Abstract
Background:
Viral hepatitis is a major public health problem throughout the world. It is the inflammation of the liver due to the infection of any of the five main hepatic viruses A to E and it affects the liver through different modes of transmission. This study mainly aims at the frequency and distribution of viral hepatitis based on age and sex during a time period of 5 years.
Materials and Methods:
This is a hospital-based retrospective study of 5 years at a tertiary level hospital in Kerala state in India. Medical records department of the hospital follow the guidelines of International Classification of Diseases-10 for coding the diseases. The data on frequency and distribution of viral hepatitis based on age and sex during a period of 5 years from April 2005 to March 2010 were collected and analyzed and ‘z’ test was used for finding out the difference in proportions.
Result:
Out of 818 cases, 76.03% were males and 23.96% were females. The preponderance of males was apparent in all types of viral hepatitis infection. The high risk groups were the adults in the age group of 20-39 years. The main cause in the present study was hepatitis E virus (HEV) and followed by hepatitis A virus (HAV). Of total viral hepatitis cases, 31.54% were due to HAV, 6.35% hepatitis B virus, 0.85% hepatitis C virus and 61.24% were due to HEV respectively. In the present study, there was no case of hepatitis D virus has reported. The case fatality rate of viral hepatitis in the present study was minor than 1% (0.98%); whereas males were 0.96%; females of 1.02%.
Conclusion:
Taking the safety measures including vaccination and proper management of waste materials are the only solution to control or eradicate this infection.
Keywords: Age, Sex and cause, Viral hepatitis
INTRODUCTION
Viral hepatitis can be defined as inflammation of liver due to infection of any of the hepatotropic viruses. WHO executive board[1] certify that 10 lakhs people die each year due to the causes related to viral hepatitis which is 2.7% of the total deaths occur in the world. Some groups are at high risk of contracting this disease compared with others. Hepatitis A virus (HAV) and hepatitis E virus (HEV) are found higher in communities where food and sanitation are poor and HBV and HCV infections are seen more common among the recipients of organs, blood and tissues and also among the health care providers who sustain accidental needle stick injuries while caring these patients.[2] Pregnant women also at high risk of infection because HEV affects young males and females. Arun Kumar Mitra et al.[3] had revealed in their study on liver disorders during pregnancy and their management that pregnant women were at high risk for acute and fulminant hepatitis compared to non-pregnant women because mortality rate in pregnancy reported as 25% while it was 0.65% in non-pregnant women even though the incidence of acute viral HEV is same in both pregnant and non-pregnant women.
Acute infections show the symptoms such as jaundice, dark urine, extreme fatigue, nausea, vomiting and abdominal pain.[4] HAV or infectious hepatitis is an acute infectious disease of liver caused by the HAV through fecal-oral route. HAV is a small, non-enveloped, single stranded ribonucleic acid (RNA) virus and it is transmitted to man by the ingestion of contaminated food and water[5] or direct contact with an infectious person and it has only a short period of incubation. Hepatitis B virus (HBV) is an infectious inflammatory illness of the liver caused by the HBV.[6] It is a complex, 42-nm double shelled deoxyribonucleic acid virus and the infection spread either from carriers or from cases.[7] It is transmitted through parenteral or perinatal route or through sexual transmission. Hepatitis C virus (HCV) is a single stranded RNA virus which is transmitted through transfusion of blood or blood products or sexual transmission.[8] hepatitis D virus (HDV) is caused by a small enveloped RNA virus and its transmission resembles HBV.[8] HEV is a viral hepatitis or liver inflammation caused by infection with a virus called HEV. HEV is a positive-sense single-stranded RNA icosahedral virus with a 7.5 kb genome.[8] It is transmitted through the consumption of contaminated water or food. The main aim of the study is to find out the frequency and distribution of different types of viral hepatitis based on age, sex and its infective causes during a time period of 5 years.
MATERIALS AND METHODS
The present study was conducted at Malankara Orthodox Syrian Church Medical College Hospital in Kolenchery, Kerala state in South India. The data were retrieved from medical records department with the approval of hospital ethical committee. Medical records department follow the guidelines of International Classification of Diseases-10 for coding the diseases. The present study aims at the frequency and distribution of viral hepatitis based on age, sex during a time period of 5 years from April 2005 to March 2010. The data related to viral hepatitis (B15-B19) were collected and analyzed and “z” test was used to find out the difference in proportions.
RESULT
Out of 818 confirmed hepatic patients in the study hospital, 622 (76.03%) were males and 196 (23.96%) were females [Figure 1]. Out of 818 reported hepatitis cases, 244 cases (29.82%) were in the age group of < 19 years, 447 (54.64%) were in 20-39 years, 94 (11.49%) cases were in 40-59 years, 30 cases (3.66%) were in 60-79 years and 3 cases (0.36%) were in the age group of 80 years and above. The high proportion of cases was observed in the 20-39 years and low in 80rs and above. The preponderance of males was observed in all age groups. As shown in the Table 1, <19 years, 71.31% were males and 28.68% were females. In the age group of 20-39 years, 79.19% were males and 20.80% were females. In 40-59 years, 69.14% were males and 30.85% were females. In 60-79 years, 86.66% were males and 13. 33% were females. In the age group of 80 years and above all the cases were males. The age groups <19 years (P = 0.02) and 20-39 years (P = 0.00) showed significant difference between males and females. The age wise distribution of cases according to different types of viral hepatitis has shown in Table 2. The main cause of viral hepatitis in the present study was HEV followed by HAV. Of total viral hepatitis cases, 258 cases (31.54%) were due to HAV, 52 cases (6.35%) were due to HBV, 7 cases (0.85%) were due to HCV, 0 cases due to HDV and 501 cases (61.24%) were due to HEV [Figure 1].
Figure 1.
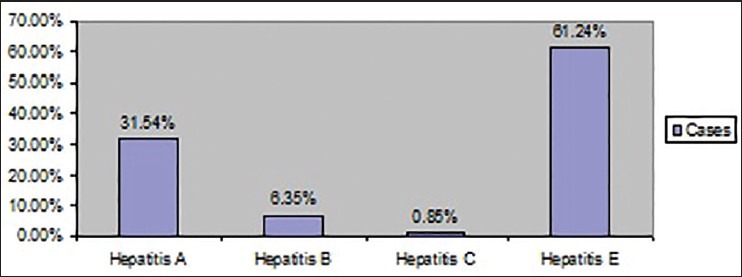
Infective causes of viral hepatitis
Table 1.
Age and sex wise distribution of viral hepatitis
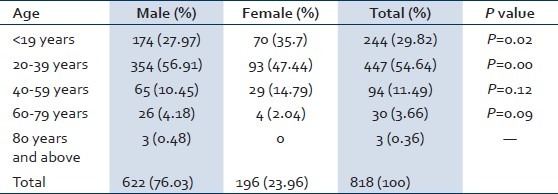
Table 2.
Distribution of viral hepatitis based on age and infective causes
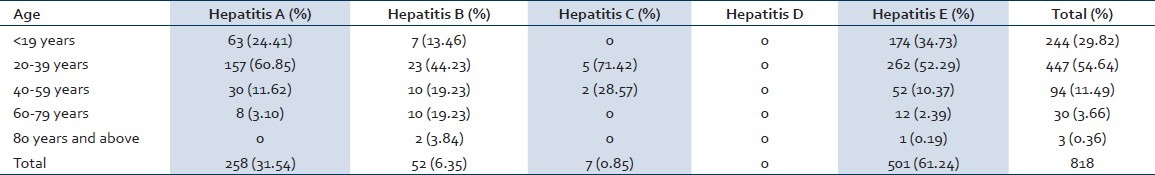
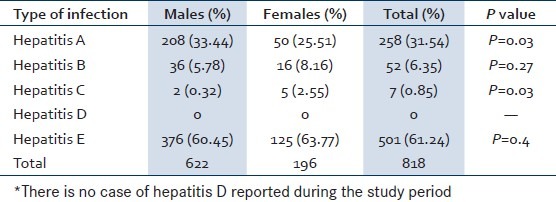
The male dominancy had observed in HAV, HBV and HEV. However, the HCV contracted high proportion of cases in females compared to males. HAV (P = 0.03) and HEV (P = 0.03) showed significant difference between males and females. Sex wise distribution of different types of viral hepatitis has specified in the Table 3.
Table 3.
Sex wise distribution of different types of viral hepatitis
The case fatality of viral hepatitis in the present study was 0.98%; while males fatality 0.96%; females of 1.02% respectively. The trend of case fatality was increased from 2005 to 2006 and continued up to 2008 and a decline of fatality has observed throughout 2009 and 2010. The trend of case fatality of viral hepatitis has shown in the Figure 2.
Figure 2.
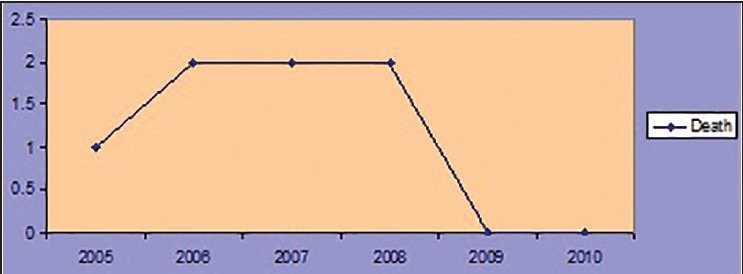
Trend of case fatality of viral hepatitis
DISCUSSION
Viral hepatitis is reporting from almost all countries in the world and it consider as a global public health problem.[9] The common infective causes of viral hepatitis are HAV, HBV, HCV, HDV and HEV. In the present study, as shown in the Figure 2, of total 818 hepatitis cases, 31.54% was due to HAV, 6.35% was due to HBV, 0.85% was due to HCV and 61.24% account for HEV respectively. HDV infection had not reported in the study hospital during the study period of 5 years. The trend of different types of viral hepatitis during the study period has shown in the Figure 3.
Figure 3.
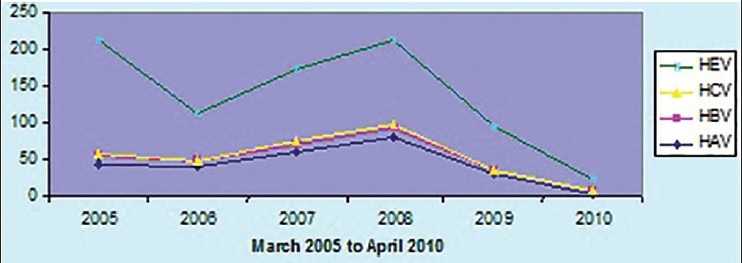
Trend of different types of viral hepatitis
HAV
Studies reveals that HAV is a predominant cause of viral hepatitis[10] and it constitute around 32.1% of the total viral hepatitis reported all over the world.[11] It is endemic in most developing countries, with frequent outbursts of minor or major outbreaks.[12] The high endemicity of hepatitis A is reporting from low socio-economic countries or regions with poor hygiene standards, where the incidence of infection with this virus reported as high among children,[13] because the infection is associated with contaminated food and water due to unhygienic environment. Studies have shown that along with the progress of socio-economic status, the provision of safe water supply and sanitation programs gets better and the infection moves from children to adult. Kunasol reports that India was high endemic for HAV.[14] Previous sero-epidemiological studies in India and in other developing countries such as Bangladesh, Bhutan and Nepal also demonstrated that 85-95% of children have been infected with the HAV infection and are immune to HAV infection by their 10 years of age.[14] However, recent studies have noticed a shift of HAV infection from child to adult[15] and there had a progress of safe water and sanitation comparatively. In the present study, the HAV infection is 24.41% in <19 years; majority of cases 60.85% were in 20-39 years; 11.62% in 40-59 years, 3.1% in 60-79 years and none of the cases has reported in the 80 years and above. Sebastian et al.[16] in his study on outbreak of HAV in central India reveals that, out of 399 confirmed cases of HAV, 65% was in 15-33 years age group. A study conducted by Arankalle et al.[17] in Kottayam district of Kerala revealing the major outbreak of HAV with more cases in adults compared with children. Hussain et al.[18] in their study in North India reveals that HAV infection in adults increasing from 3.4% in 1999 to 12.3% in 2003. Mathur and Arora in their study[19] on epidemiological transition of HAV in India supports that age of HAV acquiring is shifting from childhood to adolescent. Not only the Indian studies but the studies from all over the world also pointing the fact that along with the socio-economic improvement, infection of HAV cases increasing in the adult compared to childhood.[20] The studies reports from Korea,[15] Indonesia, Thailand, China,[21] Sri Lanka and Malaysia[22] also reports that the infection level increasing as per age increases.
In the present study of HAV infection, males are predominant than females. Out of 258 cases 80.62% were males while females were of 19.37% only. A study by Wu et al.[23] in Canada reports that males have higher proportion than females in the infection of HAV. Barrientos Gutierrez et al.[24] in their study on HAV infection reveals in their study that 77.2% of cases were males. But Faleh et al.[25] in their study on changing patterns of HAV prevalence in the Saudi population over the 18 years observed that no difference occurred among males and females in the HAV causation.
HBV or serum hepatitis
Of the 2 billion people who have been infected with the HBV in the world, more than 350 million have chronic infection and they have high risk of death from cirrhosis of liver and liver cancer and results 1 million death every year[26] and in the South East Asian Region countries only, it is the cause of 2 lakh death annually.[27] HBV has caused epidemics in Asia and Africa[28] frequently and endemic in Eastern Europe, the Mediterranean, South America and China. HBV accounts for 15-30% cases of the acute hepatitis in India[29] and it is transmitted through exposure to infectious blood, semen and other body fluids or infected mother to infants at the time of birth, contaminated infections during medical procedures and also through sharing of needles and syringes among injecting drug users[30] and also the health care providers through accidental needle stick injuries.
In the present study, 6.35% were due to HBV. Out of 52 cases of HBV, 69.23% were males and only 30.76% were females. This predominance of males in case infection is apparent in other similar studies all over the world. Tessema et al.[31] in their study in North West Ethiopia reports that HBV infection in males is 4.9% and in females are 3.3%. Another study by Baig et al. in Pakistan[32] reveals in their study on gender disparity in infections of HBV that males constitute 79.5% and females have only 20.5% of total HBV infection. Manzoor et al.[33] in their study on HBV related chronic liver diseases at Rawalpindi in Islamabad reveals the preponderance of males in their study as 64% and females only as 36%. Naz et al.[34] in their study in Muzaffarabad on prevalence of HBV among Combined Military Hospital reports that 68.3% were males and 31.7% of females in HBV infection. Khan et al.[35] in their study on HBV infection among different sex and age groups in Pakistani Punjab reporting 68.15% of males infection and 31.85% female infection of HBV. Study by Abel Girma and Ayele and Solomon Gebre Selassie in Ethiopia,[36] and Ahmad et al.[37] in Pakistan also support the male predominance in HBV infection.
In the present study, the highest frequency of cases of HBV occurred 44.23% in 20-39 years followed by 40-59 years and 60-79 years equally as 19.23%. In the age group of <19 years, the proportions of cases were 13.46% and less infection of cases were reported in 80 years and above which was 3.84%. Khan et al.[35] in their study on HBV infection among different sex and age groups in Pakistani Punjab strongly supporting the findings of present study which reveal the highest percentage of HBV infection which was occurred in the age group of 21-30 years (34.93%) followed by 23.83% in 31-40 years. Alam et al.[38] have observed in their study on Molecular epidemiology of HBV genotypes in Pakistan that high proportion of cases were in the age group of 20-40 years which was followed by 41-60 years. Cisneros-Castolo et al.[39] also admit in their study on Prevalence of HBV infection and related risk factors in a rural community of Mexico that more number of cases caused in adults up to the age of 40 years.
HCV affects 175 million people worldwide and it is a leading cause of liver transplantation.[40] In the present study, 0.85% of cases were due to HCV. Of the total cases, 28.57% were in males and 71.42% were in females. On the contrast to the HAV and B infection, the dominance of females in HCV infection has observed in this study. A study conducted by Charles et al.[41] on Seroprevalence of HCV and associated factors in urban areas of Antananarivo, Madagascar also reveals the preponderance of females in HCV infection. Mohammad Saleem et al.[42] also admit the high proportion of female cases in their study on frequency of HCV in district headquarters hospital, Kotli, Azad Kashmir.
In the present study highest proportion of cases; 71.42% were occurred in the age group of 20-39 years and followed by 28.57% in the 40-59 years. Mohammad Saleem et al.[42] support the findings of present study by revealing the fact that 60.54% of cases were occurred in the age interval of 21-40 years. But Amjad Ali et al.[43] refute these findings and mentioned that majority of cases happened in 51-60 years at their study on prevalence of active HCV virus infection in Mansehra district of Pakistan.
HEV is a major problem in the areas where water and sanitation problems exist.[44] It reports from Algeria, Bangladesh, China, Ethiopia, Indonesia, Iran, Libyan Arab Jamahiriya, Mexico, Myanmar, Nepal, Pakistan, Somalia and Central Asian republic of US. In India, it is the 30-70% cause of acute sporadic hepatitis and is the major cause of liver failure.[45] In the present study, HEV is the main cause of viral hepatitis; it is 61.24% of the total infection. Studies conducted by Chadha et al.[46] in Pune, Das et al.[47] in urban Delhi, Khuroo et al.[48] in North India also supports these findings.
In the present study, males are more infected than females in HEV infection. A study conducted by Manmohan et al.[49] in Tamil Nadu on retrospective hospital-based study of infective causes of Jaundice in Tamil Nadu revealed that men are more infected than females in HEV infection. In another study by Dhamdhere and Nadkarni[50] on infectious hepatitis at Aurangabad also observed that predominance of men in the infection of HEV. Mishra et al.[51] in their hospital-based study of HEV in Bangalore also noted the high male preponderance where 72 male infected while 35 female infected by this virus. In the present study highest proportion of cases (52.29%) were occurred in adult aged 20-39 years. Nargis et al.[52] also reveals high proportion of cases in 20-24 years which is 71.9%.
In the present study, the major cause of viral hepatitis is HEV (61.24%) and it is followed by HAV (31.54%). HAV and HEV types of viral hepatitis appears as a widespread problem in developing countries where there are problems in providing safe drinking water and adequate sewage disposal.[12] Wierzba and Panzner[53] reports on the International Symposium of the hepatitis that HEV epidemics occur in areas where the hygiene and sanitation problems exist especially the countries in Asia, the Middle East, Africa and Latin America. Indian studies conducted by the Acharya et al.[54] and Nanda et al.[45] also observed that enteric hepatitis is highly endemic in India. In the present study main cause of viral hepatitis was HEV which is followed by HAV. Manmohan et al.[49] in their study on viral hepatitis reveals that more prominent cause of infection is HEV which is 37.49% and it is followed by HAV with 32%. It is supported by the other studies conducted by Chadha et al.[46] in Pune, Das et al.[47] in urban Delhi, Tandon et al.[29] in North India and Madan et al.[55] in their study on detection of HCV and HEV genomes in sera of patients with acute viral hepatitis. The main cause of transmission of HAV and HEV types of viral hepatitis was the improper disposal of waste material and there by contamination of water sources. This is one of the burning public health issues in the present India. People reside in the township area or in the cities dispose all sorts of waste materials including nappies containing feces in the public places due to lack of proper waste management facilities in their household area. These disposed waste materials function as a source of enteric hepatitis and other water borne and vector borne diseases and contaminate the overflowing rainy water in the monsoon and it is getting mixed with the safe drinking water and spreads the infection in the general population easily and leads to the outbreaks of epidemics. These enteric hepatitis viruses especially infect the children who are at high risk because of their low immunity status. As contaminated food and water is the main mode of transmission much emphasize to be given for environmental and personal hygiene practices to prevent the fecal oral transmission of this pathogen.
CONCLUSION
In the present study, the main causes of viral hepatitis are HAV and HEV. The preponderance of males was observed in all age groups and in all types of viral hepatitis (HAV, HBV and HEV) except HCV where the proportion of females was comparatively high. Even though all types of viral hepatitis are being public health problem to the community, the HEV stands as continuous burden through the outbreaks of epidemics and its endemicity. This could be controlled by ensuring the safe drinking water supply and good standards of sanitation and of personal and food hygiene. The way to interrupt the transmission of HBV and HCV types of viral hepatitis is taking the safety measures before receiving blood and blood products or giving care to the infected patients. However, this hospital study shows a decline in the infection of all types of viral hepatitis from 2008 to 2010. Awareness building in the community about the safety measures and proper management of waste materials are the main remedies to control or pull out the roots of this infection.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.WHO Executive Board. Viral hepatitis. Report by the secretariat, 2009. EB126/15.12. 2009. Nov, [Last accessed on 3 May 2012]. Available from: http://www.apps.who.int/gb/ebwha/pdf_files/EB 126/B 126_15-en pdf .
- 2.Abou MA, Eltahir YM. Seropositivity of hepatitis B virus and hepatitis C virus dual infection among blood donors in Nyala teaching hospital. Virol J. 2009;6:227. doi: 10.1186/1743-422X-6-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arun Kumar Mitra, Patki PS, Mitra SK. Liver disorders during pregnancy and their management. Antiseptic. 2008;105:193–6. [Google Scholar]
- 4.Ryder SD, Beckingham IJ. ABC of diseases of liver, pancreas, and biliary system: Acute hepatitis. BMJ. 2001;322:151–3. doi: 10.1136/bmj.322.7279.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton FL. Viral hepatitis in a community. Health Serv Rep. 1973;88:236–40. [PMC free article] [PubMed] [Google Scholar]
- 6.Barker LF, Shulman NR, Murray R, Hirschman RJ, Ratner F, Diefenbach WC, et al. Transmission of serum hepatitis.1970. JAMA. 1996;276:841–4. [PubMed] [Google Scholar]
- 7.Seeger C, Mason WS. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuckerman AJ. Encyclopedia of Life Sciences. London, UK: John Wiley & Sons, Ltd.; 2011. [Last accessed on 2009 October]. Hepatitis viruses. Available from: http://www.els.net . [Google Scholar]
- 9.World Health Organization. Viral Hepatitis WHA 63.18. Geneva, Switzerland: 2010. May 21, Sixty Third World Health Assembly. [Google Scholar]
- 10.Karagöz G, Ak O, Ozer S. The coexistence of hepatitis A and infectious mononucleosis. Turk J Gastroenterol. 2005;16:102–4. [PubMed] [Google Scholar]
- 11.Singh J, Prakash C, Gupta RS, Bora D, Jain DC, Datta KK. Epidemiology of endemic viral hepatitis in an urban area of India: A retrospective community study in Alwar. Bull World Health Organ. 1997;75:463–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Park K. M/s. Banarasidas Bhanot Jabalpur. 19th ed. 2007. Park's Text Book of Preventive and Social Medicine; pp. 173–9. [Google Scholar]
- 13.Steffen R. Changing travel-related global epidemiology of hepatitis A. Am J Med. 2005;118(Suppl 10A):46S–9. doi: 10.1016/j.amjmed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Kunasol P, Cooksley G, Chan VF, Isahak I, John J, Loleka S, et al. Hepatitis A virus: Declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 1998;29:255–62. [PubMed] [Google Scholar]
- 15.Kim YJ, Lee HS. Increasing incidence of hepatitis A in Korean adults. Intervirology. 2010;53:10–4. doi: 10.1159/000252778. [DOI] [PubMed] [Google Scholar]
- 16.Sebastian B, Mathai S, Mathew G, Ouseph M, Balakrishnan P. An outbreak of hepatitis A in central India: changing patterns. Indian J Gastroenterol. 2001;20:132–5. [Google Scholar]
- 17.Arankalle VA, Sarada Devi KL, Lole KS, Shenoy KT, Verma V, Haneephabi M. Molecular characterization of hepatitis A virus from a large outbreak from Kerala, India. Indian J Med Res. 2006;123:760–9. [PubMed] [Google Scholar]
- 18.Hussain Z, Das BC, Husain SA, Murthy NS, Kar P. Increasing trend of acute hepatitis A in north India: Need for identification of high-risk population for vaccination. J Gastroenterol Hepatol. 2006;21:689–93. doi: 10.1111/j.1440-1746.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 19.Mathur P, Arora NK. Epidemiological transition of hepatitis A in India: Issues for vaccination in developing countries. Indian J Med Res. 2008;128:699–704. [PubMed] [Google Scholar]
- 20.Barzaga BN. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine. 2000;18(Suppl 1):S61–4. doi: 10.1016/s0264-410x(99)00467-3. [DOI] [PubMed] [Google Scholar]
- 21.Cao J, Wang Y, Song H, Meng Q, Sheng L, Bian T, et al. Hepatitis A outbreaks in China during 2006: Application of molecular epidemiology. Hepatol Int. 2009;3:356–63. doi: 10.1007/s12072-008-9116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. The global prevalence of hepatitis A virus infection and susceptibility: A systematic review. [Last accessed on 2011 November]. Available from: http://www.whqlibdoc.who.int/hq/2010/WHO/NB 10.01eng.pdf .
- 23.Wu J, Zou S, Giulivi A. Current hepatitis A status in Canada. Can J Infect Dis. 2001;12:341–4. doi: 10.1155/2001/834670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrientos-Gutiérrez T, Brizuela-Alcántara D, Chávez-Tapia NC. Hepatitis A virus infection in high-risk subjects. Ann Hepatol. 2011;10:578–9. [PubMed] [Google Scholar]
- 25.Al Faleh F, Al Shehri S, Al Ansari S, Al Jeffri M, Al Mazrou Y, Shaffi A, et al. Changing patterns of hepatitis A prevalence within the Saudi population over the last 18 years. World J Gastroenterol. 2008;14:7371–5. doi: 10.3748/wjg.14.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao MB. The prevalence of hepatitis B in India and its prevention with Ayurveda - A revisit. J New Approaches Med Health. 2012;19:4. [Google Scholar]
- 27.WHO. Health Situation in the South East Asia Region 1994-1997. New Delhi: South-East Asia Region; 1999. [Google Scholar]
- 28.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–6. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 29.Tandon BN, Gandhi BM, Joshi YK. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B virus infection in north India. Bull World Health Organ. 1984;62:67–73. [PMC free article] [PubMed] [Google Scholar]
- 30.Kidd-Ljunggren K, Holmberg A, Bläckberg J, Lindqvist B. High levels of hepatitis B virus DNA in body fluids from chronic carriers. J Hosp Infect. 2006;64:352–7. doi: 10.1016/j.jhin.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Tessema B, Yismaw G, Kassu A, Amsalu A, Mulu A, Emmrich F, et al. Seroprevalence of HIV, HBV, HCV and syphilis infections among blood donors at Gondar University Teaching Hospital, Northwest Ethiopia: Declining trends over a period of five years. BMC Infect Dis. 2010;10:111. doi: 10.1186/1471-2334-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baig S. Gender disparity in infections of Hepatitis B virus. J Coll Physicians Surg Pak. 2009;19:598–600. [PubMed] [Google Scholar]
- 33.Manzoor SA, Malik IA, Tariq Wuzt, Butt SA, Luqman M, Ahmed N. Hep B related chronic liver disease in Rawalpindi Islamabad. J Coll Physicians Surg Pak. 1997;7:43–6. [Google Scholar]
- 34.Naz S, Ahmad M, Asghar H. Prevalence of hepatitis ‘B’ among combined military hospital (CMH) Muzaffarabad. Int J Agric Biol. 2002;4:227–30. [Google Scholar]
- 35.Khan F, Shams S, Qureshi ID, Israr M, Khan H, Sarwar MT, et al. Hepatitis B virus infection among different sex and age groups in Pakistani Punjab. Virol J. 2011;8:225. doi: 10.1186/1743-422X-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayele AG, Gebre Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Adaba, Ethiopia. ISRN Trop Med 2013. 2013:1–7. [Google Scholar]
- 37.Ahmad I, Khan SB, Rahman HU, Khan MH, Anwar S. Frequency of hepatitis B and hepatitis C among cataract patients. Gomal J Med Sci. 2006;4:61–4. [Google Scholar]
- 38.Alam MM, Zaidi SZ, Malik SA, Shaukat S, Naeem A, Sharif S, et al. Molecular epidemiology of Hepatitis B virus genotypes in Pakistan. BMC Infect Dis. 2007;7:115. doi: 10.1186/1471-2334-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cisneros-Castolo M, Hernández-Ruiz L, Ibarra-Robles IE, Fernández-Gárate RH, Escobedo-De La Peña J. Prevalence of hepatitis B virus infection and related risk factors in a rural community of Mexico. Am J Trop Med Hyg. 2001;65:759–63. doi: 10.4269/ajtmh.2001.65.759. [DOI] [PubMed] [Google Scholar]
- 40.Sarbah SA, Younossi ZM. Hepatitis C: An update on the silent epidemic. J Clin Gastroenterol. 2000;30:125–43. doi: 10.1097/00004836-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ramarokoto CE, Rakotomanana F, Ratsitorahina M, Raharimanga V, Razafindratsimandresy R, Randremanana R, et al. Seroprevalence of hepatitis C and associated risk factors in urban areas of Antananarivo, Madagascar. BMC Infect Dis. 2008;8:25. doi: 10.1186/1471-2334-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saleem M, Waseem A, Sarwar J, Jamshed F, Gul N, Muhammad I. Frequency of Hepatitis C in asymptomatic patients in district headquarters hospital Kotli, Azad Kashmir. J Ayub Med Coll. 2011;23:59. [PubMed] [Google Scholar]
- 43.Ali A, Ahmad H, Ali I, Khan S, Zaidi G, Idrees M. Prevalence of active hepatitis c virus infection in district Mansehra Pakistan. Virol J. 2010;7:334. doi: 10.1186/1743-422X-7-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EJ, Schwab KJ. Deficiencies in drinking water distribution systems in developing countries. J Water Health. 2005;3:109–27. [PubMed] [Google Scholar]
- 45.Nanda SK, Yalcinkaya K, Panigrahi AK, Acharya SK, Jameel S, Panda SK. Etiological role of hepatitis E virus in sporadic fulminant hepatitis. J Med Virol. 1994;42:133–7. doi: 10.1002/jmv.1890420207. [DOI] [PubMed] [Google Scholar]
- 46.Chadha MS, Walimbe AM, Chobe LP, Arankalle VA. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978-81 and 1994-97. Indian J Gastroenterol. 2003;22:11–5. [PubMed] [Google Scholar]
- 47.Das K, Agarwal A, Andrew R, Frösner GG, Kar P. Role of hepatitis E and other hepatotropic virus in aetiology of sporadic acute viral hepatitis: A hospital based study from urban Delhi. Eur J Epidemiol. 2000;16:937–40. doi: 10.1023/a:1011072015127. [DOI] [PubMed] [Google Scholar]
- 48.Khuroo MS, Kamili S, Dar MY, Moecklii R, Jameel S. Hepatitis E and long-term antibody status. Lancet. 1993;341:1355. [PubMed] [Google Scholar]
- 49.Manmohan G, Patil R, Khan MI, Gupta SK. Retrospective hospital based study of infective causes of jaundice in Tamilnadu, India. Calicut Med J. 2011;9:1–4. [Google Scholar]
- 50.Dhamdhere MR, Nadkarni MG. Infectious hepatitis at Aurangabad. Report of an outbreak. Indian J Med Sci. 1962;16:1006–15. [PubMed] [Google Scholar]
- 51.Mishra B, Srinivasa H, Muralidharan S, Charles S, Macaden RS. A hospital based study of hepatitis E by serology. Indian J Med Microbiol. 2003;21:115–7. [PubMed] [Google Scholar]
- 52.Begum N, Devi SG, Husain SA, Ashok Kumar, Kar P. Seroprevalence of subclinical HEV infection in pregnant women from north India: A hospital based study. Indian J Med Res. 2009;130:709–13. [PubMed] [Google Scholar]
- 53.Wierzba TF, Panzner U. Report on the international symposium on hepatitis E, Seoul, South Korea, 2010. Emerg Infect Dis. 2012;18:5. doi: 10.3201/eid1805.111916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acharya SK, Batra Y, Bhatkal B, Ojha B, Kaur K, Hazari S, et al. Seroepidemiology of hepatitis A virus infection among school children in Delhi and north Indian patients with chronic liver disease: Implications for HAV vaccination. J Gastroenterol Hepatol. 2003;18:822–7. doi: 10.1046/j.1440-1746.2003.03051.x. [DOI] [PubMed] [Google Scholar]
- 55.Madan K, Gopalkrishna V, Kar P, Sharma JK, Das UP, Das BC. Detection of hepatitis C and E virus genomes in sera of patients with acute viral hepatitis and fulminant hepatitis by their simultaneous amplification in PCR. J Gastroenterol Hepatol. 1998;13:125–30. doi: 10.1111/j.1440-1746.1998.tb00626.x. [DOI] [PubMed] [Google Scholar]


