Abstract
Management of brachial plexus injury is a demanding field of hand and upper extremity surgery. With currently available microsurgical techniques, functional gains are rewarding in upper plexus injuries. However, treatment options in the management of flail and anaesthetic limb are still evolving. Last three decades have witnessed significant developments in the management of these injuries, which include a better understanding of the anatomy, advances in the diagnostic modalities, incorporation of intra-operative nerve stimulation techniques, more liberal use of nerve grafts in bridging nerve gaps, and the addition of new nerve transfers, which selectively neurotise the target muscles close to the motor end plates. Newer research works on the use of nerve allografts and immune modulators (FK 506) are under evaluation in further improving the results in nerve reconstruction. Direct reimplantation of avulsed spinal nerve roots into the spinal cord is another area of research in brachial plexus reconstruction.
KEY WORDS: Brachial plexus injuries, nerve grafts, recent advances, reimplantation of avulsed spinal nerve roots, selective nerve transfers
INTRODUCTION
Brachial plexus injuries represent devastating injuries with unpredictable outcomes. The results of brachial plexus repair have considerably improved with the introduction of newer diagnostic modalities, microsurgical techniques, and magnification. Introduction of micro surgical techniques in neurolysis, nerve repair, nerve grafting, and nerve transfer has made possible to restore a functioning limb in many of the patients with brachial plexus injuries, which was considered a difficult or impossible task just few years back.
CURRENT CONCEPTS IN TIMING OF REPAIR
Brachial plexus injuries are treated as an immediate, early, or delayed repair. Penetrating injuries, e.g., a stab injury to the brachial plexus, are best treated by an immediate repair, when a direct approximation of nerve ends is feasible.[1] Such repairs are likely to result in good functional outcomes, especially when applied for upper plexal lesions. An early repair indicates a repair performed in 8-12 weeks of injury. The indications include a flail limb with severe deafferentation pain, presence of pseudomeningoceles on magnetic resonance (MR) myelography, a positive Horner's sign, and an associated diaphragmatic palsy. These signs indicate a severe trauma to the plexus with little or no potential for spontaneous recovery. An early intervention, in such cases, is expected to regain some useful functions. A majority of brachial plexus lesions are the result of traction injuries and require a period of observation for up to 3-5 months. By this time, tissue oedema has resolved with recovery of neuropraxic injuries, allowing a clear demarcation between the injured and noninjured nerves. Missile injuries of the brachial plexus differ considerably from the traction injuries.[2,3] Low velocity missiles have a good prognosis on conservative management. Contrary to this high velocity missiles produce considerable composite tissue damage via shock waves and temporary cavitation. These injuries are better assessed at 3-4 months after injury. Patients with little or no recovery are the candidates for surgical repair.
DIAGNOSTIC MODALITIES
Imaging studies
Currently, plain X-ray films, computed tomography (CT) myelography, and magnetic resonance imaging (MRI) are being utilized as the main diagnostic tools. Raised hemidiaphragm on plain X-ray films of the chest is suggestive of phrenic nerve injury. Presence of pseudomeningocele on myelography indicates a root avulsion injury.[4] MRI provides general information about various components of the brachial plexus. MR myelography is gradually replacing CT myelography in the diagnosis of root avulsions. However, false-positive pseudomeningoceles have been found in patients with intact rootlets, and false-negative results have been reported during surgical exploration. One report[5] mentioned the diagnostic accuracy as 74% when three-dimensional MRI reports were compared with intra-operative findings. In another report radiological evaluation of 7 patients (34 spinal nerve roots) resulted in a diagnostic accuracy of 88%.[6]
DIFFUSION-WEIGHTED MAGNETIC RESONANCE NEUROGRAPHY
In conventional MRI study, a combination of fat- suppressed T2-weighted and T1-weighted sequences are used to localize the lesion to the nerve roots or to a more distal location. However, these techniques fail to produce three-dimensional images which depict the entire length of the nerve sheaths. Furthermore, vascular structures such as veins, which are in close vicinity of nerves, are difficult to distinguish from the neural structures, because of their similar signal intensity on T2-and T1-weighted MRI.[7] Diffusion-weighted MR neurography may provide improved contrast between the brachial plexus and the surrounding structures, and may allow a three-dimensional evaluation of the brachial plexus.[8]
HIGH-RESOLUTION MAGNETIC RESONANCE NEUROGRAPHY
Magnetic resonance neurography is a valuable tool in defining peripheral nerve anatomy and brachial plexus [Figure 1]. Recently introduced high-resolution 3T MR neurography with three-dimensional imaging[9] is capable of delineating the condition of nerve roots (avulsions or ruptures), defining the location and extent of injury in the distal part of plexus, and also the regional denervation muscle changes. An abnormal enhancement of paraspinal muscles indirectly indicates a root avulsion injury.
Figure 1.
Magnetic resonance neurography showing normal brachial plexus
ELECTRODIAGNOSTIC STUDIES
Electrodiagnostic studies, in the diagnosis and treatment of brachial plexus injuries, are of immense help, when there are a good coordination and discussion between the surgeon and the neuropathologist.
ELECTROMYOGRAPHY
A normal muscle is silent at rest and active during contraction on insertion of electromyography (EMG) needles. Positive sharp wave and fibrillations indicate a denervated muscle, when performed 2-4 weeks after the injury. Polyphasic low amplitude tracings are features of reinnervation. An electromyographic evaluation of paraspinal muscles can differentiate root avulsions from root ruptures. Furthermore, fibrillation potentials and positive sharp waves which are present in axonotmesis and neurotmesis are absent in neuropraxia injuries.
SURGICAL MANAGEMENT OF BRACHIAL PLEXUS INJURIES
Restoration of shoulder stability and abduction is an important goal in the rehabilitation of patients with devastating brachial plexus injuries. Restoration of shoulder abduction provides greater range of motion to the arm and forearm. Suprascapular and axillary are the target nerves in achieving these functions. Results in nerve transfers have shown that neurotisation of the suprascapular nerve provides more reliable and effective abduction than neurotisation of the axillary nerve.[10] Suprascapular neurotisation also restores external rotation that maximizes abduction by preventing the impingement of the greater tuberosity of humerus against the coracoacromial arch. In the context of a meta-analysis, Merrell et al.[11] reported that the best nerve transfer for restoration of shoulder abduction was the spinal accessory to suprascapular nerve. Conventionally, spinal accessory to suprascapular nerve transfers have been performed by anterior approaches. The results in shoulder function following anterior transfers are variable. Transection of spinal accessory nerve at proximal level leads to denervation of upper trapezius, which plays an important role in shoulder function. In general, distal transection spares the important branches to trapezius and preserves its function of shoulder stabilization and elevation. Many times with severe traction injuries suprascapular nerve are retracted down and not found by the standard supraclavicular approach. Its proximal part may also be involved in scar tissue and a distal transection near healthy nerve will need a graft with poor results. Furthermore, suprascapular nerve may be injured at two or three levels. Mikami et al.[12] mention its involvement at the suprascapular notch and spinoglenoid notch. Considering all these factors Malessy et al.[13] concluded that the reanimation of shoulder function in patients with severe brachial plexus traction injury, following suprascapular nerve neurotization was disappointingly low.
DORSAL APPROACH IN SHOULDER REANIMATION
Dorsal or posterior approach[14,15] in spinal accessory nerve into the suprascapular nerve is expected to lack some of the drawbacks of the anterior transfers. This technique neurotises suprascapular nerve by distal spinal accessory nerve through a transverse incision over the scapular spine. Hence, function of most of trapezius is preserved. The distal transfer is close to the target muscles and identifies, if any, a distal injury to the suprascapular nerve (e.g., near the suprascapular notch). The number of myelinated axons in the distal spinal accessory nerve has been found to be adequate to reinnervate the supraspinatus and infraspinatus muscles.[14] In addition, the dissection is in a safe and relatively avascular zone. We have adopted this technique in partial as well as total brachial plexus injuries with good results. This technique is gaining popularity as a standard technique in spinal accessory nerve to suprascapular nerve transfer [Figure 2].
Figure 2.
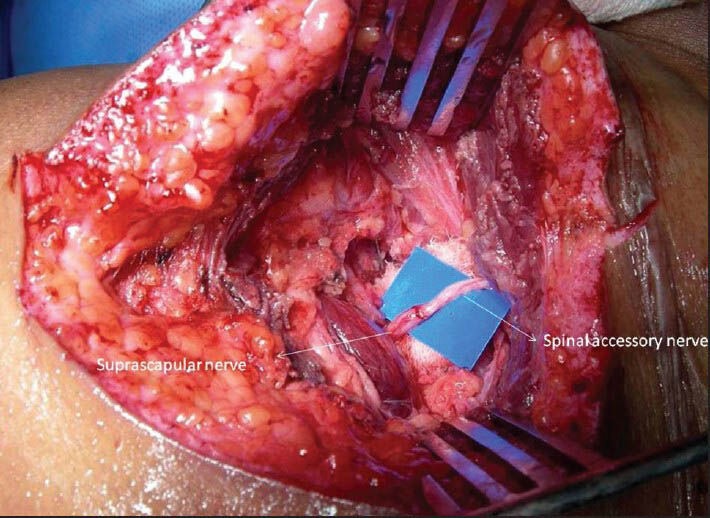
Transfer of spinal accessory nerve to suprascapular nerve by dorsal approach
NEW NERVE TRANSFERS
In the partial plexus injuries, in last few years, few selective nerve transfers have been described to restore the desired function. These nerve transfers are performed close to the target muscles and allow an early return of function. In upper plexal injuries the restoration of elbow flexion is an important goal, which has been achieved with many donor nerves including the intercostal nerves, medial pectoral nerve, phrenic nerve, thoracodorsal nerve, spinal accessory nerve, and recently introduced ulnar and median nerve fascicular transfer. Transfer of a single fascicle of ulnar nerve to the motor branch of biceps[16] and a fascicle of the median nerve to the brachialis motor branch[17] have produced the most promising results as there is no wastage of any donor nerve fibres to the sensory part of musculocutaneous nerve [Figure 3]. This technique is simple and requires no special reeducation of the muscle. Sparing of 1 or 2 fascicle from the ulnar and median nerves does not result in any subjective deficit of hand function. We have found that there is no added advantage in fascicular selection using a nerve stimulator, while performing the Oberlinnerve transfers.[18]
Figure 3.
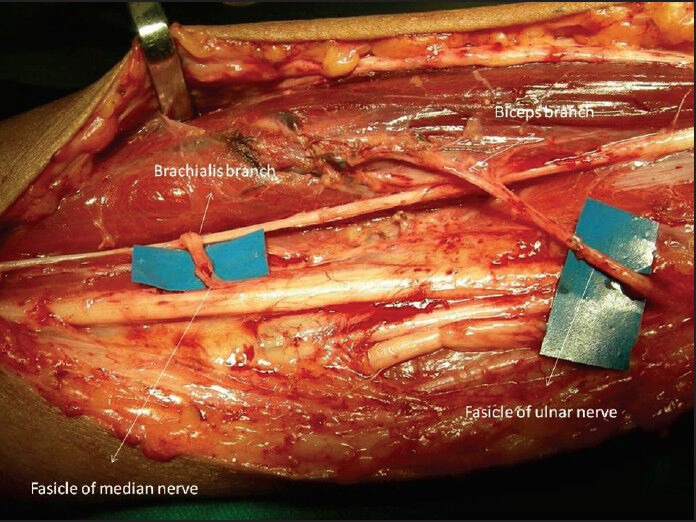
Bifascicular transfer of ulnar and median nerve fasicles to the biceps and brachialis branches
In shoulder reanimation, a simultaneous transfer of suprascapular nerve and axillary nerve yields much better results. Axillary nerve neurotisation through the anterior approach not only requires nerve grafts but also results in dilution of nerve fibers reaching the deltoid muscle.[19] A posterior approach[15,20] allows the transfer of a long head of triceps branch (radial nerve) to the anterior branch of the axillary nerve that innervates the anterior and middle parts of the deltoid muscle [Figure 4]. This transfer is again close to the target muscle (deltoid) and avoids the misdirection of the regenerated axons into the cutaneous branch and teres minor. The functioned loss is minimal and is compensated by the remaining two heads of triceps muscle.
Figure 4.
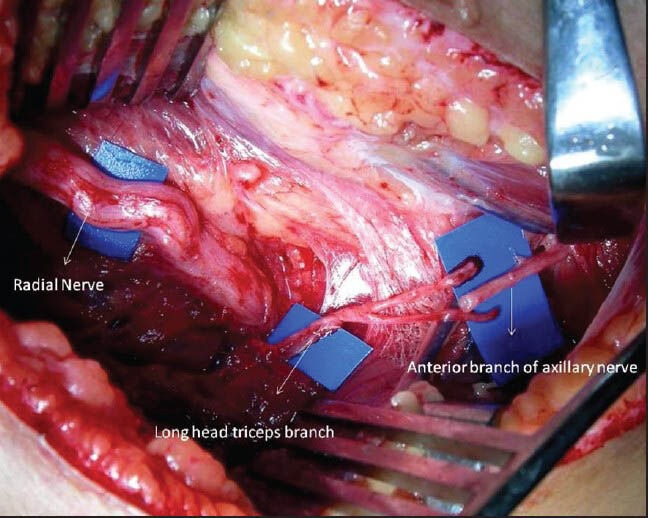
Transfer of long head triceps branches of radial nerve to the anterior branch of axillary nerve
In upper root avulsion injuries, serratus anterior muscle is dysfunctional resulting into winging of scapula. Serratus anterior muscle is needed to rotate the scapula during the last degrees of shoulder abduction. Therefore the repair of long thoracic nerve is mandatory for achieving optimum shoulder function. In recent, Uerpairojkit et al.[21] have described a new nerve transfer, that is, transfer of either medial or lateral branch of the thoracodorsal nerve to the long thoracic nerve with promising results [Figure 5].
Figure 5.
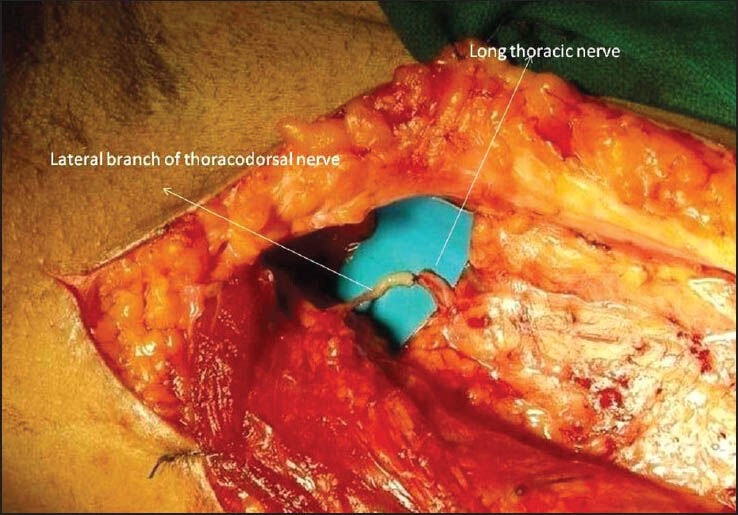
Transfer of lateral branch of thoracodorsal nerve to the long thoracic nerve
Zheng et al.[22] have described a new nerve transfer in the management of isolated lower trunk injury by transferring the brachialis muscle branch of musculocutoneous nerve to the posterior fascicular group of the median nerve. At the level of brachialis motor branch, median nerve is consistently arranged in three fascicular groups. Microanatomic studies have shown that the posterior fascicular group is mainly composed of anterior interosseous nerve innervating the finger flexors. To restore finger flexion, motor branch to the brachioradialis muscle has been transferred to the anterior interosseous nerve [Figure 6] in the management of lower plexus lesions.[23]
Figure 6.
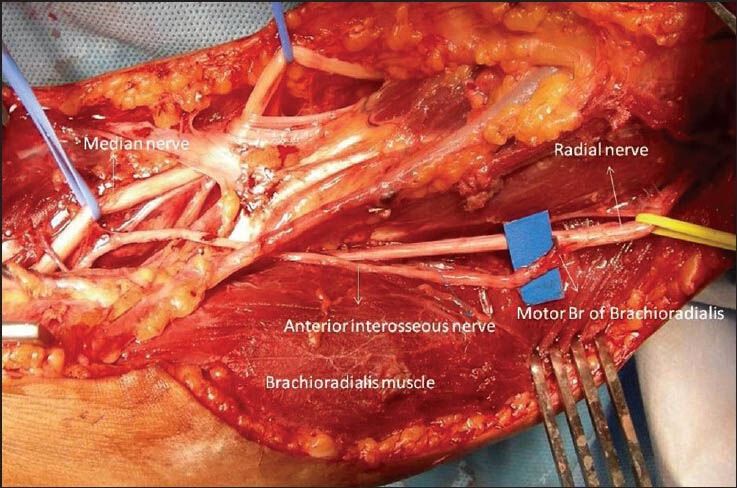
Transfer of motor branch of brachioradialis muscle to the anterior interosseous nerve
PRESPINAL ROUTE IN CONTRALATERAL C7 NERVE ROOT TRANSFER
The use of contralateral C7 transfer is necessary in global palsies when local options for nerve transfers are limited. Under such conditions contralateral C7 transfer is one of the viable options in restoring basic functions. However, to reduce the distance to the target nerves, grafts connected with the contralateral nerve root, have been placed underneath the anterior scalene and longus colli muscles, and then passed through the retro-oesophageal space to neurotise the recipient nerve. In a recent report,[24] where contralateral C7 root was used to neurotise the upper trunk and C5/C6 nerve roots, the mean length of suaral nerve graft could be reduced to 6.8 ± 1.9 cm.
VASCULARISED NERVE GRAFTS
Vascularised nerve grafts may be suitable in a scarred bed and to reconstruct large nerve defects. Vascularised nerve grafts were introduced by Taylor and Ham in 1976.[25] Though the initial results were encouraging,[26] but the technique continues to be controversial. A vascular complication might result in the complete loss of the graft. However, for bridging the long defects (30 cm or more), such as in the contralateral transfer, vascularised nerve grafts might prove to be more useful.[27,28] In global brachial plexus with C8 and T1 root avulsions, pedicled vascularised ulnar nerve has been used for a contralateral C7 root transfer to the median nerve.[29]
ENDOSCOPIC HARVESTING OF SURAL NERVE
Endoscopic harvesting of the sural nerve graft has been devised to[30] overcome the potential drawbacks of the open technique. It is associated with less morbidity, more aesthetic advantages, and greater patient satisfaction.
NERVE CONDUITS
Direct neurorrhaphy is feasible only in recent, clean and sharp nerve transections. Very often nerve grafts are required to bridge the retracted nerve ends. Use of nerve grafts not only results in donor site morbidity, but also leads to loss of several axons across the nerve graft coaptation sites. At times autologous nerve graft transplantation is not feasible due to limited numbers of donor nerves available. This problem has led to the development of new techniques for bridging the nerve gap.[31] There is now considerable evidence that peripheral nerves have the potential to regenerate in an appropriate microenvironment. Natural or synthetic guidance channels are being developed as an alternative to nerve grafts. These nerve conduits direct the axonal sprouts from the proximal nerve end to the distal and also provide a channel for the diffusion of neurotropic and neurotrophic factors secreted by the Schwann cells of the injured distal nerve end and minimize infiltration of fibrous tissue.[32] Tubes made of biological materials such as collagen have been used with more success for distances of less than 3 cm.[33] Wolfe et al. published their experience using bioabsorbable nerve conduits assisted repair as an adjunct to brachial plexus neurorraphy with evidence of clinical and electromyographic recovery.[34] However, further evaluation will be required in terms of their efficacy, especially in the management of obstetric brachial plexus palsy where nerve gap is small.
FIBRIN GLUE IN NERVE REPAIR
Conventionally, nerve grafts have been sutured with a synthetic microsuture, which may induce considerable fibrotic and inflammatory reactions at the coaptation site. This could seriously hamper regeneration of nerve fibres. In 1988, Narakas[35] revived the use of fibrin glue in nerve repair. Since then its use has steadily gained. One study[36] has compared the use of fibrin glue and microsutures in the repair of rat median nerve and found that nerve repairs performed with fibrin sealants produced less inflammatory response and fibrosis, and resulted in better axonal regeneration and better fibre alignment than the nerve repairs performed with microsutures alone. In addition, the fibrin sealant techniques were faster and easier to perform [Figure 7]. In last few years it is being increasingly used in nerve coaptations.[37]
Figure 7.
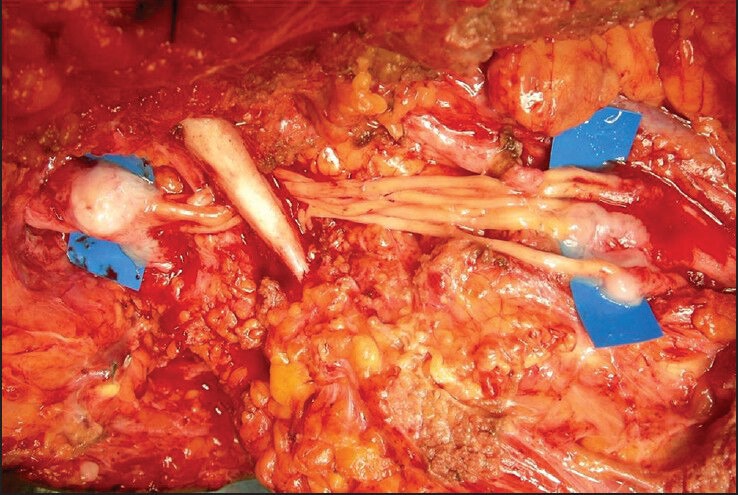
Fibrin glue fixation of multiple sural nerve grafts
DEVELOPMENTS IN GLOBAL BRACHIAL PLEXUS PALSY
Certain exciting developments have taken place in the management of global brachial plexus injuries. While selective nerve transfers can restore shoulder and elbow functions achieving hand functions remains a problem in such patients.
Free functioning muscle transfers
Encouraged by results of free gracilis muscle transfer to restore hand functions in late 90's,[38] Doi et al. described their experience with double free functioning muscle transfer in achieving elbow flexion and hand functions They were able to restore excellent to good elbow flexion in 96% of patients and >30° of total active finger motion was restored in 65% with second muscle transfer.[39] Dodakundi et al.[40] also reported that double free functioning muscle transfer significantly improved the disabilities of the arm, shoulder and hand score in 36 patients with traumatic total brachial plexus palsy. When gracilis was used to achieve elbow flexion and finger extension as in Doi transfer, it was found less effective in achieving two simultaneous functions. The combined gracilis adductor longus triple free functioning muscle transfer was described by Tu and Sananpanich.[41] The triple muscle transfer could achieve satisfactory finger extension and good hook grip of hand with good elbow flexion. Authors have used this transfer for primary distal to proximal method of reconstruction in brachial plexus injuries and in failed previous nerve transfer surgeries.[42]
Contralateral C7 root transfer to paralyzed lower trunks
Wang et al.[43] report reinnervation of the thenar muscle in five patients, who underwent the contralateral C7 nerve root transfers for repair of total brachial plexus root avulsions. The patients were followed up from 2 to 9 years after surgery. The strength of abductor pollicis brevis muscle with Grade M2 was found in four patients and Grade M1 in one with EMG showing motor unit potential. These findings show a definite reinnervation of thenar muscles after repair of the median nerve with the contralateral C7 nerve root transfer, and provide evidence for further investigation of reconstruction of the brachial plexus root avulsion injury with this procedure.
RE-IMPLANTATION OF AVULSED SPINAL ROOTS IN TO THE SPINAL CORD
A strategy to restore motor function in the affected arm by reimplanting into the spinal cord the avulsed ventral roots or autologous nerve grafts connected distally to the avulsed roots has been popularized by Carlstedt et al.[44,45] They have noticed useful recovery in shoulder and proximal arm muscles with alleviation of pain. However, the sensory recovery has been poor. Nevertheless this is a fascinating modality of achieving functions. Further studies will be necessary to prove its usefulness.
CONCLUSION
Brachial plexus injuries represent devastating injuries with a poor prognosis. The results of brachial plexus repair have considerably improved with the introduction of microsurgical techniques and magnification. Shorter defects (as in obstetric palsy) are being bridged with nerve conduits. Use of fibrin glue in nerve coaptation has considerably reduced to operating time. Nerve allografts with new immunosuppressant (FK-506) are being used where there is paucity of autografts. Direct replantation of avulsed spinal roots into the spinal cord is a new area of research in brachial plexus reconstruction.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Songcharoen P. Management of brachial plexus injury in adults. Scand J Surg. 2008;97:317–23. doi: 10.1177/145749690809700408. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari PS, Sadhotra LP, Bhargava P, Bath AS, Mukherjee MK, Singh P, et al. Management of missile injuries of the brachial plexus. Indian J Neurotrauma. 2006;3:49–54. [Google Scholar]
- 3.Kline DG. Timing for brachial plexus injury: A personal experience. Neurosurg Clin N Am. 2009;20:24–6. doi: 10.1016/j.nec.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Roger B, Travers V, Laval-Jeantet M. Imaging of posttraumatic brachial plexus injury. Clin Orthop Relat Res. 1988;237:57–61. [PubMed] [Google Scholar]
- 5.Bhandari PS, Sadhotra LP, Bhargava P, Bath AS, Mukherjee MK, Bhatti T, et al. Surgical outcomes following nerve transfers in upper brachial plexus injuries. Indian J Plast Surg. 2009;42:150–60. doi: 10.4103/0970-0358.59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abul-Kasim K, Backman C, Björkman A, Dahlin LB. Advanced radiological work-up as an adjunct to decision in early reconstructive surgery in brachial plexus injuries. J Brachial Plex Peripher Nerve Inj. 2010;5:14. doi: 10.1186/1749-7221-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freund W, Brinkmann A, Wagner F, Dinse A, Aschoff AJ, Stuber G, et al. MR neurography with multiplanar reconstruction of 3D MRI datasets: An anatomical study and clinical applications. Neuroradiology. 2007;49:335–41. doi: 10.1007/s00234-006-0197-6. [DOI] [PubMed] [Google Scholar]
- 8.Takahara T, Hendrikse J, Yamashita T, Mali WP, Kwee TC, Imai Y, et al. Diffusion-weighted MR neurography of the brachial plexus: Feasibility study. Radiology. 2008;249:653–60. doi: 10.1148/radiol.2492071826. [DOI] [PubMed] [Google Scholar]
- 9.Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34:486–97. doi: 10.3174/ajnr.A3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang DC, Lee GW, Hashem F, Wei FC. Restoration of shoulder abduction by nerve transfer in avulsed brachial plexus injury: Evaluation of 99 patients with various nerve transfers. Plast Reconstr Surg. 1995;96:122–8. doi: 10.1097/00006534-199507000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Merrell GA, Barrie KA, Katz DL, Wolfe SW. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am. 2001;26:303–14. doi: 10.1053/jhsu.2001.21518. [DOI] [PubMed] [Google Scholar]
- 12.Mikami Y, Nagano A, Ochiai N, Yamamoto S. Results of nerve grafting for injuries of the axillary and suprascapular nerves. J Bone Joint Surg Br. 1997;79:527–31. doi: 10.1302/0301-620x.79b4.7481. [DOI] [PubMed] [Google Scholar]
- 13.Malessy MJ, de Ruiter GC, de Boer KS, Thomeer RT. Evaluation of suprascapular nerve neurotization after nerve graft or transfer in the treatment of brachial plexus traction lesions. J Neurosurg. 2004;101:377–89. doi: 10.3171/jns.2004.101.3.0377. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari PS, Deb P. Dorsal approach in transfer of the distal spinal accessory nerve into the suprascapular nerve: Histomorphometric analysis and clinical results in 14 cases of upper brachial plexus injuries. J Hand Surg Am. 2011;36:1182–90. doi: 10.1016/j.jhsa.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 15.Bhandari PS, Deb P. Posterior approach for both spinal accessory nerve to suprascapular nerve and triceps branch to axillary nerve for upper plexus injuries. J Hand Surg Am. 2013;38:168–72. doi: 10.1016/j.jhsa.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Oberlin C, Béal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J Hand Surg Am. 1994;19:232–7. doi: 10.1016/0363-5023(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 17.Tung TH, Novak CB, Mackinnon SE. Nerve transfers to the biceps and brachialis branches to improve elbow flexion strength after brachial plexus injuries. J Neurosurg. 2003;98:313–8. doi: 10.3171/jns.2003.98.2.0313. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari PS, Deb P. Fascicular selection for nerve transfers: The role of the nerve stimulator when restoring elbow flexion in brachial plexus injuries. J Hand Surg Am. 2011;36:2002–9. doi: 10.1016/j.jhsa.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Hung LK, Zhang GM, Lao J. Applied anatomy of the axillary nerve for selective neurotization of the deltoid muscle. Clin Orthop Relat Res. 2001;390:244–51. doi: 10.1097/00003086-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 20.Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: A report of 7 cases. J Hand Surg Am. 2003;28:633–8. doi: 10.1016/s0363-5023(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 21.Uerpairojkit C, Leechavengvongs S, Witoonchart K, Malungpaishorpe K, Raksakulkiat R. Nerve transfer to serratus anterior muscle using the thoracodorsal nerve for winged scapula in C5 and C6 brachial plexus root avulsions. J Hand Surg Am. 2009;34:74–8. doi: 10.1016/j.jhsa.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Zheng XY, Hou CL, Gu YD, Shi QL, Guan SB. Repair of brachial plexus lower trunk injury by transferring brachialis muscle branch of musculocutaneous nerve: Anatomic feasibility and clinical trials. Chin Med J (Engl) 2008;121:99–104. [PubMed] [Google Scholar]
- 23.García-López A, Sebastian P, Martinez F, Perea D. Transfer of the nerve to the brachioradialis muscle to the anterior interosseous nerve for treatment for lower brachial plexus lesions: Case report. J Hand Surg Am. 2011;36:394–7. doi: 10.1016/j.jhsa.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Yiu HW, Li P, Li Y, Wang H, Pan Y. Contralateral C7 nerve root transfer to neurotize the upper trunk via a modified prespinal route in repair of brachial plexus avulsion injury. Microsurgery. 2012;32:183–8. doi: 10.1002/micr.20963. [DOI] [PubMed] [Google Scholar]
- 25.Taylor GI, Ham FJ. The free vascularized nerve graft. A further experimental and clinical application of microvascular techniques. Plast Reconstr Surg. 1976;57:413–l26. [PubMed] [Google Scholar]
- 26.Bonney G, Birch R, Jamieson AM, Eames RA. Experience with vascularized nerve grafts. Clinics in Plastic Surgery. 1984;11:137–142. [PubMed] [Google Scholar]
- 27.Doi K, Kuwata N, Kawakami F, Tamaru K, Kawai S. The free vascularized sural nerve graft. Microsurgery. 1984;5:175–84. doi: 10.1002/micr.1920050403. [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa T, Nakamura S, Manabe T, Mikawa Y. Vascularized nerve grafts for the treatment of large nerve gap after severe trauma to an upper extremity. Arch Orthop Trauma Surg. 2004;124:209–13. doi: 10.1007/s00402-003-0617-6. [DOI] [PubMed] [Google Scholar]
- 29.Waikakul S, Orapin S, Vanadurongwan V. Clinical results of contralateral C7 root neurotization to the median nerve in brachial plexus injuries with total root avulsions. J Hand Surg Br. 1999;24:556–60. doi: 10.1054/jhsb.1999.0264. [DOI] [PubMed] [Google Scholar]
- 30.Park SB, Cheshier S, Michaels D, Murovic JA, Kim DH. Endoscopic harvesting of the sural nerve graft: Technical note. Neurosurgery. 2006;58:ONS–E180. doi: 10.1227/01.NEU.0000193527.31749.2F. [DOI] [PubMed] [Google Scholar]
- 31.Deal DN, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20:63–8. doi: 10.5435/JAAOS-20-02-063. [DOI] [PubMed] [Google Scholar]
- 32.Agnew SP, Dumanian GA. Technical use of synthetic conduits for nerve repair. J Hand Surg Am. 2010;35:838–41. doi: 10.1016/j.jhsa.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Ashley WW, Jr, Weatherly T, Park TS. Collagen nerve guides for surgical repair of brachial plexus birth injury. J Neurosurg. 2006;105:452–6. doi: 10.3171/ped.2006.105.6.452. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe SW, Strauss HL, Garg R, Feinberg J. Use of bioabsorbable nerve conduits as an adjunct to brachial plexus neurorrhaphy. J Hand Surg Am. 2012;37:1980–5. doi: 10.1016/j.jhsa.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Narakas A. The use of fibrin glue in repair of peripheral nerves. Orthop clin North Am. 1988;19:187–98. [PubMed] [Google Scholar]
- 36.Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante RL, et al. Fibrin glue: An alternative technique for nerve coaptation — Part II. Nerve regeneration and histomorphometric assessment. J Reconstr Microsurg. 2006;22:123–8. doi: 10.1055/s-2006-932507. [DOI] [PubMed] [Google Scholar]
- 37.Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127:2381–90. doi: 10.1097/PRS.0b013e3182131cf5. [DOI] [PubMed] [Google Scholar]
- 38.Doi K, Hattori Y, Kuwata N, Soo-heong T, Kawakami F, Otsuka K, et al. Free muscle transfer can restore hand function after injuries of the lower brachial plexus. J Bone Joint Surg Br. 1998;80:117–20. doi: 10.1302/0301-620x.80b1.8073. [DOI] [PubMed] [Google Scholar]
- 39.Doi K, Muramatsu K, Hattori Y, Otsuka K, Tan SH, Nanda V, et al. Restoration of prehension with the double free muscle technique following complete avulsion of the brachial plexus. Indications and long-term results. J Bone Joint Surg Am. 2000;82:652–66. [PubMed] [Google Scholar]
- 40.Dodakundi C, Doi K, Hattori Y, Sakamoto S, Fujihara Y, Takagi T, et al. Outcome of surgical reconstruction after traumatic total brachial plexus palsy. J Bone Joint Surg Am. 2013;95:1505–12. doi: 10.2106/JBJS.K.01279. [DOI] [PubMed] [Google Scholar]
- 41.Tu YK, Sananpanich K. Venice Italy: 2008. Gracilis-adductor longus muscle transfer for patients with brachial plexus inuries. In: Micro Reconstructive Surgery, The First Sino-European Meeting on Brachial Plexus Micro-Reconstructive Surgery. [Google Scholar]
- 42.Tu YK, Chung KC. Surgical procedures for recovery of hand functions. In: Chung KC, Yang LJ, McGillicuddy JE, editors. Practical Management of Paediatric and Adult Brachial Brachial Plexus Injuries St Louis, Oxford. London, New York: Elsevier Saunders; 2012. [Google Scholar]
- 43.Wang L, Zhao X, Gao K, Lao J, Gu YD. Reinnervation of thenar muscle after repair of total brachial plexus avulsion injury with contralateral C7 root transfer: Report of five cases. Microsurgery. 2011;31:323–6. doi: 10.1002/micr.20836. [DOI] [PubMed] [Google Scholar]
- 44.Carlstedt T, Anand P, Hallin R, Misra PV, Norén G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93(2 Suppl):237–47. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- 45.Carlstedt T. Nerve root replantation. Neurosurg Clin N Am. 2009;20:39–50. doi: 10.1016/j.nec.2008.07.020. [DOI] [PubMed] [Google Scholar]


