imaging with contrast CT scan can be done to rule out bowel ischemia. Treatment of zygomycosis involves a combination of surgical debridement of involved tissues in conjunction with liposomal amphotericin B which is considered the drug of choice. Our patient had isolated invasive gastric mucormycosis with contiguous spread through vascular invasion and thrombosis involving splenic artery, which resulted in infarction and necrosis of the spleen. The natural history of the disease and the multiple co-morbidities with cardiac and renal dysfunction has contributed for our patient early mortality. As the patient died in the early post-operative period, the diagnosis was verified histologically after his death and therefore anti-fungal treatment was not started.
CONCLUSION
Perforated gastric ulcer is a very rare presentation of gastric mucormycosis which can be complicated by angio-invasion, thrombosis and infarction of the spleen. High index of clinical suspicion along with endoscopic biopsy, fungal culture and CT to rule out other causes of the acute abdomen can lead to early diagnosis.
Surgical debridement followed by antifungal therapy can improve the survival rate.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 4.Gregory JE, Golden A, Haymaker W. Mucormycosis of the central nervous system: a report of three cases. Bull Johns Hopkins Hosp. 1943;73:405–19. [Google Scholar]
- 5.Paulo De Oliveira JE, Milech A. A fatal case of gastric mucormycosis and diabetic ketoacidosis. Endocr Pract. 2002;8:44–6. doi: 10.4158/EP.8.1.44. [DOI] [PubMed] [Google Scholar]
- 6.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 7.Lyon DT, Schubert TT, Mantia AG, Kaplan MH. Phycomycosis of the gastrointestinal tract. Am J Gastroenterol. 1979;72:379–94. [PubMed] [Google Scholar]
- 8.Spellberg B. Gastrointestinal mucormycosis: An evolving disease. Gastroenterol Hepatol (N Y) 2012;8:140–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Jung JH, Choi HJ, Yoo J, Kang SJ, Lee KY. Emphysematous gastritis associated with invasive gastric mucormycosis: A case report. J Korean Med Sci. 2007;22:923–7. doi: 10.3346/jkms.2007.22.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Rikabi AC, Al-Dohayan AD, Al-Boukai AA. Invasive mucormycosis in benign gastric ulcer. Saudi Med J. 2000;21:287–90. [PubMed] [Google Scholar]
- 11.Thomson SR, Bade PG, Taams M, Chrystal V. Gastrointestinal mucormycosis. Br J Surg. 1991;78:952–4. doi: 10.1002/bjs.1800780819. [DOI] [PubMed] [Google Scholar]


