Abstract
Osteomyelitis is a significant cause of morbidity in children throughout the world. Multiple imaging modalities can be used to evaluate for suspected osteomyelitis, however magnetic resonance imaging (MRI) has distinct advantages over other modalities given its ability to detect early changes related to osteomyelitis, evaluate the true extent of disease, depict extraosseous spread of infection, and help guide surgical management. MRI has assumed a greater role in the evaluation of osteomyelitis with the increase in musculoskeletal infections caused by methicillin-resistant Staphylococcus aureus which have unique imaging features that are well-demonstrated with MRI. This review focuses primarily on the use of MRI in the evaluation of osteomyelitis in children and will include a discussion of the clinically important and characteristic findings on MRI of acute bacterial osteomyelitis and related conditions.
Keywords: Magnetic resonance imaging, Osteomyelitis, Pediatrics, Infectious diseases
Core tip: Osteomyelitis is a significant cause of morbidity and mortality in children. Plain radiography and radionuclide bone scintigraphy, which have been the traditional imaging modalities for detecting osteomyelitis, both have significant limitations. Magnetic resonance imaging (MRI) is increasingly relied upon for detecting osteomyelitis in children, due to its superior soft tissue contrast for detecting early disease and extraosseous complication, as well as its lack of ionizing radiation exposure to patients. This article focuses on basic and advanced MRI techniques for evaluating osteomyelitis, as well as MRI imaging features of disease and their impact on clinical management.
INTRODUCTION
Musculoskeletal infection is a significant cause of morbidity and mortality in children throughout the world. This category of disease encompasses both osteomyelitis and septic arthritis, however this review will be primarily focused on the former. Osteomyelitis is typically categorized as either hematogenous or non-hematogenous. Hematogenous osteomyelitis typically occurs when circulating pathogenic organisms take up residence in the metaphyses of long bones due to sluggish circulation in these regions. Non-hematogenous osteomyelitis, on the other hand, results from direct inoculation of organisms into bone due to penetrating trauma, open fractures, etc. Acute hematogenous osteomyelitis (AHO) is the most common type of musculoskeletal infection in children with an estimated incidence of 1 case per 5000 children per year in the United States[1]. It is primarily a disease of young children with approximately half of all cases occurring in children 5 years of age or younger[2]. Some recent studies have indicated that the incidence of AHO is increasing with a concurrent increase in the number of cases due to methicillin-resistant Staphylococcus aureus (S. aureus) (MRSA) infections[3]. The signs and symptoms of AHO in children are nonspecific and as such, imaging frequently plays a significant role in the diagnosis and management of this condition.
EPIDEMIOLOGY
Infection by S. aureus is the most common cause of osteomyelitis. Community acquired S. aureus is implicated in most cases with 30% of these cases caused by community acquired MRSA (CA-MRSA)[4,5]. Other organisms that cause osteomyelitis include Streptococcus pneumoniae, Streptococcus pyogenes, Pseudomonas aeruginosa and Bartonella henselae. Salmonella is an important cause of osteomyelitis in patients with Sickle cell disease. Gram-negative bacteria and group B streptococci are common causes in newborns and Kingella Kingae in the first two years of age[5].
PATHOPHYSIOLOGY
Hematogenous osteomyelitis is the most common type of osteomyelitis in children[4]. This occurs when an infection elsewhere in the body spreads to the bone via the bloodstream. Risk factors for development of hematogenous osteomyelitis include trauma, prematurity, urinary tract infections, vascular catheters and immunodeficiencies. The blood vessels in the metaphyses have sluggish flow and discontinuous endothelium, which predispose to infection[4]. The most common bones to be affected are the fastest growing bones that have highly vascularized long bone metaphyses and metaphyseal equivalents. Common sites include the distal femur, proximal tibia, proximal humerus and distal radius. Most cases start with a focal infection in the metaphyseal marrow which progresses to local decalcification and bony destruction. Occasionally, multiple foci may be infected which eventually coalesce. This infection can spread within the marrow cavity and as the pressure increases within the marrow cavity, the infection can spread through Haversian canals in the cortex into the subperiosteal space, giving rise to a subperiosteal abscess. Similarly, the infection can traverse the periosteum and infect the adjacent soft tissues leading to pyomyositis. Infection may also spread across the physis into the epiphysis and joint space[4].
The first stage of osteomyelitis occurs with vascular congestion, intravascular thrombosis and increased intraosseous pressure. Next is the suppurative stage where pus traverses the Haversian canals and forms a subperiosteal abscess. Subsequently a sequestrum may form when the periosteal and endosteal blood supply is compromised from increased pressure and vascular obstruction. This may lead to formation of an involucrum: new bone growing from the periosteum. Depending on medical or surgical treatment at this point the infection may resolve or progress with complications.
The site of osteomyelitis varies with patient age and is related to the blood supply. In early infancy osteomyelitis occurs in epiphyses and metaphyses and epiphyseal-equivalent regions. Transphyseal vessels are present in infants younger than 18-24 mo of age, which allow easier spread of infection across the physis from the metaphysis to the epiphysis[4,6]. This is the reason that infantile osteomyelitis frequently involves the epiphysis and joint space. It is important to note that this is not the most common cause of septic arthritis, which more often results from direct hematogenous synovial seeding[4]. During early infancy, isolated involvement of the epiphyseal growth plate can occur. Infection of the epiphyseal growth plate during infancy can result in growth disturbance. In the 2-16 years age group, osteomyelitis is most often located in the metaphyses[6].
IMAGING APPROACH TO OSTEOMYELITIS
Osteomyelitis in children demonstrates abnormalities on nearly all imaging modalities, including radiography, ultrasound, computed tomography, radionuclide bone scintigraphy, and magnetic resonance imaging (MRI). The conventional approach to the imaging evaluation of suspected AHO in the past has been radiography followed by bone scintigraphy if the radiographs were negative. In this algorithm, MRI was typically been reserved for cases of poor treatment response or suspected vertebral diskitis-osteomyelitis. However, due to multiple factors, including the rise of rapidly aggressive and invasive musculoskeletal infections with CA-MRSA, this approach may no longer be ideal[7] (Figure 1).
Figure 1.
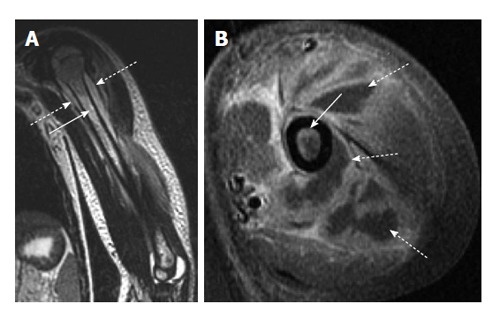
Acute osteomyelitis secondary to methicillin-resistant Staphylococcus aureus infection. A: Coronal T2-weighted image of the left humerus shows bone marrow edema (solid arrow) as well as a periosteal fluid collection (dashed arrow) consistent with periosteal abscess; B: Axial T1-weighted post-contrast image shows both enhancement of the bone marrow (solid arrow) as well as extensive periosteal and soft tissue abscess formation (dashed arrows) which is characteristic of methicillin-resistant Staphylococcus aureus infection.
As a first line modality radiography is useful for excluding other differential diagnoses such as trauma or tumor, however radiographs are insensitive for the detection of early osteomyelitis. Radiography may be normal in cases of osteomyelitis up to 14 d after the onset of infection and even then, only 20% of cases demonstrate radiographic abnormalities after this two-week delay[8]. Additionally, the early radiographic findings, including soft tissue swelling, vague bony lucency, and periosteal reaction, may be subtle and may not reflect the true extent of disease.
Triple-phase bone scintigraphy using 99mTc-methylene diphosphonate (99mTc-MDP) can demonstrate evidence of infection as soon as 24 h after onset and also has the advantage of being able to depict multiple sites of infection. Osteomyelitis typically manifests as increased radiotracer uptake on all phases (angiographic, blood pool, and delayed) of the triple-phase examination. However 99mTc-MDP scintigraphy is limited by poor anatomic detail and is insensitive for the detection of abscesses and extraosseous involvement. Furthermore, the sensitivity of 99mTc-MDP scintigraphy for the diagnosis of osteomyelitis, which in the past has been reported to be as high as 80%[9], may be decreasing with the increasing incidence of MRSA infections that tend to have significant soft-tissue involvement[7]. Positron emission tomography with 18-fluorodeoxyglucose appears to be sensitive (95%) and specific (87%) for the diagnosis of osteomyelitis[9], however it has limited availability and involves a significant amount of radiation exposure. Scintigraphic studies using white blood cells labeled with indium-111 or 99mTc hexamethylpropyleneamine oxime require relatively large volumes of blood and are not used frequently in younger children.
In contrast to the modalities listed above, MRI is both sensitive for the detection of early osteomyelitis (Figure 2) and can also accurately depict the extent of disease as well as any associated abscess or soft-tissue extension without the risks associated with radiation exposure. MRI combines high-resolution anatomic delineation of the medullary space, cortex, and periosteum with high soft tissue contrast for detection of edema and fluid. Pre-operative MRI has been shown to reduce operative time and extent of surgical exposure in cases requiring surgical debridement[10]. MRI does have distinct disadvantages in children including long scan times and susceptibility to motion artifacts which necessitate sedation or anesthesia in young children (approximately 6 mo to 8 years of age). Additionally, MRI is contraindicated in some patients with metallic foreign bodies and certain types of implanted hardware. However, the overall superiority of MRI in evaluating osteomyelitis is reflected in recent clinical practice guidelines which indicate that MRI is the imaging modality of choice for the detection of osteomyelitis and associated infection of the extraosseous soft tissues[11]. As such, the current best imaging approach for suspected osteomyelitis is radiography followed by MRI.
Figure 2.
Early acute osteomyelitis. A: Coronal proton-density weighted image of the right ankle of a 12-year-old male shows a subtle area of decreased intramedullary signal along the lateral aspect of the calcaneus (long arrow); B: Axial T2-weighted image with fat suppression demonstrates more conspicuous bone marrow edema (arrowhead); C: Coronal post-contrast T1-weighted image with fat suppression shows an area of enhancement corresponding to the bone marrow edema. Bone biopsy revealed bacterial osteomyelitis.
MRI TECHNIQUE
Multiple variations of MRI protocols for the evaluation of osteomyelitis exist, however the essential sequences include both multiplanar T1 and T2-weighted fast-spin echo or turbo spin-echo (FSE/TSE) sequences and short-tau inversion recovery (STIR) or T2-weighted FSE/TSE sequences with fat-suppression (T2-FS). STIR and T2-FS sequences are particularly helpful for increasing the conspicuity of bone marrow edema and fluid collections. There is some controversy regarding when to use gadolinium in infants and children with suspected osteomyelitis. Intravenous gadolinium contrast does not appear to improve the sensitivity or specificity for the diagnosis of osteomyelitis overall. Recent studies suggest that if the fluid-sensitive images (e.g., STIR, T2-FS) are normal, gadolinium enhancement provides no additional diagnostic value[12,13]. If the fluid-sensitive images are abnormal, however, gadolinium enhancement is of value in increasing confidence in the diagnosis of an abscess (if present) and planning of the approach to abscess aspiration and drainage[12]. Despite these recent studies that suggests that gadolinium contrast administration may not be needed for all cases, there are some specific indications for which contrast is always indicated. In cases of suspected vertebral osteomyelitis, contrast is necessary to assist in the differentiation of abscess in the epidural space or paravertebral masses from inflammatory masses[4] (Figure 3). Additionally, epiphyseal growth plate involvement by osteomyelitis may sometimes only be seen on gadolinium enhanced T1 sequences and not seen on non-contrast T1 and fluid sensitive sequences or on radiography or bone scintigraphy. Active epiphyseal infection manifests as one or more areas of decreased or no enhancement of the epiphyseal cartilage which otherwise should enhance uniformly[14,15]. As mentioned above, infection of the epiphyseal growth plate during infancy can result in growth disturbance and therefore gadolinium use in this age group is advised.
Figure 3.
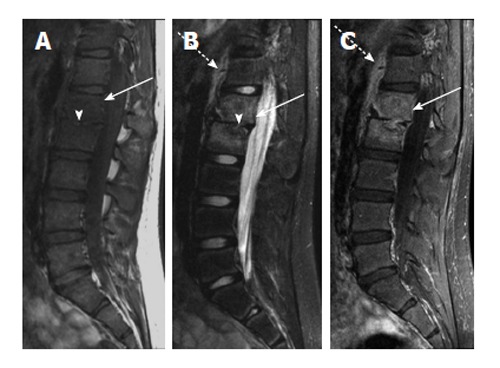
Chronic vertebral diskitis-osteomyelitis secondary to S. typhi. A: Sagittal T1-weighted image shows abnormally decreased T1 marrow signal in the L1 and L2 vertebral bodies (arrow) and loss of the L1-2 disk space (arrowhead); B: Sagittal T2-weighted image with fat suppression shows abnormally increased T2 signal in the L1 and L2 vertebral bodies (solid arrow) with loss of normal T2 intervertebral disk signal (arrowhead). T2 hyperintensity anterior to the spine (dashed arrow) likely represents adjacent soft tissue edema; C: Sagittal T1-weighted image after intravenous contrast shows intramedullary enhancement in the L1 and L2 vertebral bodies (solid arrow) with soft tissue enhancement anterior to the spine (dashed arrow).
Because it is frequently difficult to precisely localize sites of involvement by clinical exam, especially in infants, an initial large field-of-view coronal STIR or T2-FS sequence of the general region of concern can be used to help localize the site(s) of disease followed by a tailored evaluation of the involved areas. In cases of suspected osteomyelitis affecting the lower extremities, imaging of the contralateral extremity may also be considered: abnormalities in the contralateral extremity are common, however they may not affect clinical management[16]. Whole-body MRI (WBMRI) may be indicated in cases of suspected multifocal involvement such as in cases of severe CA-MRSA infections, which frequently involve multiple sites, or in cases of suspected chronic multifocal recurrent osteomyelitis (CRMO). WBMRI is typically performed using a series of coronal STIR acquisitions obtained in multiple anatomic stations using receiver coils spanning the entire body, with the scan table moved through the magnet between stations. The images from each station are then digitally fused at points of overlap to create a single whole body image stack.
MRI FEATURES OF ACUTE OSTEOMYELITIS
Because MRI is able to detect early marrow involvement, it is an important modality for detection of osteomyelitis in early stages. Additionally, MRI is helpful for detection of fluid collections and abscesses that may occur in the marrow, subperiosteal region or in soft tissues. Anatomical information provided by MR can be helpful for drainage and surgical treatment. T1 fat saturation gadolinium-enhanced images will show non-enhancement of fluid and pus with peripheral enhancement.
The earliest finding of osteomyelitis on MRI is bone marrow edema and T2 and STIR sequences are very important for detecting these early changes (Figures 2 and 4). MRI is also sensitive for detection of periosteal elevation and the presence of a subperiosteal fluid collection or abscess (Figure 5). Distinguishing normal hematopoietic marrow from abnormal marrow can be challenging in certain situations because of the normal hematopoietic marrow often seen in the metaphyses in children. Normal hematopoietic marrow T1 signal should be hyperintense relative to muscle. If there is marrow infiltration or edema, the T1 signal is generally isointense or hypointense to muscle (Figure 6). Normal hematopoietic marrow should appear similar in adjacent or contralateral metaphyses. Imaging of the contralateral body part is often helpful for this and is more easily obtained during imaging of the pelvis and lower extremities. Since detailed small structure anatomical information is less important during evaluation of osteomyelitis (OM), imaging with a body coil should be considered if large field of view imaging would be helpful.
Figure 4.
Acute osteomyelitis of the hip. A: Coronal fast spin echo inversion recovery image shows T2-hyperintense marrow edema in the left femoral metaphysis (solid arrow) with a small round very intense focus of T2 signal (arrowhead) which is consistent with an intraosseous abscess. Surrounding soft tissue edema (dashed arrow) is also noted; B: Coronal T1-weighted post-contrast image in the same patient shows enhancement in the metaphyseal bone marrow (solid long arrow), peripheral enhancement of the abscess (arrowhead), and surrounding soft tissue enhancement (dashed arrow). Note enhancement of the left femoral head (solid short arrow) which indicates adequate perfusion.
Figure 5.
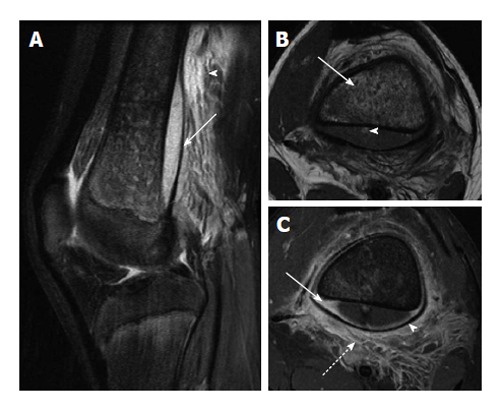
Osteomyelitis with subperiosteal abscess. A: Sagittal T2-weighted image with fat suppression shows a large subperiosteal abscess (solid arrow) with adjacent soft tissue edema (arrowhead); B: Axial T1-weighted image shows heterogeneous T1 hypointense marrow signal (solid arrow). Note small T1 hyperintense focus in the posterior subperiosteal fluid collection (arrowhead) indicative of a fat globule; C: Axial T1-weighted post-contrast image demonstrates peripheral enhancement of the subperiosteal abscess (solid arrow), significant periosteal elevation (arrowhead) and adjacent soft tissue inflammation (dashed arrow).
Figure 6.
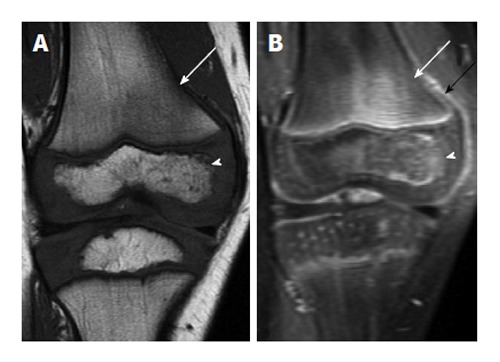
Early acute osteomyelitis. A: Coronal T1-weighted image of the right knee of a 4-year-old male shows ill-defined areas of low T1 signal in the bone marrow in the lateral femoral metaphysis (arrow) and lateral epiphysis (arrowhead); B: Coronal T1-weighted post-contrast image in the same patient shows associated enhancement in these areas (white arrow and arrowhead) as well as some periosteal reaction, indicated by periosteal enhancement (black arrow).
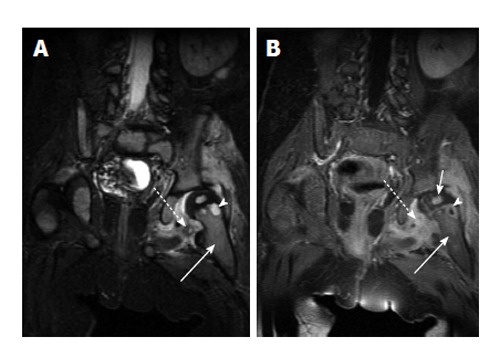
Pelvic osteomyelitis often occurs in metaphyseal equivalents such as the ischiopubic synchondrosis, pubic symphysis, triradiate cartilage, iliac apophyses and adjacent to the sacroiliac joint. Involvement of adjacent soft tissues, muscles and bowel is not uncommon and MRI is helpful in imaging the extent and often the source of infection.
CHRONIC OSTEOMYELITIS
Chronic infections of bone may be indolent and have minor signs, symptoms and serologic abnormalities. However, cases of chronic osteomyelitis may be severe, incapacitating and difficult to treat. Chronic osteomyelitis may occur after the following: acute osteomyelitis, trauma (Figure 7), joint replacement, orthopedic hardware (ex. used in fracture reduction), and mycobacterium tuberculosis or syphilis infections. Chronic osteomyelitis is defined if symptoms persist after one month of appropriate antibiotic treatment or if there is persistent infection after one month of inadequate treatment.
Figure 7.
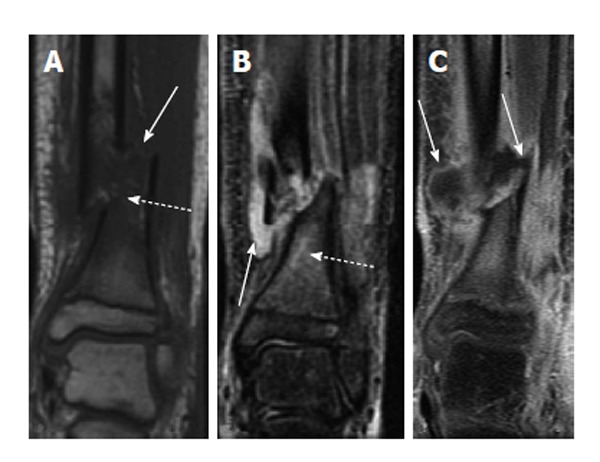
Osteomyelitis secondary to open fracture. A: Coronal T1-weighted image of the left distal tibia shows a displaced fracture of the distal tibial metadiaphysis (solid arrow) with associated bone marrow edema (dashed arrow); B: Coronal T2-weighted image of the same patient shows a fluid collection adjacent to the fracture (solid arrow) with T2 hyperintense marrow edema (dashed arrow); C: Coronal T1-weighted post-contrast image shows peripheral enhancement surrounding the above-mentioned fluid collection, consistent with an abscess (solid arrows).
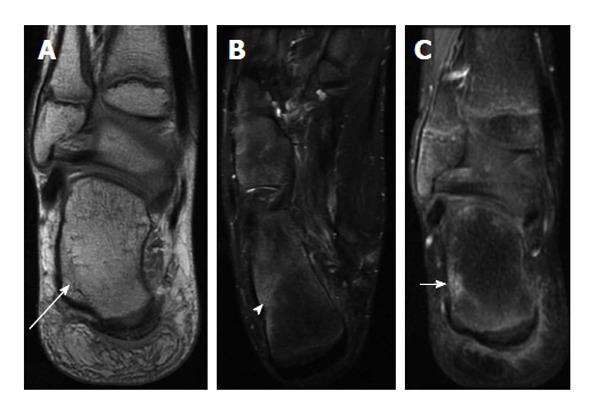
Imaging chronic osteomyelitis is helpful to evaluate the extent of infection within bone and surrounding soft tissues, identification of an abscess, sequestrum and sinus tract (Figure 8). Sequestra of cortical bone appear as low signal on T1, T2 and STIR and do not enhance. Imaging findings of chronic osteomyelitis include Brodie abscess, thick periosteum, sequestrum/necrotic bone fragments, and cloaca/draining tract (Figure 9). Sequestra of cancellous bone also do not enhance but are relatively hyperintense to cortical sequestra on T1, T2 and STIR. Granulation tissue, soft tissue inflammation and sinus tracts are all T1 hypointense, T2 and STIR hyperintense and enhance with gadolinium.
Figure 8.
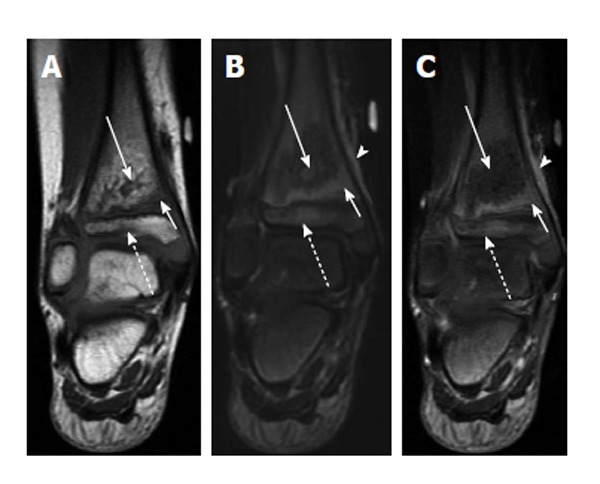
Transphyseal methicillin-resistant Staphylococcus aureus osteomyelitis with intraosseous abscess. A: Coronal T1-weighted image of the right ankle shows an area of T1 hyperintensity in the distal tibial metaphysis with central T1 hypointensity (solid long arrow) indicative of abscess formation. T1 hypointensity is seen surrounding this area in metaphysis (solid short arrow) and in the epiphysis (dashed arrow) indicative of transphyseal spread; B: Coronal T2-weighted image with fat suppression shows an area of T2 hypointensity with central T2 hyperintensity (solid long arrow) corresponding to the areas of abnormal T1 signal in A. T2 hyperintensity in the distal metaphysis (solid short arrow) and epiphysis (dashed arrow) are consistent with edema. T2 hyperintensity in periosteum and adjacent soft tissues indicating inflammation (arrowhead); C: Coronal T1-weighted image post contrast shows a lack of enhancement in the central distal metaphysis consistent with necrosis and abscess formation (solid long arrow). Enhancement is seen peripherally in the distal metaphysis and epiphysis (solid short arrow and dashed arrow). Enhancement in periosteum and adjacent soft tissues indicating inflammation (arrowhead).
Figure 9.
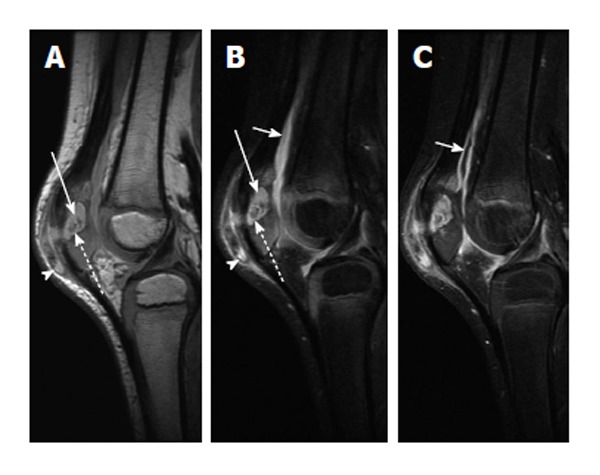
Chronic patellar osteomyelitis with abscess and sequestrum. A: Sagittal proton-density-weighted image of the right knee shows an abscess cavity in the patella (solid arrow) with a central low-density focus consistent with a sequestrum (dashed arrow). There is also disruption of the anterior cortex with spread of infection into the prepatellar bursa (arrowhead); B: Sagittal T2-weighted image with fat suppression again shows the intraosseous abscess (solid long arrow) with central T2 hypointense sequestrum (dashed arrow) and extension of infection into the prepatellar soft tissues (arrowhead). A knee joint effusion is more apparent on this image (solid short arrow); C: Sagittal T1-weighted post-contrast image shows enhancement of the synovium (solid short arrow) indicative synovitis, likely from intra-articular extension of infection.
Brodie’s abscesses are usually metaphyseal and appear as a fluid filled cavity with an enhancing lining, rim of low signal sclerosis and peripheral edema (Figure 10). A “penumbra sign” has been described with Brodie abscess and results from the lining of granulation tissue around the abscess that is T1 hyperintense relative to the abscess cavity. A “double-line sign” has also been described on T2 and STIR images representing hyperintense granulation tissue surrounded by low signal sclerosis.
Figure 10.
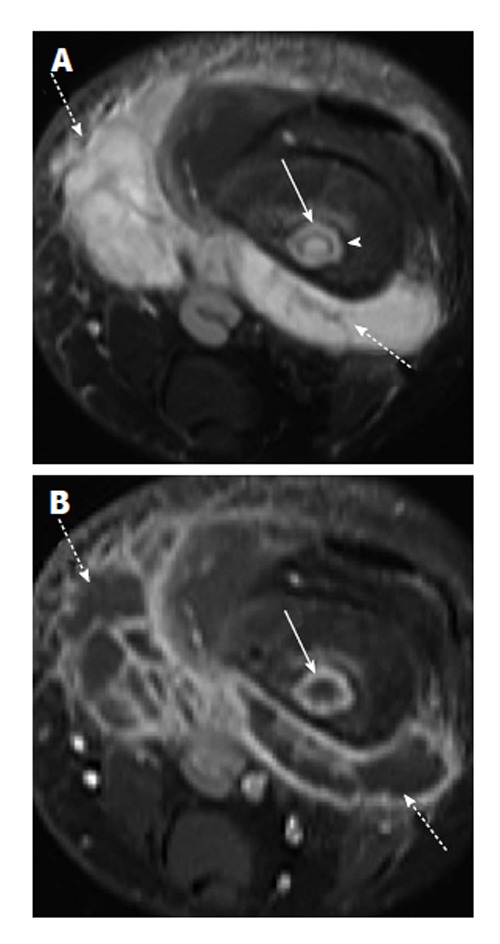
Osteomyelitis with intraosseous and soft tissue abscess secondary to methicillin-resistant Staphylococcus aureus infection. A: Axial T2-weighted fat suppressed image shows intraosseous abscess cavity (solid arrow) with rim of surrounding edema and large surrounding soft tissue fluid collection (dashed arrow). Note T2 hypointense rim (arrowhead) forming the “double-line” sign; B: Axial T1-weighted post-contrast image shows peripheral enhancement associated with the intraosseous abscess (solid arrow) and soft tissue abscesses (dashed arrows).
Sclerosing osteomyelitis of Garre is a type of chronic bone infection manifesting primarily with bony sclerosis.
CHRONIC RECURRENT MULTIFOCAL OSTEOMYELITIS
Chronic recurrent multifocal osteomyelitis is a non-bacterial, noninfectious inflammation of bone that has been characterized as an auto-inflammatory disease. Cultures do not show an infectious source and biopsy shows nonspecific inflammation. Antibiotics do not alter the course of the disease and symptoms are better treated with anti-inflammatory medications. Frequent sites of involvement include the metaphyses of the long bones, clavicles, spine and pelvis. Other sites include the mandible, scapula, ribs, sternum, hands and feet. Radiographic findings typically include lysis and sclerosis. Since this disease frequently involves multiple sites, some of which are asymptomatic, WBMRI is recommended both to help aid in the diagnosis with multifocal involvement and document the extent of disease. During the active phase, there is bone marrow edema and often periostitis and soft tissue inflammation. MRI can show transphyseal disease, which can lead to physeal bars affecting growth and angular deformities (Figure 11). Joint effusions, synovitis cartilage and subchondral bone erosions may also be seen. Larger fluid collections, abscess, sinus tracts and sequestra are not typical features of CRMO and are more often seen with bacterial osteomyelitis[17].
Figure 11.
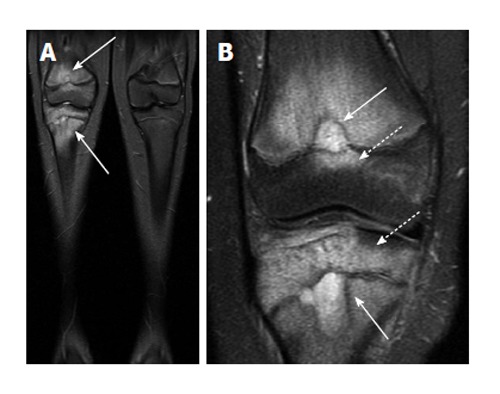
Chronic recurrent multifocal osteomyelitis. A: Coronal short-tau inversion recovery image from whole body magnetic resonance imaging (lower extremity station) shows areas of bone marrow edema in the distal femur and proximal tibia; B: Coronal T2-weighted fat suppressed image with smaller field of view again demonstrates the bone marrow edema as well as two areas of very hyperintense T2 signal in the femur and tibia which may represent intraosseous abscesses (solid arrows), though these are not typically found in chronic multifocal recurrent osteomyelitis. Involvement of the epiphysis is apparent at both sites (dashed arrows).
DIAGNOSTIC CHALLENGES
Differentiating osteomyelitis from Ewing sarcoma can often be challenging. The fact that children often do not present with the classic signs and symptoms of an infection makes the clinical differentiation between these two diagnoses difficult. Plain film findings of both osteomyelitis and Ewing sarcoma are often similar with an aggressive intramedullary process destroying normal cancellous and cortical bone creating a moth eaten and permeative appearance. MRI can be helpful in differentiating between these two aggressive diseases. Both may produce periostitis, periosteal elevation, adjacent soft tissue mass and effacement of fat planes. Soft tissue enhancement, cystic and necrotic foci and cortical destruction are found in both diseases and are less reliable at differentiation. The presence of fat globules within the infiltrating marrow process or in a subperiosteal location is a feature of osteomyelitis more often than neoplasia[18] (Figure 5). A recent study by Henninger et al[19] showed that all cases of Ewing sarcoma had a sharp or defined margin of the bone lesion between normal bone and edematous/affected bone on T1 which was not present in cases of osteomyelitis (Figure 12).
Figure 12.
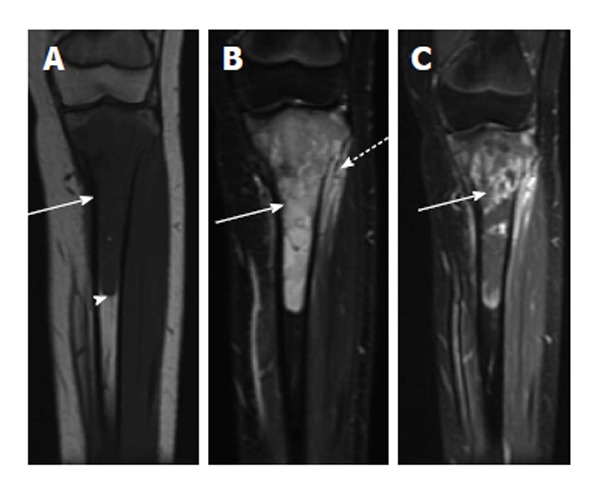
Ewing sarcoma. A: Coronal T1-weighted image of the left tibia shows a long segment of intramedullary T1 hypointensity (solid arrow). Note abrupt transition to normal marrow signal inferiorly (arrowhead); B: Coronal fast multi-planar inversion recovery image of the same patient shows very intense T2 signal in the marrow cavity (solid arrow) with extraosseous extension (dashed arrow); C: T1-weighted post-contrast image shows very heterogeneous intramedullary enhancement associated with this lesion (solid arrow).
Langerhans cell histiocytosis often has a very similar appearance to osteomyelitis with an aggressive lytic lesion with ill-defined borders and surrounding inflammatory changes (Figure 13). A process centered in the diaphysis favors langerhans cell histiocytosis over OM. However, differentiation from Langerhans cell histiocytosis, lymphoma and leukemia can be challenging and biopsy is necessary for definitive diagnosis. Fractures, bone infarcts and healed osteomyelitis may pose a diagnostic challenge in differentiating between active osteomyelitis due to common features.
Figure 13.
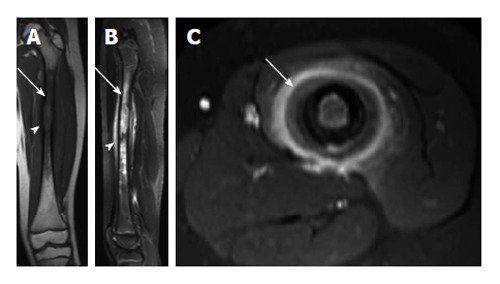
Langerhans cell histiocytosis. A: Coronal T1-weighted image of the left femur shows a long segment of marrow T1 hypointensity (arrow) and cortical erosion/expansion (arrowhead); B: Sagittal short-tau inversion recovery image from the same patient shows very intense T2-signal in the marrow cavity (arrow). T2 hyperintensity in the periosteum (arrowhead) is indicative of periosteal reaction; C: Axial T1-weighted image post contrast shows intense enhancement of the periosteum (arrow).
ADVANCED MRI TECHNIQUES
As noted above, the current protocols for MRI of suspected osteomyelitis utilize standard T1 and T2-weighted, STIR, and sometimes contrast-enhanced sequences. More advanced imaging techniques such as diffusion-weighted imaging (DWI), dynamic contrast-enhanced (DCE) MRI, and MR spectroscopy can be used as well, however their role is currently not well defined. A potential application for DWI would be characterization of fluid collections associated with osteomyelitis as abscesses characteristically demonstrate restricted diffusion on DWI. Additionally, DCE-MRI could potentially be used to identify areas of soft tissue necrosis, femoral/humeral head avascular necrosis, or possibly increase sensitivity for detection of CA-MRSA infection in non-ossified growth cartilage. Whole body MRI with digital merging of multiple anatomic stations can be helpful for assessment of multiple sites of disease as well as suspected cases of CRMO. Further research is needed to define the utility of these imaging techniques in this setting.
Footnotes
P- Reviewer: Lichtor T S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
References
- 1.Steer AC, Carapetis JR. Acute hematogenous osteomyelitis in children: recognition and management. Paediatr Drugs. 2004;6:333–346. doi: 10.2165/00148581-200406060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Nelson JD. Acute osteomyelitis in children. Infect Dis Clin North Am. 1990;4:513–522. [PubMed] [Google Scholar]
- 3.Arnold SR, Elias D, Buckingham SC, Thomas ED, Novais E, Arkader A, Howard C. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–708. doi: 10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo D. Infection: musculoskeletal. Pediatr Radiol. 2011;41 Suppl 1:S127–S134. doi: 10.1007/s00247-011-2001-y. [DOI] [PubMed] [Google Scholar]
- 5.Kalyoussef S, Tolan RW, Noel GJ. Pediatric Osteomyelitis. 2014. Available from: http: //emedicine.medscape.com/article/967095-overview. [Google Scholar]
- 6.DiPoce J, Jbara ME, Brenner AI. Pediatric osteomyelitis: a scintigraphic case-based review. Radiographics. 2012;32:865–878. doi: 10.1148/rg.323115110. [DOI] [PubMed] [Google Scholar]
- 7.Browne LP, Mason EO, Kaplan SL, Cassady CI, Krishnamurthy R, Guillerman RP. Optimal imaging strategy for community-acquired Staphylococcus aureus musculoskeletal infections in children. Pediatr Radiol. 2008;38:841–847. doi: 10.1007/s00247-008-0888-8. [DOI] [PubMed] [Google Scholar]
- 8.Capitanio MA, Kirkpatrick JA. Early roentgen observations in acute osteomyelitis. Am J Roentgenol Radium Ther Nucl Med. 1970;108:488–496. doi: 10.2214/ajr.108.3.488. [DOI] [PubMed] [Google Scholar]
- 9.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937–1949. doi: 10.2967/jnumed.110.076232. [DOI] [PubMed] [Google Scholar]
- 10.Kan JH, Hilmes MA, Martus JE, Yu C, Hernanz-Schulman M. Value of MRI after recent diagnostic or surgical intervention in children with suspected osteomyelitis. AJR Am J Roentgenol. 2008;191:1595–1600. doi: 10.2214/AJR.08.1115. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 12.Averill LW, Hernandez A, Gonzalez L, Peña AH, Jaramillo D. Diagnosis of osteomyelitis in children: utility of fat-suppressed contrast-enhanced MRI. AJR Am J Roentgenol. 2009;192:1232–1238. doi: 10.2214/AJR.07.3400. [DOI] [PubMed] [Google Scholar]
- 13.Kan JH, Young RS, Yu C, Hernanz-Schulman M. Clinical impact of gadolinium in the MRI diagnosis of musculoskeletal infection in children. Pediatr Radiol. 2010;40:1197–1205. doi: 10.1007/s00247-010-1557-2. [DOI] [PubMed] [Google Scholar]
- 14.Browne LP, Guillerman RP, Orth RC, Patel J, Mason EO, Kaplan SL. Community-acquired staphylococcal musculoskeletal infection in infants and young children: necessity of contrast-enhanced MRI for the diagnosis of growth cartilage involvement. AJR Am J Roentgenol. 2012;198:194–199. doi: 10.2214/AJR.10.5730. [DOI] [PubMed] [Google Scholar]
- 15.Guillerman RP. Osteomyelitis and beyond. Pediatr Radiol. 2013;43 Suppl 1:S193–S203. doi: 10.1007/s00247-012-2594-9. [DOI] [PubMed] [Google Scholar]
- 16.Metwalli ZA, Kan JH, Munjal KA, Orth RC, Zhang W, Guillerman RP. MRI of suspected lower extremity musculoskeletal infection in the pediatric patient: how useful is bilateral imaging? AJR Am J Roentgenol. 2013;201:427–432. doi: 10.2214/AJR.12.9644. [DOI] [PubMed] [Google Scholar]
- 17.Khanna G, Sato TS, Ferguson P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics. 2009;29:1159–1177. doi: 10.1148/rg.294085244. [DOI] [PubMed] [Google Scholar]
- 18.Davies AM, Hughes DE, Grimer RJ. Intramedullary and extramedullary fat globules on magnetic resonance imaging as a diagnostic sign for osteomyelitis. Eur Radiol. 2005;15:2194–2199. doi: 10.1007/s00330-005-2771-4. [DOI] [PubMed] [Google Scholar]
- 19.Henninger B, Glodny B, Rudisch A, Trieb T, Loizides A, Putzer D, Judmaier W, Schocke MF. Ewing sarcoma versus osteomyelitis: differential diagnosis with magnetic resonance imaging. Skeletal Radiol. 2013;42:1097–1104. doi: 10.1007/s00256-013-1632-5. [DOI] [PubMed] [Google Scholar]


