Abstract
Oligodendrocytes are myelin-forming glia that ensheath the axons of neurons in the CNS. Recent studies have revealed that Wnt/β-catenin signaling plays important roles in oligodendrocyte development and myelin formation. However, there are conflicting reports on the specific function of Wnt signaling components in oligodendrocyte specification and differentiation. In the present study, we demonstrate that activation of β-catenin in neural progenitor cells before gliogenesis inhibits the generation of oligodendrocyte progenitors (OLPs) in mice. Once OLPs are formed, β-catenin becomes necessary for oligodendrocyte differentiation. Disruption of β-catenin signaling instead leads to a significant delay of oligodendrocyte maturation. These findings suggest that Wnt/β-catenin pathway regulates oligodendrocyte development in a stage-dependent manner.
Keywords: β-catenin, oligodendrocyte differentiation, OLPs, spinal cord, Wnt
Introduction
Myelin ensheathment of neuronal axons enables rapid and accurate transmission (salutatory conduction) of electrical currents along axons. In the CNS, myelin sheaths are elaborated by specialized glial cells termed oligodendrocytes (OLs) (Baumann and Pham-Dinh, 2001). Impairment of OL function is found in many neurological disorders including multiple sclerosis (Van der Walt et al., 2010; Prineas and Parratt, 2012), schizophrenia, and bipolar disorder (Tkachev et al., 2003). Elucidation of signaling pathways that control OL differentiation and myelin formation is a crucial prerequisite for developing novel strategies for myelin repair in these neurological diseases.
Recent studies have revealed that canonical Wnt signaling plays vital roles in OL development. Despite the extensive work outlining the function of Wnt signaling in OL development, there are some conflicting reports on the role of the Wnt pathway in oligodendrogenesis. For instance, inactivation of Wnt/β-catenin signaling with dominant-negative forms of Tcf/Lef (Ye et al., 2009; Langseth et al., 2010) or Wnt antagonism (Shimizu et al., 2005; Langseth et al., 2010) increases the production of OL progenitors (OLPs). Activation of Wnt/β-catenin signaling by ways such as ΔExon3 mutation of β-catenin (Fancy et al., 2009; Feigenson et al., 2009; Ye et al., 2009), Wnt3a treatment (Shimizu et al., 2005; Feigenson et al., 2009; Azim and Butt, 2011), loss of function of the Wnt pathway inhibitor Apc (ApcMin; Fancy et al., 2009), or Apc knock-out (Lang et al., 2013) significantly inhibit the maturation of OLs. Moreover, stabilization of Axin2 via inhibiting tankyrase with small molecule XAV939, or reduction of β-catenin concentration, accelerates OLP differentiation and myelination after hypoxic and demyelinating injury (Fancy et al., 2011). Together, these studies demonstrate that increased Wnt signaling inhibits the production and differentiation of OLP. However, the following studies illustrate that Wnt signaling is vital for the production and differentiation of OLP. For instance, activation of Wnt signaling in postnatal brain by inhibiting Gsk3β or Wnt3a treatment increases OLPs and promotes myelination (Azim and Butt, 2011), whereas overexpression of the dominant-negative form of Tcf7l2/Tcf4 decreases the number of Olig2 and Pdgfrα-positive cells (Ortega et al., 2013). Wnt/β-catenin signaling has also been shown to be an essential direct driver of myelin gene expression in both Schwann cells and OLs (Tawk et al., 2011; Makoukji et al., 2012). In addition, Tcf7l2/Tcf4 is expressed specifically in premyelinating OLs in spinal cord (Fu et al., 2009), and knock-out of Tcf7l2 causes a myelin deficit phenotype (Fu et al., 2009; Ye et al., 2009). These studies suggest that the Wnt/β-catenin pathways functions to promote OL differentiation.
To address the perplexing roles of Wnt/β-catenin signaling in OL development, we investigated OL specification and differentiation in various genetically modified mutant mice with both gain of function (β-catenin ΔExon3, Axin2−/−) and loss of function (β-catenin ΔExon2–6) of β-catenin. Activation of β-catenin signaling resulted in an inhibition of the specification of OLP from neural stem cells. Conversely, loss of β-catenin function had little effect on OLP generation, but caused a significant delay and reduction of OL differentiation. Together, these results demonstrate the stage-specific effects of Wnt/β-catenin signaling on OL development.
Materials and Methods
Animals.
Use of the animals was approved by the Committee of Laboratory Animals, Hangzhou Normal University. Olig1Cre without Neo cassette has been previously described (Xin et al., 2005). Mouse lines for Axin2LacZ (Lustig et al., 2002), β-cateninloxP(Exon3) (Harada et al., 1999), β-cateninloxP(Exon2–6) (Brault et al., 2001), Rosa26R-LacZ (Soriano, 1999), and Rosa26Sor-GNZ (Schüller et al., 2008) were obtained from The Jackson Laboratory. Mice of either sex were used for sampling.
In situ hybridization.
Samples were fixed in 4% paraformaldehyde/PBS at 4°C overnight, followed by 20% sucrose infusion for cryoprotection, and finally embedded in Tissue-Tek O.C.T Compound (Sakura Finetek). Samples were cryosectioned at 18 μm for In situ hybridization (ISH). DIG-labeled RNA probes were transcribed by T7, T3, or SP6 RNA polymerase using DIG RNA Labeling Mix or Fluorescein RNA Labeling Mix (Roche Diagnostics). Standard ISH was performed according to manufacturer's instruction.
Quantitative reverse-transcription PCR.
RNA from the spinal cord of four wild-type (WT) mice and four β-catenin gain-of-function (CatG/+) mice was purified individually using RNAiso Plus [TaKaRa Biotechnology (Dalian)]. RNA was reverse transcribed into cDNA using PrimeScript II first Strand cDNA Synthesis Kit [TaKaRa Biotechnology (Dalian)]. Real-time PCR was performed using SsoFast EvaGreen Supermix with CFX96 Real-Time PCR Detection System (Bio-Rad). Primer sequences used for gene expression analysis were obtained from PrimerBank (Wang et al., 2012). Student's t test was used for statistical analysis.
Results
Activation of Wnt/β-catenin signaling impaired the generation of OLPs
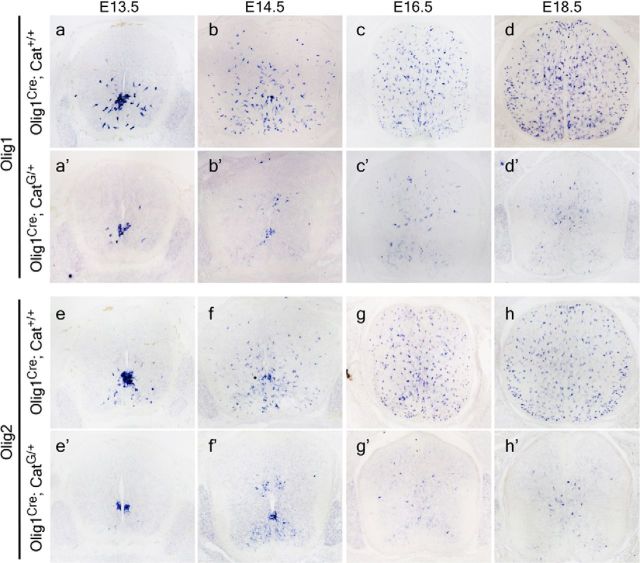
To systematically investigate the role of Wnt/β-catenin signaling in OLP specification, we first examined the expression of several OLP markers in the spinal cords of β-cateninloxP(Exon3)/Olig1Cre transgenic mice in which β-catenin is selectively activated in the ventral pMN domain and its OLP progenies. At embryonic day 13.5 (E13.5), while many Olig1+ and Olig2+ cells were produced from the ventral pMN domain in the control tissues (Fig. 1a,e), only a few Olig1+/Olig2+ cells migrated away from the ventricular zone in the spinal cord of CatG/+ mice (Fig. 1a′,e′). At E14.5, Olig1/2+ cells in the control animals proliferated rapidly and dispersed into the entire tissue, but only a small number of Olig1/2+ oligodendrocyte precursor cells (OPCs) were observed adjacent to the ventral and dorsal ventricular zone in CatG/+ mice (Fig. 1b′,f′). Even at later stages, the number of Olig1/2+ cells did not appear to increase with time in CatG/+ mice despite their wider distribution (Fig. 1c–d′,g–h′). No significant difference in cell death was observed between CatG/+ mice and WT mice at these stages (data not shown).
Figure 1.
Activation of β-catenin impairs the generation and migration of Olig1 and Olig2-positive cells. Transverse sections of spinal cord from E13.5 (a–a′, e–e′), E14.5 (b–b′, f–f′), E16.5 (c–c′, g–g′), and E18.5 (d–d′, h–h′), WT (a–h), and CatG/+ (a′–h′) mice are subjected to ISH with Olig1 and Olig2 riboprobes as OL lineage cell markers. The total number of Olig1 and Olig2-positive cells in CatG/+ mice is severely reduced.
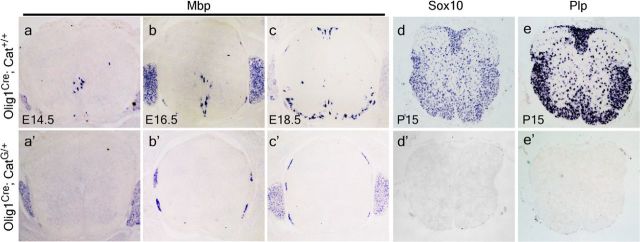
In agreement with the reduced generation of Olig1/2+ cells, expression of two OLP markers, Sox10 and Pdgfrα, was also impaired by catenin activation. Sox10+ and Pdgfrα+ cells were only detected in the ventral ventricular zone of E13.5 CatG/+ spinal cord, contrary to their wide distribution in control tissues (Fig. 2a–a′,d–d′). At E16.5 and later stages, Sox10+ and Pdgfrα+ cells were not found in the CatG/+ tissues (Fig. 2b–c′,e–f′), indicating that Sox10+ and Pdgfrα+ cells failed to migrate out of the ventricular zone and progress along the OLP lineage. Together, these results suggest that activation of the β-catenin pathway dramatically inhibits the specification of neural stem cell-derived OLPs. Concomitant with the lack of OLPs, expression of the mature OL marker, Mbp or Plp, was completely absent at all stages (from E14.5 to P15 when animals fail to survive) in the spinal cord tissue of CatG/+ mice (Fig. 3).
Figure 2.
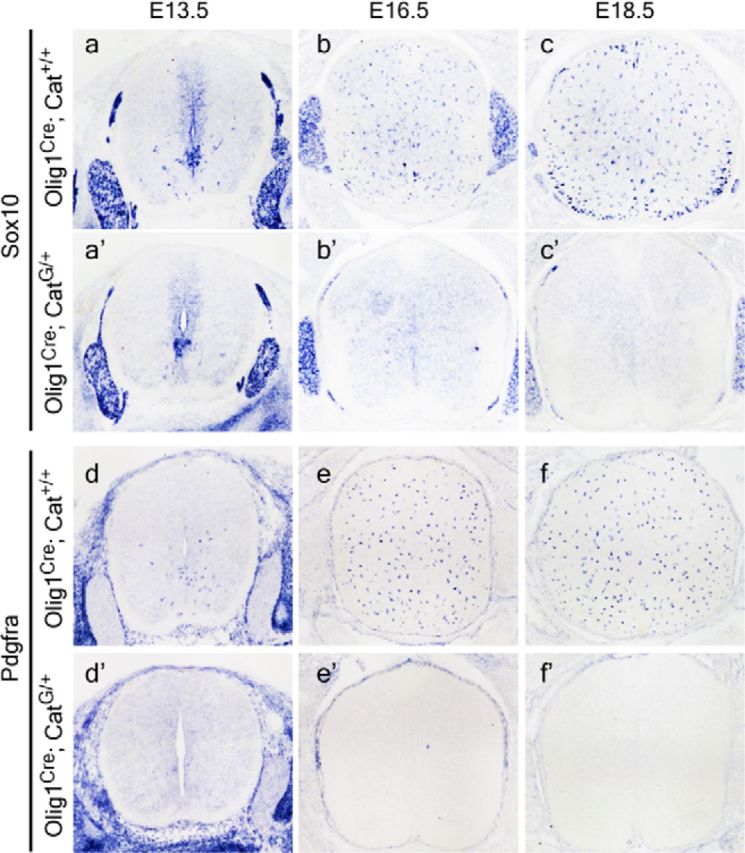
Activation of Wnt/β-catenin signaling inhibits the generation of OLPs. Transverse sections of spinal cord from E13.5 (a–a′, d–d′), E16.5 (b–b′, e–e′), and E18.5 (c–c′, f–f′) of WT (a–f) and CatG/+ (a′–f′) mice are subjected to ISH with Sox10 and Pdgfrα riboprobes. Lack of expression of Sox10 and Pdgfrα in the spinal cord of CatG/+ mice (b′–f′) indicated that specification of OLPs from neural stem cells was impaired.
Figure 3.
Lack of OLs in CatG/+ mice. Transverse sections of spinal cord from E14.5 (a–a′), E16.5 (b–b′), E18.5 (c–c′), and P15 (d–e′) of WT (a–e) and CatG/+ (a′–e′) mice are subjected to ISH with Sox10, Mbp, or Plp riboprobes as OLP or mature OL marker. Expression of Sox10, Mbp, and Plp is absent in CatG/+ tissue.
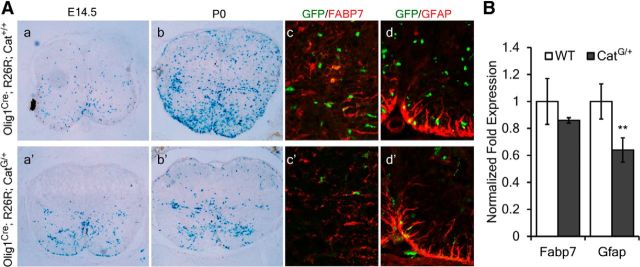
Intriguingly, in the CatG/+ spinal cord, the Olig1+/Olig2+ cells derived from the pMN domain followed a radial migratory trajectory similar to astrocyte precursor cells (Fig. 1b′,f′), raising the possibility that these cells may have changed identity to astrocyte precursor cells. To examine this possibility, we traced the fate of these cells in Olig1Cre; R26R; CatG/+ transgenic mice. Consistent with the ISH study, LacZ+ cells in E14.5 CatG/+ embryos were restricted to the ventral spinal cord, in contrast to the widespread distribution in the WT spinal cord (Fig. 4a–a′). Even at P0, the LacZ+ cells remained largely confined to the ventral spinal cord in CatG/+ tissue as would be expected for astrocytes, whereas LacZ+ cells in the control tissue were widely dispersed (Fig. 4b–b′). However, double immunofluorescent labeling in Olig1Cre; R26R; CatG/+ mice revealed that expression of two astrocyte markers Fabp7 and Gfap was not increased (Fig. 4c,d,c′,d′); instead, Gfap expression was significantly reduced (Fig. 4B). These results suggest the Olig1-Cre+ cells may become an unknown glial cell type or unknown stage of glial development, as suggested by a different study (Ye et al., 2009).
Figure 4.
Altered migration pattern of LacZ+ cells in CatG/+ mice. A, Spinal cord tissues from Olig1Cre; Rosa26 (a–d) or Olig1Cre; Rosa26; CatG/+ (a′–d′) are stained for β-galactosidase activity (a, b, a′, b′) or subjected to immunofluorescent labeling with FABP7 or GFAP (c, d, c′, d′). LacZ+ cells in CatG/+ background are less abundant and largely confined to the ventral spinal cord. B, Quantitative reverse-transcription PCR analysis of the expression level of Fabp7 and Gfap in E18.5 spinal cord from WT and CatG/+ mice. All values are presented as means ± SD. **p < 0.01.
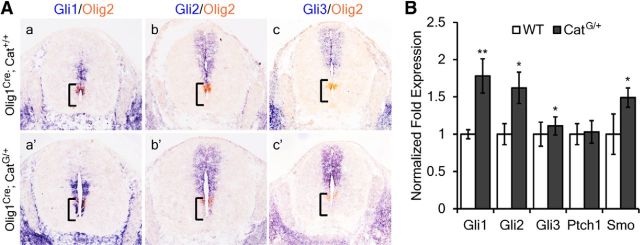
It has been previously shown that OLP generation in the ventral spinal cord is promoted by Shh signaling from the ventral midline structures (Pringle et al., 1996). To test the possibility that Wnt signaling represses OLP generation by inhibiting Shh signaling, we examined the expression of three Gli genes (Gli1–3), the downstream components of Shh pathway, in the transgenic spinal cord. It was found that expression of all Gli genes, especially Gli1, which functions as a transcriptional activator for Shh signaling, was upregulated in the spinal cord of CatG/+ mice (Fig. 5). Moreover, the expression of Smo and Ptch1, the coreceptors for Shh, was not reduced in the spinal cord of CatG/+ mice (Fig. 5). Together, these results indicated that Shh signaling was not compromised by increased Wnt/β-catenin activity.
Figure 5.
Expression of Gli genes in the CatG/+ embryonic spinal cords. A, Transverse sections of spinal cord from E13.5 of WT (a–c) and CatG/+ (a′–c′) mice are subjected to ISH with Gli1, Gli2, and Gli3 riboprobes, followed by ISH with Olig2 riboprobe. B, Quantitative reverse-transcription PCR analysis of the expression level of Gli1, Gli2, Gli3, Smo, and Ptch1 in E18.5 spinal cord from WT and CatG/+ mice. All values are presented as means ± SD. *p < 0.05; **p < 0.01.
Axin2LacZ/LacZ mice showed reduced OLP cell number and delayed maturation of OL
Considering that β-catenin with ΔExon3 mutation may result in dysregulation of the cadherin/catenin-mediated cell—cell adhesion signaling pathway (Nelson and Nusse, 2004) or partial loss of its function, we used the Axin2LacZ/LacZ mice to further verify the effects of increased Wnt/β-catenin signaling on OL development. Consistent with the finding in CatG/+ embryos, a significantly smaller number of Sox10 and Pdgfrα-positive cells were produced at E13.5 from the ventricular zone in the spinal cord of Axin2-null mice than in their WT or heterozygous littermates (Fig. 6a–f,B). However, expression of both Sox10 and Pdgfrα returned to normal in Axin2-null mice at P0, probably due to the increased proliferation of Pdgfrα+ OLPs in the mutants (Fig. 6g–l). Differentiation of OLs was also inhibited in the Axin2 mutants, as evidenced by the dramatically decreased number of Tcf7l2/Tcf4+, Mbp+, and Plp+ mature OL cells at P0 (Fig. 7).
Figure 6.
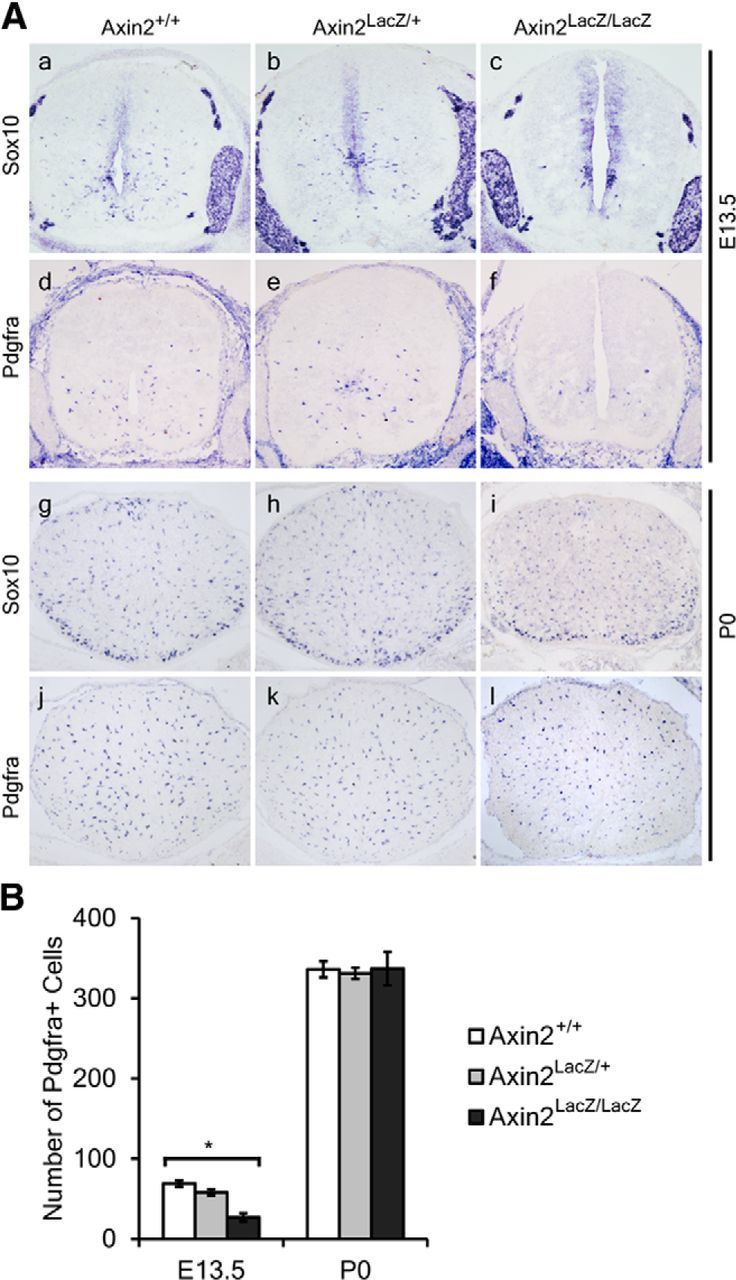
Reduced generation of OLPs in Axin2 mutant mice. A, Transverse sections of spinal cord from E13.5 (a–f) and P0 (g–l) WT (a, d, g, j), heterozygous (b, e, h, k) and mutant (c, f, i, l) Axin2 mice are subjected to ISH with Sox10 (a–c, g–i) and Pdgfrα (d–f, j–l) riboprobes as OLP markers. B, Quantification of the Pdgfrα+ cells in E13.5 and P0 spinal cord from WT heterozygous and mutant Axin2 mice. All values are presented as means ± SD. *p < 0.05.
Figure 7.
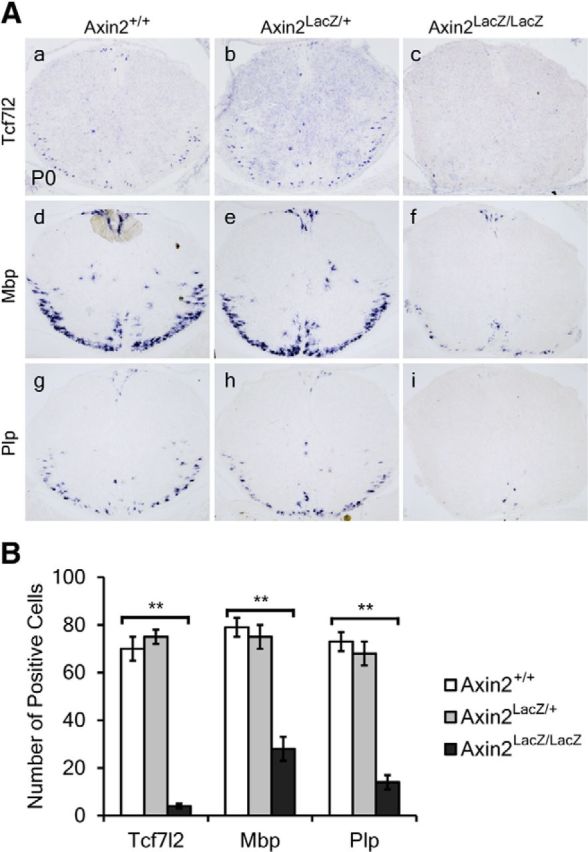
Delay of OL differentiation in Axin2 mutant mice. A, Transverse sections of spinal cord from P0 WT (a, d, g), heterozygous (b, e, h), and mutant (c, f, i) Axin2 mice are subjected to ISH with Tcf7l2 (a–c), Mbp (d–f), and Plp (g–i) riboprobes. B, Quantification of the Tcf7l2+, Mbp+, and Plp+ cells in P0 spinal cord from WT, heterozygous, and mutant Axin2 mice. All values are presented as means ± SD. **p < 0.01.
Together, data from both the CatG/+ and Axin2 mutant mice demonstrate that activation of β-catenin signaling at the early stage of glial development represses the specification and generation of OLPs from neural progenitor cells and their subsequent differentiation.
β-catenin is required for the timely differentiation of OLPs
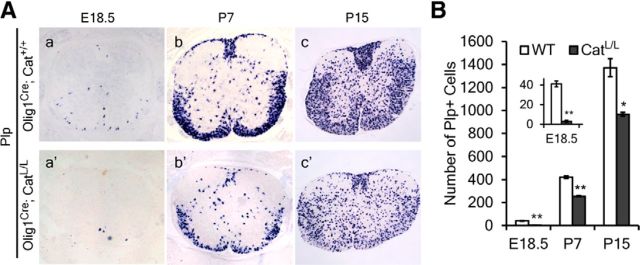
We next examined if β-catenin is required for the development of OLs by selectively disrupting the function of β-catenin in OLPs in the Olig1Cre/β-cateninloxP(Exon2–6)/loxP(Exon2–6) conditional mutant mice (CatL/L). Compared with WT littermates, a mild but significant increase of Pdgfrα-positive cells was observed in the spinal cord of CatL/L mutant mice at E12.5 (Fig. 8b–b′,B), suggesting that OLPs were specified precociously. At E18.5, CatL/L mice displayed a normal number of Sox10+ and Pdgfrα+ OPCs in the spinal cord (Fig. 8c–d′,B), but the expression of mature OL marker Plp was dramatically reduced (Fig. 9a–a′,B). However, at later stages (P7 and P15), the number of differentiated OLs in CatL/L mice increased markedly, but remained significantly smaller than that in WT mice (Fig. 9b–c′,B). Thus, maturation of OLs was delayed rather than absent in the CatL/L mice, indicating that Wnt/β-catenin signaling is required for the timely differentiation of OLPs, but not absolutely essential for their maturation.
Figure 8.
Precocious generation of OLPs in β-catenin mutant mice. A, ISH showed that at E12.5 more Sox10+ and Pdgfrα+ cells migrated away from the ventricular zone in CatL/L mice (a′, b′) than in WT mice (a, b). At E18.5, a similar number of Sox10+ and Pdgfrα+ cells was observed in CatL/L mice (c′, d′) and WT mice (c, d). B, Quantification of the Pdgfrα+ cells in E12.5 and E18.5 spinal cord from WT and CatG/+ mice. All values are presented as means ± SD. **p < 0.01.
Figure 9.
Delay of OL differentiation in β-catenin mutant mice. A, Transverse sections of spinal tissues from E18.5 (a–a′), P7 (b–b′), and P15 (c–c′) control (a–c) and CatL/L (a′–c′) mice are subjected to ISH with Plp riboprobe. Expression of Plp is delayed and reduced. B, Quantification of the Plp+ cells in WT and CatG/+ spinal cord tissues. All values are presented as means ± SD. *p < 0.05; **p < 0.01.
Discussion
In this study, we systematically investigated the seemingly discrepant roles of Wnt/β-catenin signaling in OL development by examining OL specification and differentiation under various Wnt/β-catenin signaling conditions. Our results suggest that Wnt/β-catenin signaling exhibits a stage-specific function in the control of OL lineage development.
Activation of Wnt/β-catenin signaling in neural progenitor cells inhibits the specification of OLPs
Previous studies have demonstrated that declined expression of Wnt in the dorsal spinal cord shows a strong correlation with the emergence of OLPs, suggesting an inhibitory role for Wnt/β-catenin signaling in OLP specification (Shimizu et al., 2005; Ye et al., 2009; Langseth et al., 2010). In agreement with these earlier studies, our work shows that loss of β-catenin function also leads to precocious expression of OLP markers in the ventral spinal cord. Moreover, increased β-catenin function or expression in the CatG/+ transgenic mice (Figs. 1, 2) or Axin2 mutant mice (Fig. 6) suppresses the fate specification of OLPs from neural stem cells, as shown by the lack or reduction of expression of Sox10 and Pdgfrα in spinal cord tissues. Since expression of the Shh pathway components, Smo, Ptch1, and Gli genes, is not compromised in CatG/+ mice (Fig. 7), it is possible that Wnt/β-catenin signaling inhibits the specification of OLPs from neural stem cells through some unknown factors that override the positive effects of Shh signaling on specification of OLPs.
Interestingly, Ortega et al. (2013) demonstrated that activation of canonical Wnt signaling in culture and in adult brain by Wnt3a treatment significantly increased the number of Olig2+ and Pdgfrα+ cells, and inhibition of canonical Wnt signaling by overexpression of dnTcf4 decreased the number of these cells. Their findings differ from this and other studies, which showed that inhibition of Wnt signaling by disruption of β-catenin, and overexpression of dnTcf4, dnLef1, or Dkk1 increases OLP generation during development (Ye et al., 2009; Langseth et al., 2010). At this stage, we do not understand what causes this discrepancy; one possibility is that neural stem cells in developing embryos may behave differently from those in adult tissues or in culture.
It was previously observed that reduced or delayed generation of OLPs is invariably associated with delayed differentiation of OLs in many unrelated mutant mice, such as Nkx6.1 and Gli2 mutants (Liu et al., 2003; Qi et al., 2003), suggesting that an intrinsic timing mechanism may also operate during the in vivo development of OLs. Thus, the absent expression of OL differentiative markers in the CatG/+ and Axin2−/− tissues is likely to be secondary to the defective generation of OLPs, and therefore should not be interpreted as inhibition of OL differentiation by β-catenin. In further support of this notion, activation of β-catenin after OLP specification in CnpCre; CatG/+ mice only led to a mild delay of OLP differentiation and axonal myelination (Feigenson et al., 2009).
Wnt/β-catenin signaling is required for the timely differentiation of OLPs
It was previously shown that selective inhibition of Wnt components blocks the expression of myelin protein zero (Mpz) and peripheral myelin protein 22 (Pmp22) in Schwann cells as well as Plp in OLs, while activation of Wnt signaling by Wnt1 treatment increased the expression of Mpz, Pmp22, and Plp (Tawk et al., 2011). Moreover, treatment with Wnt1 enhanced the recruitment of β-catenin to the Tcf/Lef transcription factor binding sites present in the promoters of Mpz and Pmp22 (Tawk et al., 2011). These results suggest that Wnt/β-catenin signaling may directly drive the expression of myelin gene expression and is essential for OLP differentiation (Tawk et al., 2011). Consistently, Tcf7l2 and β-catenin colocalize in the nuclei of premyelinating OLs (Fu et al., 2009, 2012). In addition, BAT-gal reporter mice show that Wnt/β-catenin signaling is active during developmental myelination and remyelination (Fancy et al., 2009). More importantly, mutation of Tcf7l2/Tcf4 gene results in an inhibition of OL maturation (Fu et al., 2009; Ye et al., 2009). In the present study, we have demonstrated that the Olig1Cre-mediated disruption of β-catenin in OLPs does not alter the expression of OLP markers such as Sox10 and Pdgfrα, but causes a significant delay in the expression of mature OL markers such as Mbp and Plp (Fig. 9). Thus, Wnt/β-catenin signaling is required for the timely differentiation of OLPs.
These observations, together with others, suggest that Wnt/β-catenin signaling fulfills multiple functions during OL development. At early CNS development, Wnt/β-catenin signaling in the dorsal neural tube functions to inhibit OLP specification from neural progenitor cells, and that the generation of OLPs is accompanied by declined expression of Wnts. After OPCs are produced and migrate into the white matter regions, Tcf7l2/Tcf4 expression is upregulated and coordinates with β-catenin to promote differentiation of OLPs. Finally, once OLs are fully differentiated, Apc is upregulated in mature OLs to suppress Wnt/β-catenin signaling (Lang et al., 2013).
Footnotes
This work was supported by National Key Basic Research Program of China (2012CB910402; 2013CB531300), the National Natural Science Foundation of China (Grant 31101642, 31372150), the Key Project of Zhejiang Provincial Natural Science Foundation of China (2100730), the Zhejiang Provincial Science and Technology Key Project (2011C13030), the NMSS (RG-3275, RG-4727), and the NIH (R01-NS37717, R01-NS072427 and R01-NS075243).
The authors declare no competing financial interests.
References
- Azim K, Butt AM. GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia. 2011;59:540–553. doi: 10.1002/glia.21122. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson K, Reid M, See J, Crenshaw EB, 3rd, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Fu H, Cai J, Clevers H, Fast E, Gray S, Greenberg R, Jain MK, Ma Q, Qiu M, Rowitch DH, Taylor CM, Stiles CD. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–11408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Kesari S, Cai J. Tcf7l2 is tightly controlled during myelin formation. Cell Mol Neurobiol. 2012;32:345–352. doi: 10.1007/s10571-011-9778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J, Maeda Y, Bannerman P, Xu J, Horiuchi M, Pleasure D, Guo F. Adenomatous polyposis coli regulates oligodendroglial development. J Neurosci. 2013;33:3113–3130. doi: 10.1523/JNEUROSCI.3467-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langseth AJ, Munji RN, Choe Y, Huynh T, Pozniak CD, Pleasure SJ. Wnts influence the timing and efficiency of oligodendrocyte precursor cell generation in the telencephalon. J Neurosci. 2010;30:13367–13372. doi: 10.1523/JNEUROSCI.1934-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Cai J, Hu X, Tan M, Qi Y, German M, Rubenstein J, Sander M, Qiu M. Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development. 2003;130:6221–6231. doi: 10.1242/dev.00868. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoukji J, Belle M, Meffre D, Stassart R, Grenier J, Shackleford G, Fledrich R, Fonte C, Branchu J, Goulard M, de Waele C, Charbonnier F, Sereda MW, Baulieu EE, Schumacher M, Bernard S, Massaad C. Lithium enhances remyelination of peripheral nerves. Proc Natl Acad Sci U S A. 2012;109:3973–3978. doi: 10.1073/pnas.1121367109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega F, Gascón S, Masserdotti G, Deshpande A, Simon C, Fischer J, Dimou L, Chichung Lie D, Schroeder T, Berninger B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat Cell Biol. 2013;15:602–613. doi: 10.1038/ncb2736. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Parratt JD. Oligodendrocytes and the early multiple sclerosis lesion. Ann Neurol. 2012;72:18–31. doi: 10.1002/ana.23634. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Guthrie S, Roelink H, Lumsden A, Peterson AC, Richardson WD. Determination of neuroepithelial cell fate: induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev Biol. 1996;177:30–42. doi: 10.1006/dbio.1996.0142. [DOI] [PubMed] [Google Scholar]
- Qi Y, Tan M, Hui CC, Qiu M. Gli2 is required for normal Shh signaling and oligodendrocyte development in the spinal cord. Mol Cell Neurosci. 2003;23:440–450. doi: 10.1016/S1044-7431(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kagawa T, Wada T, Muroyama Y, Takada S, Ikenaka K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Tawk M, Makoukji J, Belle M, Fonte C, Trousson A, Hawkins T, Li H, Ghandour S, Schumacher M, Massaad C. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis. J Neurosci. 2011;31:3729–3742. doi: 10.1523/JNEUROSCI.4270-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Van der Walt A, Butzkueven H, Kolbe S, Marriott M, Alexandrou E, Gresle M, Egan G, Kilpatrick T. Neuroprotection in multiple sclerosis: a therapeutic challenge for the next decade. Pharmacol Ther. 2010;126:82–93. doi: 10.1016/j.pharmthera.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012;40:D1144–1149. doi: 10.1093/nar/gkr1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yue T, Ma Z, Wu FF, Gow A, Lu QR. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J Neurosci. 2005;25:1354–1365. doi: 10.1523/JNEUROSCI.3034-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]