Abstract
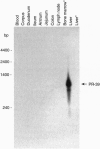
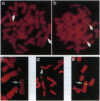
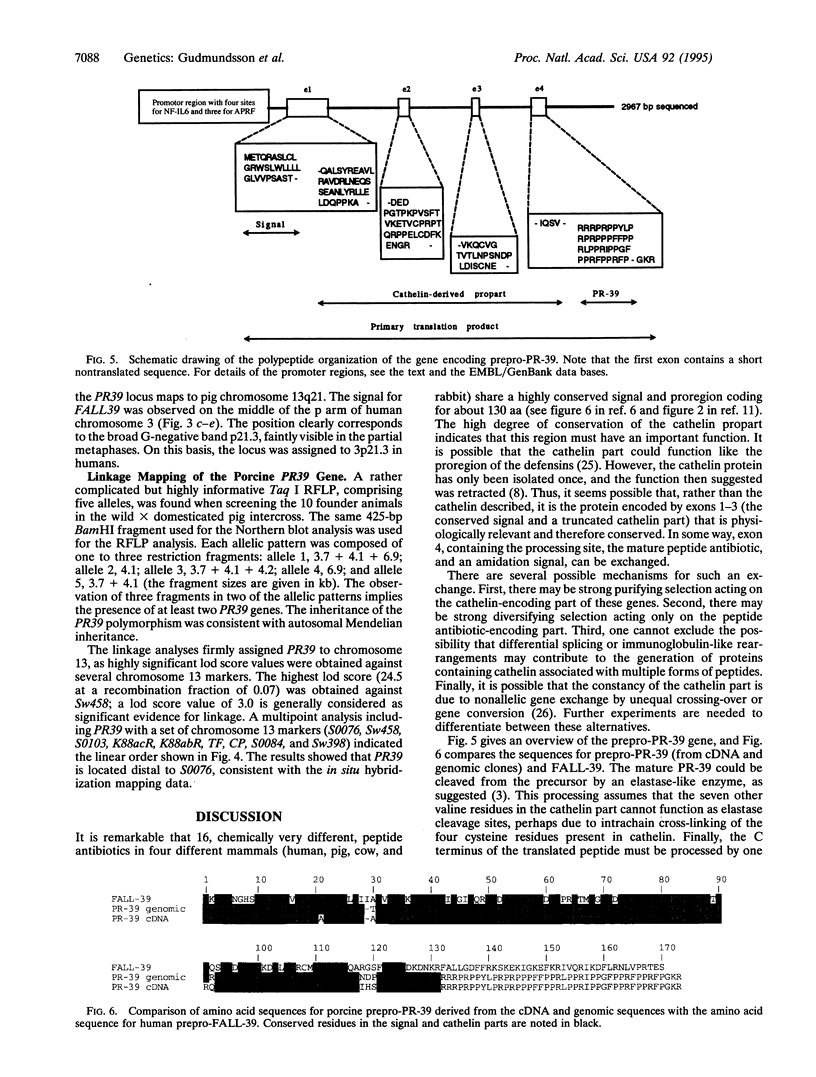
PR-39 is a porcine 39-aa peptide antibiotic composed of 49% proline and 24% arginine, with an activity against Gram-negative bacteria comparable to that of tetracycline. In Escherichia coli, it inhibits DNA and protein synthesis. PR-39 was originally isolated from pig small intestine, but subsequent cDNA cloning showed that the gene is expressed in the bone marrow. The open reading frame of the clone showed that PR-39 is made as 173-aa precursor whose proregion belongs to the cathelin family. The PR39 gene, which is rather compact and spans only 1784 bp has now been sequenced. The coding information is split into four exons. The first exon contains the signal sequence of 29 residues and the first 37 residues of the cathelin propart. Exons 2 and 3 contain only cathelin information, while exon 4 codes for the four C-terminal cathelin residues and the mature PR-39 peptide extended by three residues. The sequenced upstream region (1183 bp) contains four potential recognition sites for NF-IL6 and three for APRF, transcription factors known to regulate genes for both cytokines and acute phase response factors. Genomic hybridizations revealed a fairly high level of restriction fragment length polymorphism and indicated that there are at least two copies of the PR39 gene in the pig genome. PR39 was mapped to pig chromosome 13 by linkage and in situ hybridization mapping. The gene for the human peptide antibiotic FALL-39 (also a member of the cathelin family) was mapped to human chromosome 3, which is homologous to pig chromosome 13.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerberth B., Gunne H., Odeberg J., Kogner P., Boman H. G., Gudmundsson G. H. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerberth B., Lee J. Y., Bergman T., Carlquist M., Boman H. G., Mutt V., Jörnvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991 Dec 18;202(3):849–854. doi: 10.1111/j.1432-1033.1991.tb16442.x. [DOI] [PubMed] [Google Scholar]
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Andersson L., Haley C. S., Ellegren H., Knott S. A., Johansson M., Andersson K., Andersson-Eklund L., Edfors-Lilja I., Fredholm M., Hansson I. Genetic mapping of quantitative trait loci for growth and fatness in pigs. Science. 1994 Mar 25;263(5154):1771–1774. doi: 10.1126/science.8134840. [DOI] [PubMed] [Google Scholar]
- Archibald A. L., Haley C. S., Brown J. F., Couperwhite S., McQueen H. A., Nicholson D., Coppieters W., Van de Weghe A., Stratil A., Winterø A. K. The PiGMaP consortium linkage map of the pig (Sus scrofa). Mamm Genome. 1995 Mar;6(3):157–175. doi: 10.1007/BF00293008. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Agerberth B., Boman A. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect Immun. 1993 Jul;61(7):2978–2984. doi: 10.1128/iai.61.7.2978-2984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Smyth D. G. Peptide amidation. Trends Biochem Sci. 1991 Mar;16(3):112–115. doi: 10.1016/0968-0004(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Del Sal G., Storici P., Schneider C., Romeo D., Zanetti M. cDNA cloning of the neutrophil bactericidal peptide indolicidin. Biochem Biophys Res Commun. 1992 Aug 31;187(1):467–472. doi: 10.1016/s0006-291x(05)81517-7. [DOI] [PubMed] [Google Scholar]
- Diamond G., Jones D. E., Bevins C. L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H., Johansson M., Chowdhary B. P., Marklund S., Ruyter D., Marklund L., Bräuner-Nielsen P., Edfors-Lilja I., Gustavsson I., Juneja R. K. Assignment of 20 microsatellite markers to the porcine linkage map. Genomics. 1993 May;16(2):431–439. doi: 10.1006/geno.1993.1207. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Ono M., Povsic T., Page C., Eriksson E., Klagsbrun M., Bernfield M. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Rayner J. R., Valore E. V., Tumolo A., Talmadge K., Fuller F. The structure of the rabbit macrophage defensin genes and their organ-specific expression. J Immunol. 1989 Aug 15;143(4):1358–1365. [PubMed] [Google Scholar]
- Johansson M., Ellegren H., Andersson L. Comparative mapping reveals extensive linkage conservation--but with gene order rearrangements--between the pig and the human genomes. Genomics. 1995 Feb 10;25(3):682–690. doi: 10.1016/0888-7543(95)80011-a. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992 Nov 15;267(32):23216–23225. [PubMed] [Google Scholar]
- Lenarcic B., Ritonja A., Dolenc I., Stoka V., Berbic S., Pungercar J., Strukelj B., Turk V. Pig leukocyte cysteine proteinase inhibitor (PLCPI), a new member of the stefin family. FEBS Lett. 1993 Dec 27;336(2):289–292. doi: 10.1016/0014-5793(93)80822-c. [DOI] [PubMed] [Google Scholar]
- Linzmeier R., Michaelson D., Liu L., Ganz T. The structure of neutrophil defensin genes. FEBS Lett. 1993 Apr 26;321(2-3):267–273. doi: 10.1016/0014-5793(93)80122-b. [DOI] [PubMed] [Google Scholar]
- Liu L., Ganz T. The pro region of human neutrophil defensin contains a motif that is essential for normal subcellular sorting. Blood. 1995 Feb 15;85(4):1095–1103. [PubMed] [Google Scholar]
- Maeda N., Smithies O. The evolution of multigene families: human haptoglobin genes. Annu Rev Genet. 1986;20:81–108. doi: 10.1146/annurev.ge.20.120186.000501. [DOI] [PubMed] [Google Scholar]
- Mariani P., Johansson M., Ellegren H., Harbitz I., Juneja R. K., Andersson L. Multiple restriction fragment length polymorphisms in the porcine calcium release channel gene (CRC): assignment to the halothane (HAL) linkage group. Anim Genet. 1992;23(3):257–262. doi: 10.1111/j.1365-2052.1992.tb00138.x. [DOI] [PubMed] [Google Scholar]
- O'Brien S. J., Womack J. E., Lyons L. A., Moore K. J., Jenkins N. A., Copeland N. G. Anchored reference loci for comparative genome mapping in mammals. Nat Genet. 1993 Feb;3(2):103–112. doi: 10.1038/ng0293-103. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Kopitar M., Jerala R., Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. 1989 Sep 25;255(2):211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- Sparkes R. S., Kronenberg M., Heinzmann C., Daher K. A., Klisak I., Ganz T., Mohandas T. Assignment of defensin gene(s) to human chromosome 8p23. Genomics. 1989 Aug;5(2):240–244. doi: 10.1016/0888-7543(89)90052-9. [DOI] [PubMed] [Google Scholar]
- Storici P., Del Sal G., Schneider C., Zanetti M. cDNA sequence analysis of an antibiotic dodecapeptide from neutrophils. FEBS Lett. 1992 Dec 14;314(2):187–190. doi: 10.1016/0014-5793(92)80971-i. [DOI] [PubMed] [Google Scholar]
- Storici P., Scocchi M., Tossi A., Gennaro R., Zanetti M. Chemical synthesis and biological activity of a novel antibacterial peptide deduced from a pig myeloid cDNA. FEBS Lett. 1994 Jan 17;337(3):303–307. doi: 10.1016/0014-5793(94)80214-9. [DOI] [PubMed] [Google Scholar]
- Storici P., Zanetti M. A cDNA derived from pig bone marrow cells predicts a sequence identical to the intestinal antibacterial peptide PR-39. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1058–1065. doi: 10.1006/bbrc.1993.2358. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Akira S., Yoshida K., Umemoto M., Yoneda Y., Shirafuji N., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995 Jan 27;80(2):353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Tossi A., Scocchi M., Zanetti M., Storici P., Gennaro R. PMAP-37, a novel antibacterial peptide from pig myeloid cells. cDNA cloning, chemical synthesis and activity. Eur J Biochem. 1995 Mar 15;228(3):941–946. doi: 10.1111/j.1432-1033.1995.tb20344.x. [DOI] [PubMed] [Google Scholar]